Abstract
Liquid sodium is used as coolant in fast reactors (FRs), because of its high thermal conductivity and wide temperature range of liquid phase. It has superior thermal medium properties but its chemical reactivity with water and oxygen is very high. Hence, FR plants have to take safety measures regarding these reactions. To develop FR systems for practical use, a better physical and chemical understanding of reaction phenomena and more mechanistic analyses for accidents are desired. Oxidation in the early stage of sodium combustion is especially important regarding the aspect of reaction continuity. The purpose of this study is to understand the sodium reaction precisely in order to apply the knowledge of the sodium reaction to promoting further safety of FRs. In the authors' previous study, it was recognized that dendritic oxide had an important role in the sodium oxidation reaction such as supplying unreacted sodium to the reaction interface. In this study, a mechanistic model for supplying sodium through the dendritic oxide, which is influenced by physical properties and environmental factors, was proposed from the observation results of growth of the dendritic oxide. This mechanistic model provides an explanation of the oxidation behavior, which is consistent with the observation results from the oxidation reaction of sodium with suspended nanoparticles, that sodium with suspended nanoparticles showed a suppression effect on the oxidation reaction due to the difference of dendritic oxide growth behavior.
1. Introduction
Liquid sodium is used as a coolant in fast reactors (FRs). Sodium has many advantages including superior thermal medium properties and compatibility with structural materials. However, it has also the serious disadvantage of high chemical reactivity with oxygen and water. The purpose of this study is to understand the sodium reaction precisely and apply the acquired knowledge to promote further safety of FRs.
The authors previously observed the sodium oxidation reaction, focusing on what occurs at the reaction interface [1]. They showed that changes of the reaction by controlling the oxygen fraction and sodium initial temperature could be systematically obtained. On the other hand, the application of an atomic interaction between nanoparticles and sodium has been studied as a reaction control [2,3]. Trial samples of sodium with suspended titanium nanoparticles were used to confirm that they had different physical properties from pure sodium, such as a decreased evaporation rate and increased surface tension caused by the atomic interaction between the nanoparticles and sodium [4]. From the results of these studies, it became clear that decreased evaporation rate and increased surface tension contribute to reaction control.
In this article, the authors describe the analysis of factors which dominate the oxidation reaction behavior, focusing on the growth behavior of dendritic oxide during oxidation which has a role in an intermediate reaction between sodium and oxygen as mentioned later. The suppression effect of sodium with suspended nanoparticles on the reaction behavior is clarified and the sodium oxidation reaction becomes better understood mechanistically.
2. Characteristic behavior in the sodium oxidation reaction
2.1. Dendritic oxide which forms and grows during the oxidation reaction
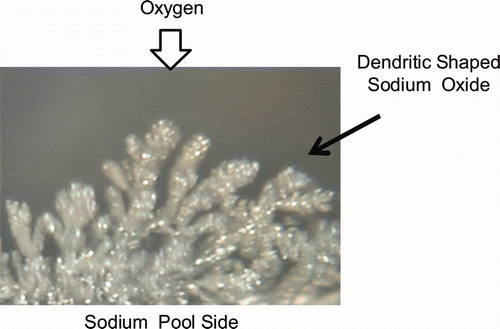
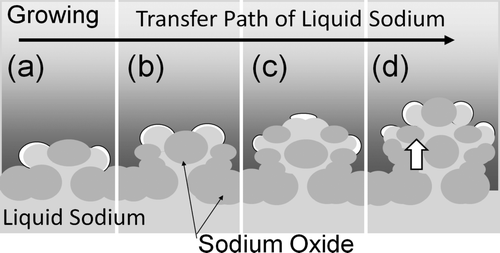
Sodium shows characteristic behavior in the oxidation reaction. In the pool fire process of a sodium pool, sodium has a metallic luster at first and the dendritic oxide as shown in grows on the pool surface when the oxygen is supplied. The reaction progresses with coexisting sodium and dendritic oxide which continues to form and grow. Generally, the oxidation reaction products become an interruption factor for continuity of the oxidation reaction because the chemical stability of oxidation products toward oxygen is high.
However, the sodium reaction product has a dendritic shape and it promotes continuity of the oxidation reaction by sucking sodium up to the reaction interface. For this reason, clarification of the generation and growth mechanism of dendritic oxide is very important for understanding the sodium oxidation reaction.
2.2. Observation of growth behavior of dendritic oxide
Some experiments to understand the growth behavior of dendritic oxide which forms in sodium oxidation reaction were carried out. Because dendritic oxide grows during the course of the reaction, its observation from a fixed point is difficult. Furthermore, because the observed reaction interface is very narrow and a clear view of it is prevented by the sodium oxide aerosol, making observations at the reaction interface is not easy. Therefore, using the way of experimental technique conducted by Ara et al. [4], a stainless steel mesh (thickness = 0.2 mm) was soaked in sodium and then the mesh with the adhered sodium was heated to the initial temperature and oxygen was supplied to the system. The growth behavior of dendritic oxide was recorded using a high-speed camera. In this experimental method, a very small area of the dendritic oxide could be observed by changing the conditions for oxygen fraction and sodium initial temperature.
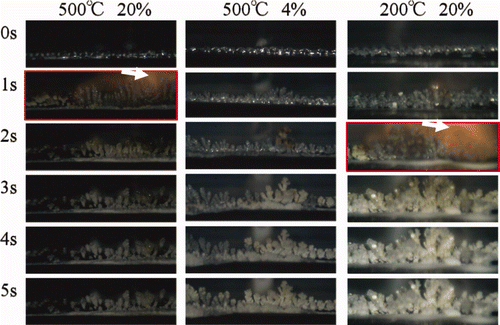
By considering the operating temperature of FRs, the reference experimental conditions were set at 20% oxygen fraction and 500°C initial temperature. shows observations of two different growth behaviors. The relative growth speed of dendritic oxide can be compared by the largeness of oxides after 1 s. The relative growth speed depended significantly on oxygen fraction and was higher in the 20% oxygen fraction than that in the 4% oxygen fraction. Ignition behavior with an orange-colored emission in the gas phase area (indicated by the arrows) was observed only in the 20% oxygen fraction. The shape of reaction product was needle shaped and did not spread at the tip in the 20% fraction. On the other hand, the shape of reaction product became spreading at the tip in the 4% fraction. The relative growth speed also depended on initial temperature. The relative speed at the low initial temperature of 200°C was similar to the speed in the low oxygen fraction of 4% and shape of reaction product was also spreading at the tip.
2.3. Growth model of dendritic oxide
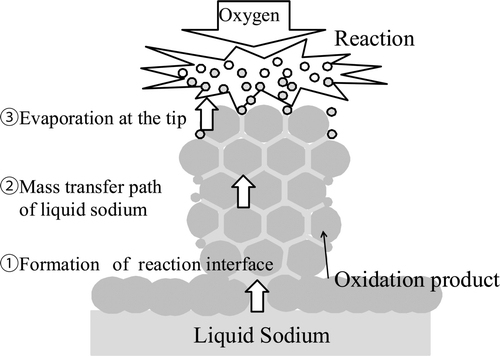
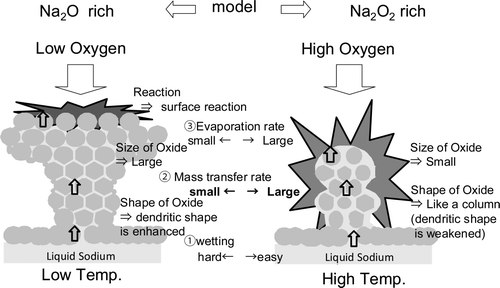
From the observations, dendritic oxide seemed to grow in an intermediate reaction between sodium and oxygen. In this section, the role of the physical properties regarding the growth of the dendritic oxide is estimated from the three points as shown in .
2.3.1. Interface formation of liquid sodium
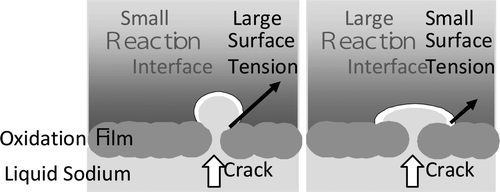
A schematic of the interface formation with an atmosphere is shown in . The reaction product forms as a smooth and white oxidation film on the surface of the liquid sodium at first. After that, the oxidation film becomes cracked and then the flow path which connects between a reaction interface and sodium in the pool (indicated by the heavy arrow in ) is formed. The reaction interface where sodium meets oxygen occurs in the outer surface of ellipsoidal liquid sodium on the oxidation film. When the sodium temperature is low at the first stage of reaction because heat generation is defeated by heat release, the reaction product is deposited on the sodium side, and dendritic oxide grows on the oxidation film because sodium vapor which does not have enough thermal energy cannot move into the gas phase.
The shape of the reaction interface is decided by the balance between gravity and surface tension. The reaction interface is thought to be smaller when the surface tension is larger because it becomes spherical in shape.
2.3.2. Keeping the transfer path in the dendritic oxide
To continue the reaction, sodium in the pool needs to be supplied to the reaction interface at the tip of the dendritic oxide which grows during the reaction; i.e. the transfer path of sodium must be kept in the dendritic oxide.
The dendritic oxide consists of various sodium oxides that are formed depending on the environmental factors such as oxygen fraction and temperature. Liquid sodium has known solubility for oxygen and this solubility depends largely on temperature [5]. The oxygen solubility at 500°C (1200 ppm) is one hundred times larger than the solubility at 200°C (12 ppm).
There was no blockage of the transfer path at high temperature because hardly any sodium flowing in the path is oxidized due to the high oxygen solubility. If oxidation occurs, the temperature rises due to the reaction heat and the amount of flowing sodium in the dendritic oxide becomes large because sodium temperature becomes high due to the reaction heat. The reaction in the growth expansion stage is assumed to progress in a cycle like that shown in .
From another viewpoint, the kinds of oxide are thought to have an influence on keeping the supplying path. It is confirmed that sodium oxides consist mainly of sodium peroxide and sodium monoxide. From the standpoint of thermodynamics, sodium peroxide is formed when the oxygen fraction is sufficiently large, and sodium monoxide is formed under a relatively low oxygen fraction. The melting points of sodium peroxide and monoxide are 675°C and 1275°C, respectively.
It is considered that the kinds of oxides largely influence the transfer of liquid sodium. The operating temperature range of FRs is 200–550°C; however, it is easy to assume that the temperature in the reacting sodium in an accident is over the melting point of sodium peroxide which is lower than the boiling point of sodium (881°C). Sodium peroxidewhich cannot exist as a solid does not prevent the formation of the reaction interface. As a result,the dendritic oxide is thought to grow preferentially toward the direction where the oxygen fraction is high.
2.3.3. Reaction at the dendritic oxide tip
A surface reaction and a gas phase reaction of sodium vapor are occurring simultaneously at the dendritic oxide tip. At first, the surface reaction is relatively dominant. However, the gas phase reaction is thought to become dominant over time. Surface reaction and gas phase reaction can be judged from by confirming the growth of sodium oxide in the condensed phase and the generation of aerosol in gas phase. During surface reaction, oxide is taken into the condensed matter. On the other hand, during gas phase reaction, oxide is released out around the condensed matter. The amount of reaction is thought to become larger, as the evaporation rate becomes larger in thegas phase reaction. As a result, temperatures of the dendritic oxide and sodium rise and the amount of transfer sodium in the path increases. However, the dendritic oxide does not grow so much in the gas phase reaction because the reaction products are released to the gas phase area.
The reaction model described above is shown in . The sodium monoxide tends to form under low oxygen fraction; mass transfer rate of the liquid sodium in the reaction product becomes smaller. Temperature does not rise up for the reason that the reaction rate at the tip becomes small. Oxygen is mainly taken up by the reaction product as surface reaction, so the reaction product is supposed to grow like layer stack. In case of low initial sodium temperature, the growing behavior is same as under low oxygen fraction condition because lower sodium temperature and hence, lower oxygen solution results in larger production of the sodium monoxide. On the other hand, in case of high oxygen fraction and high initial sodium temperature, the reaction interface is formed on the whole of dendritic oxide because it is easy to produce sodium peroxide and move to gas phase reaction quickly. So the shape of the oxide is like a needle and the size of it is smaller than in lower case.
3. Influence of different physical properties on sodium oxidation
3.1. Different physical properties of sodium with suspended nanoparticles
To control the chemical activity of sodium, the suppression effect of sodium with suspended nanoparticles was studied. They reported that surface tension became large and evaporation rate became small, because the atomic interaction between nanoparticles and sodium was strengthened. By comparing the growth of dendritic oxide between pure sodium and sodium with suspended nanoparticles, which has different physical property from pure sodium, validity of the growth model proposed in the previous section was confirmed.
3.2. Estimation of effect on the growth behavior of dendritic oxide by changing physical properties
The physical properties of sodium with suspended nanoparticles were based on the measured data of a trial sample using titanium nanoparticles [4]. At first, the influence was estimated from the three points of the proposed growth model.
3.2.1. Decreasing the reaction interface by increasing the surface tension
The surface tension of sodium with suspended nanoparticles is larger than that of sodium by about 15–20% due to the strengthened atomic interaction between sodium and nanoparticles. On the other hand, the density is only larger than that of sodium by about 5%. Consequently, the reaction interface of sodium with suspended nanoparticles is thought to become smaller because the force pulling sodium up to the upper side is large and the interface shape is hemispherical.
3.2.2. Decreasing the amount of transfer sodium due to the different stability of oxides
There is a difference in the kinds of oxidation reaction products which surround the transfer path between pure sodium and sodium with suspended nanoparticles. The chemical stability of the kinds of oxides is very important. If the kinds of oxidation reaction product are chemically stable, then it is hard for them to be reduced by sodium which is in the transfer path.
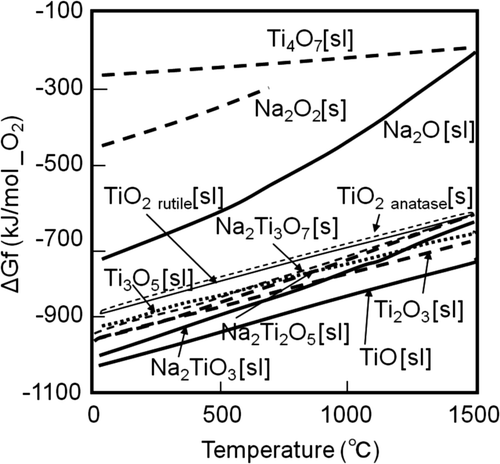
Sodium with suspended titanium nanoparticles reacts with oxygen and some kinds of titanium oxide and sodium titanate are formed. shows the chemical stability of some oxides which react with onemole of oxygen. The chemical stabilities of titanium oxides are higher than those of non-titanium oxides. In other words, it is hard to reduce the titanium oxides compared with non-titanium oxides. Therefore, the titanium oxides remain in the transfer path of liquid sodium in a solid state. As the result, it becomes harder to supply sodium with suspended nanoparticles in the transfer path than pure sodium.
3.2.3. Decreasing sodium consumption by decreasing evaporation rate
Saito and Ara [2] reported that the evaporation rate of sodium with suspended nanoparticles tends to decrease compared with that of pure sodium. This is because of the strengthened atomic interaction between sodium and nanoparticles as well as surface tension. Sodium with suspended nanoparticles suppresses the reaction because the amount of consumed sodium is decreased due to the decreased evaporation rate at the dendritic oxide tip. Because the amount of sucked up sodium becomes small, the speed of dendritic oxide growth is thought to be slow.
3.2.4. Validation of effect on the growth behavior of dendritic oxide by changing physical properties
The growth of dendritic oxide was effected by the oxygen fraction. When oxygen was supplied from above, the dendritic oxide grew upward [4]. When oxygen was supplied from the side, the dendritic oxide grew toward the side [4]. The dendritic oxide grew selectively toward the direction of higher oxygen fraction where the transfer path was easy to form. Furthermore, validation of the estimated effect was done by the observation at the tip of dendritic oxide where the interface of the reaction between sodium and oxygen was located.
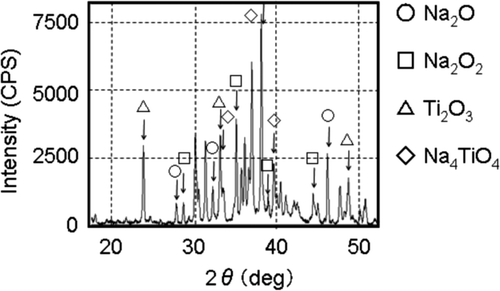
To confirm the suppression effect of sodium with suspended nanoparticles, oxidation test was conducted by Ara et al. [4] as described in chapter II, section 2. The experimental conditions were coordinated to observe the growth behavior of dendritic oxide easily, temperature was 200°C and oxygen fraction was 4%. The results are shown in . Growth of the dendritic oxide as reaction product of sodium with suspended nanoparticles was extremely suppressed. The expansion behavior of sodium with suspended nanoparticles that remained on the net was obviously different from the case of pure sodium. In the case of pure sodium, sodium with a metallic luster was observed continuously at the dendritic oxide tip. On the other hand, in the case of sodium with suspended nanoparticles, the reaction progress was slow and the reaction came to an end at an early stage without dendritic oxide growth. Furthermore, reaction products in the residue were identified by X-ray diffraction. As a result, existence of titanium oxide and sodium titanate was confirmed as shown in.
Figure 8. Growth of oxidation reaction product for sodium with suspended nanoparticles compared to sodium (initial temperature 200°C) (adapted from [4].)
![Figure 8. Growth of oxidation reaction product for sodium with suspended nanoparticles compared to sodium (initial temperature 200°C) (adapted from [4].)](/cms/asset/ffa380f3-febf-406a-b8e6-5f0dd6fe0e19/tnst_a_636557_o_f0008g.jpg)
The dendritic oxide is an intermediate in the reaction between sodium and oxygen, so the difference in growth behavior of the dendritic oxide was thought to affect the whole reaction progress. According to the experimental results by Ara et al. [4] as shown in and , the oxidation reaction product of sodium grew toward the high oxygen fraction while supplying oxygen and the reaction continued. On the other hand, the oxidation reaction product of sodium with suspended nanoparticles grew at some level toward the vertical direction and stopped, after that the reaction area spread uniformly to the horizontal direction. And the growth of dendritic oxide of sodium with suspended nanoparticles was suppressed.
The temperature course of sodium with suspended nanoparticles started to drop in temperature at an early stage and it was confirmed that the reaction ended at an early stage as predicted from the growth model of dendritic oxide. For the confirmation of this, the amount of unreacted sodium in the combustion products was detected by chemical analysis. Whereas all the sodium in the sample of pure sodium was reacted, about 20% of the sodium in the sodium with suspended nanoparticles remained in the combustion products. Therefore, it was confirmed that the reaction of sodium with suspended nanoparticles ended at an early stage with unreacted sodium remaining. This was because the growth of dendritic oxide was suppressed by the mechanism as estimated in Section 2.
4. Conclusion
As a result of this study to get a better understanding of basic sodium combustion phenomena, the following points were seen.
From experimental observations, the growth behavior of dendritic oxide was thought to have the role in an intermediate reaction between sodium and oxygen. So the growth model of dendritic oxide in an intermediate reaction was structured from the influences of:
| 1. | surface tension on forming the reaction interface of liquid sodium; | ||||
| 2. | chemical stability of the dendritic oxide while keeping its transfer path open; and | ||||
| 3. | evaporation rate in reaction at the dendritic oxide tip. | ||||
A comparison experiment was carried out using sodium with suspended nanoparticles which has different properties relating to the growth behavior of dendritic oxide such as surface tension and evaporation rate. Sodium with suspended nanoparticles has a larger surface tension and smaller evaporation rate than pure sodium because of the atomic interaction between nanoparticles and sodium. The growth of dendritic oxide which has the role of supplying sodium to the reaction interface was suppressed. As a result, the oxidation reaction was suppressed.
In this way, oxidation reaction phenomena which included the influences of physical properties or environmental factors were identified based on the proposed growth model of dendritic oxide.
Acknowledgments
The present study describes the results of the project, “Development of the chemical reactive suppression technology of liquid sodium metal based on nanotechnology”, entrusted to the Japan Atomic Energy Agency by the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).
References
- Nishimura , M , Kamide , H , Otake , S and Sugiyama , K . 2011 . Features of dendritic oxide during sodium combustion . J. Nucl. Sci. Technol , 48 : 1420 – 1427 .
- Saito , J and Ara , K . 2010 . A study of atomic interaction between suspended nanoparticles and sodium atoms in liquid sodium . Nucl. Eng. Des , 240 : 2664 – 2673 .
- Ara , K , Sugiyama , K , Kitagawa , H Nagai , M . 2010 . Study on chemical reactivity control of sodium by suspended nanoparticles I . J. Nucl. Sci. Technol , 47 : 1165 – 1170 .
- Ara , K , Sugiyama , K , Kitagawa , H Nagai , M . 2010 . Study on chemical reactivity control of sodium by suspended nanoparticles II . J. Nucl. Sci. Technol , 47 : 1171 – 1181 .
- Eichelberger , R. L. 1968 . The Solubility of Oxygen in Liquid Sodium , AI-AEC-12685 .
![Figure 10. Suppression of the growth behavior of oxidation reaction product (adapted from [4].)](/cms/asset/cd103353-0cc9-4c78-b590-e35b62b070bb/tnst_a_636557_o_f0010g.jpg)
![Figure 11. Temperature of the liquid sodium during reaction (adapted from [4].)](/cms/asset/ef025438-1756-42bd-acf0-317b84e2044f/tnst_a_636557_o_f0011g.gif)