Abstract
Thermal properties of molten and mixed core materials are required to be known for effective analysis of core damage in severe accidents at nuclear power plants. The specific heat capacity, thermal expansion coefficient, thermal diffusivity, and melting temperature were measured or estimated on the core debris samples of the Three Mile Island Unit 2 (TMI-2) reactor and simulated debris (SIMDEBRIS), which had chemical composition and porosity similar to the TMI-2 debris. The thermal diffusivity of the TMI-2 debris, which is mainly composed of (U, Zr)O2, is as low as 10–25% of UO2 at room temperature but is comparable above 1500 K. The melting temperature of SIMDEBRIS is about 2840 K, which is equivalent to the liquidus temperature of (U, Zr)O2 with the same ZrO2/UO2 ratio. Accordingly, other core materials less than 10% in weight were observed to have no influence on the melting temperature.
1. Introduction
In the accident at the Three Mile Island Unit 2 (TMI-2) reactor, the loss of coolant caused the oxidation of fuel cladding and the temperature of the reactor core was raised through the radioactive decay and the exothermic oxidation. As a consequence, the degradation of the fuel bundle, including liquefactions of fuel and core components, originated in the central upper region of the core. The liquefied materials flowed down, solidified, and formed a small blockage in the lower and cooler parts of the core. The temperature of the relocated materials continuously increased to form a large molten mass called a “molten pool” surrounded by a “hard crust”. Finally, the hard crust failed and the molten materials flowed from the molten pool to the lower head of the reactor vessel through the core barrel and the lower support assembly region [1].
Molten and mixed core materials (debris) were sampled at various positions of the TMI-2 reactor core, and the samples were examined and analyzed in the USA and European countries in order to obtain information about liquefactions and interactions among the core materials, history of the core temperature, and melt progression during the accident [1]. The Japan Atomic Energy Research Institute (JAERI, currently the Japan Atomic Energy Agency (JAEA)) participated in the TMI-2 Vessel Investigation Project conducted by the Organization for Economic Co-operation and Development and the Nuclear Energy Agency (OECD-NEA) [2] and obtained about 60 debris samples, including 10 samples that were solidified on the lower head. Those debris samples were subjected to various tests and analyses at the Reactor Fuel Examination Facility (RFEF) of JAEA [3,4].
Integrated computer codes and specialty codes have been developed for analysis of severe accidents and the prediction of the processes of core degradation and melt progression during severe accidents [5–9]. The high-temperature behavior of core materials and the thermal properties of the debris are essential for developing these computer codes, for estimating the state of reactor cores during and after severe accidents, and for discussing the measures to recover the damaged reactor cores. However, data on thermal properties of the debris are limited. In the present study, specific heat capacity, thermal expansion coefficient, thermal diffusivity, and melting temperature of the core debris sampled from the TMI-2 reactor were measured, and those of the simulated debris (SIMDEBRIS) with a chemical composition and porosity similar to the TMI-2 debris were also measured.
2. Experimental procedure
2.1. Sample
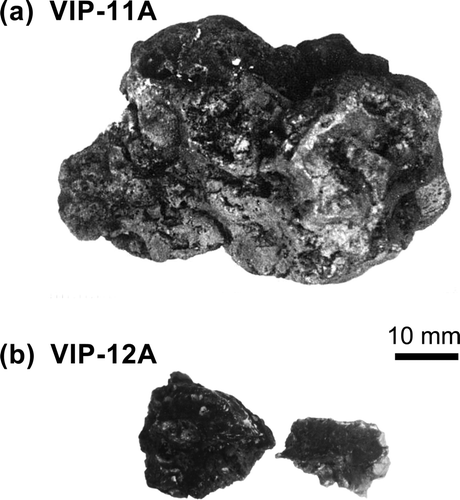
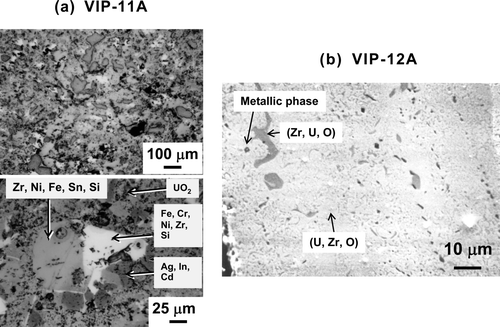
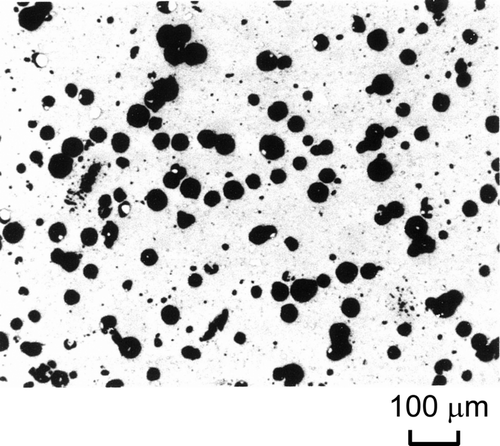
lists the TMI-2 debris samples tested in the present study. The samples were collected from the lower head of the reactor vessel and provided to the JAERI in the frame of the TMI-2 Vessel Investigation Project conducted by the OECD-NEA. The sample numbers (No. 9–12) indicate the quadrant locations from where the samples were collected. VIP-9 was collected from the southeast quadrant, VIP-10 was collected from the northwest quadrant, VIP-11 was collected from the southwest quadrant, and VIP-12 was collected from the northeast quadrant. shows the appearance of VIP-11A and VIP-12A, and shows their microstructures. The TMI-2 debris is generally a mixture of ceramic and metallic phases. The ceramic phases consist of (U, Zr)O2 and/or (Zr, U)O2, and the metallic phases consist of elements from the control rod and the other core materials (Ag, Fe, Ni, Cr, etc.). The mixing ratio of the two phases varies among the debris samples. Main components of the tested TMI-2 debris samples are shown in . For example, the metallic phases are the main components in VIP-11A, whereas the ceramic phases are the main components in VIP-12A and the other samples.
Table 1. List of TMI-2 debris samples.
The apparatus and facility have limitations in performing measurements for the TMI-2 debris, which includes irradiated fuel. Therefore, simulated fuel debris (SIMDEBRIS) samples having chemical composition and porosity similar to the TMI-2 debris were fabricated, and laboratory tests were performed to obtain the extensive data on thermal properties that are essential to accident analyses. 1ists an initial inventory of the core materials in TMI-2 [10] and the composition of SIMDEBRIS. In the preparation of SIMDEBRIS, the main elements of core materials were selected and it was assumed that all of them except silver were oxidized and uniformly mixed. Powdered raw materials of UO2, ZrO2, Fe3O4, Cr2O3, NiO, and Ag were blended, pressed, and sintered in a reducing atmosphere, as was done for the commercial UO2 fuel pellet. The density of SIMDEBRIS was changed from 8.2 to 9.0 g/cm3 by controlling the pressing and sintering processes. The average porosity of the fabricated SIMDEBRIS was measured to be 2.1–20.5% in the microstructure of the cross sections (). shows the microstructure of SIMDEBRIS-L with a density of approximately 8.2 × 106 g/m3. The Electron micro probe analysis (EMPA) of SIMDEBRIS showed that the matrix consists of U, Zr, and O, and the metallic particles of Ag, Cr, and Fe disperse in the matrix.
Table 2. Initial inventory of core materials in TMI-2 and composition of SIMDEBRIS.
2.2. Measurement and evaluation method
The thermal expansion, thermal diffusivity, and melting temperature of the TMI-2 debris and SIMDEBRIS were mostly measured at the RFEF of the JAEA. Some measurements for SIMDEBRIS were performed at Nuclear Fuel Industries Ltd. The specific heat capacity and thermal conductivity were evaluated using reference data and experimental data. Methods adopted in the present study are described in brief.
Thermal expansion of SIMDEBRIS was measured in the temperature range of 273–1873 K with a heat-up rate of 0.1 K/s in decomposed ammonia gas. Data were acquired at 1 K intervals during the heat-up and a polynomial curve for temperature dependence was obtained.
Thermal diffusivity was measured on disk samples with a thickness of 2 × 10−3 m by a laser flash method. The apparatus used for the thermal diffusivity measurement has been described elsewhere [11]. The disk sample was set in the apparatus and heated to the desired temperatures using a tungsten heater, and the sample temperature was measured with a W-5%Re/W-26%Re thermocouple placed near the sample. The measurement was performed from room temperature (RT) to approximately 1800 K at intervals of approximately 100 K in vacuum below 1 × 10−3 Pa. The front surface was heated by pulse irradiation of a ruby laser and the thermal diffusivity was determined by analyzing the temperature rise at the rear surface by the logarithmic method [12]. An In-Sb infrared detector was used to measure the rear surface temperature.
Specific heat capacity of SIMDEBRIS was determined using the following Neumann–Kopp rule.
where xn is the mole fraction of component n and Cpn is the specific heat capacity of component n. The specific heat capacity and the temperature dependence of the components listed in were quoted from the database MALT2 [13] and NIST JANAF [14].
Thermal conductivity was determined by the following equation:
where K is the thermal conductivity, α is the thermal diffusivity, Cp is the specific heat capacity and ρ is the density of the sample. The thermal conductivity of SIMDEBRIS was determined from the measured density, thermal diffusivity, and the specific heat capacity estimated as mentioned above. Since the necessary data were not fully prepared for the TMI-2 debris, the temperature dependence of the density of the TMI-2 debris was estimated from that of SIMDEBRIS and the thermal conductivity was estimated using the specific heat capacity of SIMDEBRIS.
The melting temperatures of the TMI-2 debris and SIMDEBRIS were measured with disk specimens of thickness 2 mm using a thermal arrest method. The apparatus and detailed measuring method are indicated elsewhere [15]. Before the measurement for the debris, calibration was done with Al2O3, Y2O3, and HfO2. In addition, melting temperatures of ZrO2 and UO2 were measured in the present system and found to be 2979 K and 3094 K, respectively. The representative melting temperatures reported in the previous studies are 2980 K [16] and 3120 K [17], respectively; thus, the error in the measurement is smaller than 5%.
3. Results and discussion
3.1. Density
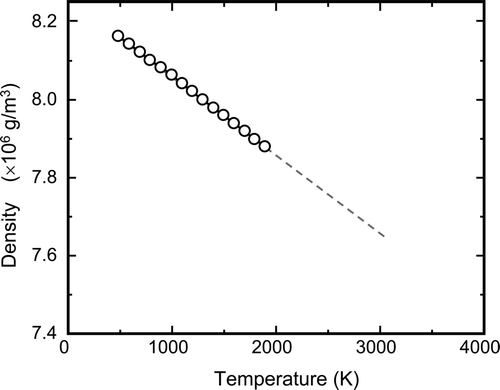
Measured densities of the TMI-2 debris samples ranged from 6.32 × 106 g/m3 to 8.25 × 106 g/m3 as listed in . The densities are between ZrO2 (6 × 106 g/m3) and UO2 (10.96 g/m3). Porosities measured on the microphotographs of the cross sections were 4.6–38.0%. Because there is no correlation between the density and the porosity, the observed variation in the density of the TMI-2 debris is thought to be basically related to that of its composition. shows the temperature dependence of the density for SIMDEBRIS-L (8.2 × 106 g/m3 at RT), which was estimated using the thermal expansion data (shown in Section 3.3). The density decreases by 0.2 × 106 g/m3 with a temperature increase of 1000 K. Data acquired above the temperature of 1873 K were obtained by extrapolating data obtained at lower temperatures.
3.2. Specific heat capacity
shows the temperature dependence of the specific heat capacity of SIMDEBRIS. Because SIMDEBRIS has a unique composition as shown in , the specific heat capacity was calculated using the Neumann–Kopp rule and the reference data for UO2, ZrO2, Fe3O4, Cr2O3, NiO, and Ag [13,14]. Although a discontinuity is seen in the temperature dependence of ZrO2 at approximately 1480 K, which is caused by the phase transformation from the monoclinic phase to the tetragonal phase, the specific heat capacity of SIMDEBRIS continuously increases with temperature. It has been reported that the specific heat capacity of UO2 increases in the temperature range below the melting temperature, but it drops by more than 30% on reaching the melting temperature and decreases with an increase in temperature above the melting temperature [18]. The phenomenon is known as “Bredig transition” which occurs in many fluorite structure compounds [19]. Although it is very difficult to measure and it was not clarified in the scope of the present study whether the Bredig transition occurs in SMDEBRIS and the TMI-2 debris, the information above the melting temperatures would be required for the severe accident analysis.
3.3. Thermal expansion
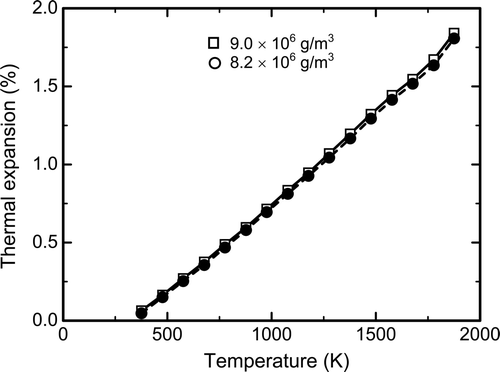
shows thermal expansion of SIMDEBRIS with two different densities (8.2 and 9.0 × 106 g/m3) for the temperature range of 373–1873 K. SIMDEBRIS expands continuously with temperature, which indicates that the influence of the phase transformations of ZrO2 on thermal expansions is negligible. The thermal expansion coefficient in the range of 373–1673 K is 11.6 × 10−6/K for the higher density SIMDEBRIS and 12.3 × 10−6/K for the lower density SIMDEBRIS. The measured data agrees well with the correlation derived by Martin for UO2 [20]. Therefore, the influence of addition of ZrO2 and minor components and difference in porosity appear to be small, and the debris which is mainly composed of (U, Zr)O2 is thought to have a thermal expansion similar to those of SIMDEBRIS and UO2. The thermal expansion of UO2 shows a large deviation above 1600 K from the trend observed at lower temperatures (<1600 K) [20]. The increase in thermal expansion coefficient observed above 1673 K in is possibly related to the reported increase in thermal expansion of UO2.
3.4. Thermal diffusivity and conductivity
shows thermal diffusivities of SIMDEBRIS and the TMI-2 debris as a function of temperature. The SIMDEBRIS data with three kinds of densities are shown as a band. The thermal diffusivity of the UO2 pellet (95% of theoretical density) measured by Hirai [21] is also shown in the figure for comparison. VIP-11A, which is mainly composed of metallic components, has higher diffusivity than the UO2 pellet. The diffusivity of VIP-11A is minimum at approximately 800 K, and it increases at higher temperatures. The thermal diffusivities of the other TMI-2 debris samples also show the temperature dependences with a minimum at approximately 800 K; however, the values are mostly as low as approximately 0.2–1 × 10−6 m2/s (which is approximately 10–25% of UO2 [21]) at lower temperatures. These temperature dependences with minimum values are clearly different from that of UO2, which shows a monotonic decrease in the examined temperature range. Consequently, there is a large difference between the thermal diffusivities of UO2 and the TMI-2 debris (except for VIP-11A) in the low temperature range, and these thermal diffusivities become comparable above 1500 K. SIMDEBRIS shows similar diffusivities to the TMI-2 debris though the diffusivity of SIMDEBRIS continuously decreases with the temperature in the examined range. The TMI-2 debris samples except for VIP-11A are a mixture of (U, Zr)O2 ceramic phases with a small amount of metallic phases. ZrO2 has the relatively low thermal diffusivity and it increases at higher temperatures [22]. Therefore, the drastic decrease at lower temperatures and slight increase at higher temperatures of the thermal diffusivity of the TMI-2 debris may be caused by addition of ZrO2. The porosity is also an effective factor in the thermal diffusivity. Therefore, the difference in porosity as well as addition of ZrO2 is considered to be one of the main causes of the difference between UO2 and the TMI-2 debris and the variation among the TMI-2 debris.
Figure 7. Thermal diffusivity of TMI-2 debris, SIMDEBRIS, and UO2 [20] as a function of temperature.
![Figure 7. Thermal diffusivity of TMI-2 debris, SIMDEBRIS, and UO2 [20] as a function of temperature.](/cms/asset/00d5b48c-d5b6-4326-8bb6-fee7c310ec49/tnst_a_636534_o_f0007g.gif)
Thermal conductivity of the TMI-2 debris was determined using the calculated thermal diffusivity, specific heat capacity, and density. As indicated in section II, SIMDEBRIS data were used to determine the specific heat capacity and the temperature dependence of the density. shows the temperature dependence of thermal conductivity of the TMI-2 debris. The thermal conductivity of UO2 was also calculated with reference data [21,22] and shown in the figure. The thermal conductivity of UO2, which is higher at RT, monotonically decreases with temperature whereas that of the TMI-2 debris, which is much lower at RT, increases with temperature. These thermal conductivities become comparable at 1500−1800 K, similar to the result of thermal diffusivity.
3.5. Melting temperature
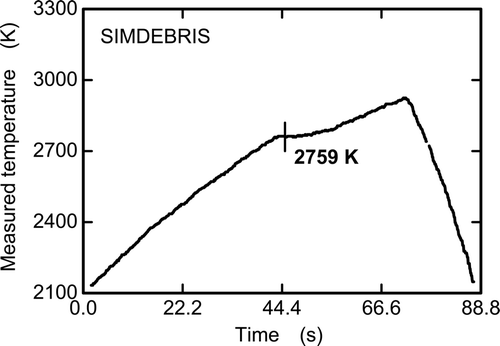
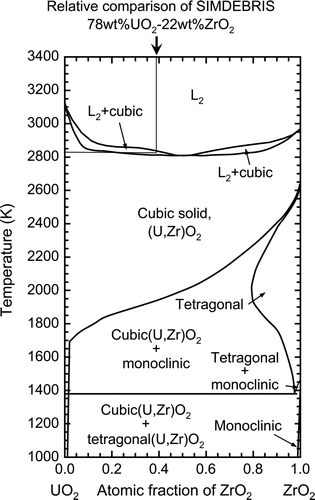
shows an example of the temperature history during the measurement of the melting temperature of SIMDEBRIS. The temperature change is observed to stagnate at a certain temperature level, which is caused by the latent heat of melting of the sample. The measured temperatures were calibrated and the melting temperatures of six SIMDEBRIS samples were determined to be in the range of 2827–2862 K (average value: 2842 K) by the thermal arrest method. The pseudo-binary phase diagram of the UO2–ZrO2 system [23–25] () indicates that the lowest melting temperature of this system is just above 2800 K. SIMDEBRIS contains minor core materials, 7% or 8% in weight. If these minor materials are eliminated from the original composition, the relative composition of SIMDEBRIS in this binary system becomes 78wt%-UO2 and 22wt%-ZrO2 (62at%-UO2 and 38at%-ZrO2). The melting temperature of the UO2–ZrO2 compounds estimated from the pseudo-binary phase diagram is approximately 2830 K. This value is in very good agreement with the average value of the measured melting temperatures of SIMDEBRIS. Therefore, it can be concluded that the melting temperature is influenced very slightly, if at all, by the other minor core materials presented in the core debris.
4. Conclusion
In the case of severe accidents occurring in nuclear power plants, data on the thermal properties of molten and mixed core materials (debris) are necessary for effectively analyzing the core damage. The specific heat capacity, thermal expansion coefficient, thermal diffusivity, and melting temperature of the core debris samples from the TMI-2 reactor were measured, and those of the SIMDEBRIS with a chemical composition and porosity similar to the TMI-2 debris were also measured. It was found that SIMDEBRIS expands continuously with temperature and the thermal expansion coefficient was nearly the same as that of UO2, at least below 1873 K. The thermal diffusivity of the debris which is mainly composed of (U, Zr)O2 was as low as approximately 10–25% of UO2 at RT but was comparable above 1500 K. The melting temperature of SIMDEBRIS was approximately 2840 K, which is nearly equal to the liquidus temperature of (U, Zr)O2 with the same ZrO2/UO2 ratio. Accordingly, the influence of the other core materials less than 10% in weight on the melting temperature is negligible.
Acknowledgments
The authors are grateful to Takashi Otomo for help with the experiments. They also acknowledge and express their appreciation for the time and effort devoted by numerous engineers and technicians at the Department of Hot Laboratories and Facilities, JAEA.
References
- Akers , D. W. , Bart , G. Bottomley , P. 1992 . TMI-2 examination results from the OECD-CSNI program NEA/CSNI/R(91)9, Committee on the Safety of Nuclear Installations, Organization for Economic Cooperation and Development
- Three Mile Island Reactor Pressure Vessel Investigation Project - Achievements and Significant Results . Proceedings of an open forum sponsored by the OECD Nuclear Energy Agency and the United States Nuclear Regulatory Commission . October . pp. 20 – 22 . Boston , , USA : OECD Publishing .
- Uetsuka , H. and Nagase , F. “Companion sample examination and related study at JAERI” . ibid , 269 – 280 .
- Uetsuka , H. , Nagase , F. and Suzuki , T. 1995 . Gamma Spectroscopy of TMI-2 Debris , Text in Japanese : Japan Atomic Energy Research Institute . JAERI-Research, 95-084
- Allison , C. M. Oct 1997 . SCDAP/RELAPS/MOD3.2 Code Manual , Oct , Nuclear Regulatory Commission . NUREG/CR-6150, INEL 96/0422, Revision 1
- Fichot , F. 2000 . ICARE/CATHARE – computer code for severe accidents in LWRs: Description of physical models IPSN Tech. Note SEMAR 00/03, Institute for Nuclear Safety and Protection (IPSN)
- Allelein , H. J. Jacq , F. Severe Accident Code ASTEC Development and Validation . Proceedings of EUROSAFE . November . pp. 18 – l9 . Paris, France
- Gauntt , R. O. Cole , R. K. May 2000 . MELCOR Computer Code Manuals, Vo1ume 2: Reference Manuals, Version 1.8.5 , May , Nuclear Regulatory Commission . Prepared by Sandia National Laboratories (SNL) for the US Nuclear Regulatory Commission, Office of Nuclear Regulatory Research, NUREG/CR-6119, Vo1.2, Rev.2
- Trambaur , T. Coupling Methods of Thermal–Hydraulic Models with Core Degradation Models in ATHLET-CD . Proceedings of the 6th international conference on nuclear engineering (ICONE6), San Diego, CA, USA, 10–14 . May .
- Akers , D. W. and McCardell , R. K. 1989 . Core materials inventory and behavior . Nucl. Technol , 87 : 4 – 223 .
- Owada , I. , Nishino , Y. , Kushida , T. , Nakamura , J. and Matsuda , T. 1994 . Characterization test of the pellet thermal conductivity measurement apparatus using unirradiated samples , Text in Japanese : Japan Atomic Energy Research Institute . Rep. JAERI-Tech, 94–028
- James , H. M. 1980 . Some extensions of the flash method of measuring thermal diffusivity . J. Appl. Phys , 51 : 4666 – 4672 .
- Yokokawa , H. , Yamauchi , S. and Matsumoto , T. 1994 . Thermodynamic database MALT2 and its applications to high temperature materials chemistry . Themochim. Acta , 245 : 45 – 55 .
- Chase , N. W. Jr. 1998 . NIST JANAF thermochemical tables . J. Phys. Chem. Ref. Data ,
- Harada , K. Melting Temperature of High Burnup UO2 Pellet . Proceedings of Enlarged Halden Programme Group Meeting . March . Lillehammer, Norway
- Yamada , T. , Mizuno , M. , Ishizuka , T. and Noguchi , T. 1988 . Liquidus curve measurement in the system zirconia-hafnia, in Advances in Ceramics , 959 – 964 . Science and technology of zirconia III .
- Popov , S. G. , Carbajo , J. J. , Ivanov , V. K. and Yodar , G. L. 2000 . Thermophysical properties of MOX and UO2 fuels including the effects of irradiation , Oak Ridge National Laboratory . ORNL/TM-2000/351
- Dworkin , A. S. and Bredig , M. A. 1968 . Diffuse transition and melting in fluorite and antifluorite type of compounds: Heat content of potassium sulfide from 298 to 1260 K . J. Phys. Chem , 72 : 1277 – 1281 .
- Fink , J. K. 2000 . Thermophysical properties of uranium dioxide . J. Nucl. Mater , 279 : 1 – 18 .
- Martin , D. G. The thermal expansion of solid UO2 and (U,Pu) mixed oxide – A review and recommendations . J. Nucl. Mater , 152 ( 1988 ) 94 – 101 .
- Hirai , M. 1990 . Thermal diffusivity of UO2–Gd2O3 pellets . J. Nucl. Mater , 170 : 247 – 254 .
- Hagrman , D. T. 1993 . SCDAP/RELAPS/MOD3.1 Code Manual, Vol. 4: MATPRO. A Library of Materials Properties for Light Water Reactor Accident Analysis USNRC Rep. NUREG/CR-6150 (EGGG-2720)
- Levin , E. M. and McMurdie , H. F. 1975 . Phase diagrams for ceramists, 1975 supplement , American Ceramic Society : Westerville, OH .
- Cohen , I. and Schaner , B. E. 1963 . A metallographic and X-ray study of the UO2-ZrO2 system . J. Nucl. Mater , 9 : 18 – 52 .
- Lambertson , W. A. and Mueller , M. H. 1953 . Uranium oxide phase equilibrium systems: III. UO2–ZrO2 . J. Am. Ceram. Soc , 36 : 365 – 368 .
![Figure 5. Estimated specific heat capacity of SIMDEBRIS in comparison with data of UO2 and ZrO2 [13,14].](/cms/asset/bf6ca8ed-678a-441e-bc24-1a9ed8607292/tnst_a_636534_o_f0005g.gif)
![Figure 8. Thermal conductivity of TMI-2 debris and UO2 [21,22] as a function of temperature. Values of TMI-2 debris were calculated with specific heat capacity and density of SIMDEBRIS.](/cms/asset/bb154663-1b7a-4467-acb5-c26fffd9a165/tnst_a_636534_o_f0008g.gif)