ABSTRACT
Introduction
Diagnosing dementia in people with severe/profound intellectual (and multiple) disabilities (SPI(M)D) is complex. Whereas existing dementia screening instruments as a whole are unsuitable for this population, a number of individual items may apply. Therefore, this study aimed to identify applicable items in existing dementia screening instruments.
Methods
Informant interviews about 40 people with SPI(M)D were conducted to identify applicable items in the Dementia Scale for Down Syndrome, Behavioral and Psychological Symptoms of Dementia in Down Syndrome II scale, Dementia Questionnaire for persons with Mental Retardation and Social competence Rating scale for people with Intellectual Disabilities.
Results
Among 193 items, 101 items were found applicable, categorized in 5 domains: behavioral and psychological functioning (60 items), cognitive functioning (25), motor functioning (6), activities of daily living (5) and medical comorbidities (5).
Conclusion
Identifying applicable items for people with SPI(M)D is an essential step in developing a dedicated dementia screening instrument for this population.
Introduction
People with intellectual disabilities (ID) grow older, which is driven by improvements in medical and social care (Bittles & Glasson, Citation2004; Coppus, Citation2013; Evans et al., Citation2013). Advancing age substantially increases the risk of developing dementia (Alzheimer’s Association, Citation2022). Consequently, dementia is becoming increasingly prevalent among people with ID. Moreover, Down syndrome (DS) is associated with an extremely high genetic risk of developing Alzheimer's disease dementia (Ballard et al., Citation2016).
Recognizing and diagnosing dementia in people with ID is a major challenge. Dementia is characterized by a decline from an individuals’ previous level of cognitive functioning, which is sufficient enough to significantly interfere with daily functioning (American Psychiatric Association, Citation2013; McKhann et al., Citation2011; World Health Organization, Citation2018). In people with ID, it is complex to differentiate cognitive limitations resulting from the underlying ID from cognitive deficits due to dementia (Ball et al., Citation2004). Dementia assessment should thus focus on recognizing a deterioration in (cognitive) functioning relative to the premorbid limitations in functioning (Prasher, Citation2009). The lower the level of baseline functioning, the more difficult the assessment becomes. Therefore, diagnosing dementia is particularly challenging in people with severe/profound intellectual (and multiple) disabilities (abbreviated as SPI(M)D), that is, an estimated intelligence quotient (IQ) of less than 35 points (Evans et al., Citation2013; McKenzie et al., Citation2018).
In the general population, direct neuropsychological tests are used to identify changes in cognitive functioning associated with dementia (Alzheimer’s Association, Citation2022; Salmon & Bondi, Citation2009). However, there are hardly any validated and feasible direct neuropsychological tests to aid the diagnosis of dementia for people with SPI(M)D (Elliott-King et al., Citation2016; Esbensen et al., Citation2017; Fletcher et al., Citation2016; Hon et al., Citation1999; Keller et al., Citation2016; McKenzie et al., Citation2018). Direct neuropsychological tests are not suitable for people with SPI(M)D, because they require skills such as proper understanding of test instructions and good verbal communication skills, which are very limited in individuals with SPI(M)D (Nieuwenhuis-Mark, Citation2009; Oliver & Kalsy, Citation2005). Consequently, floor effects occur when conducting these tests with people having SPI(M)D, making them unsuitable for detecting a decline in cognitive functioning (Elliott-King et al., Citation2016; Esbensen et al., Citation2017; Fletcher et al., Citation2016; Hon et al., Citation1999; Keller et al., Citation2016; McKenzie et al., Citation2018).
Alternatively, informant-based dementia screening instruments, that is interviews with or self-administered questionnaires filled out by direct support professionals/caregivers and/or family members, are used to aid the diagnosis of dementia. A number of informant-based instruments are available for people with ID. Recommended and commonly used instruments are, for instance, the Dementia Questionnaire for Learning Difficulties (DLD) previously referred to as the Dementia Questionnaire for Persons with Mental Retardation (DMR; Evenhuis, Citation1992; Evenhuis et al., Citation2006; Walker et al., Citation2015; Zeilinger et al., Citation2022), the Cambridge Examination for Mental Disorders of Older People with Down’s syndrome and Others with Intellectual Disabilities (CAMDEX-DS; Ball, Holland, Huppert et al., Citation2006; Zeilinger et al., Citation2022). Nevertheless, various studies indicate that such commonly used scales are not suitable for people with SPI(M)D (Elliott-King et al., Citation2016; Evenhuis, Citation1990; Hon et al., Citation1999; Margallo-Lana et al., Citation2007).
Today, no standardized dementia screening instruments dedicated to people with SPI(M)D exist. A diagnosis in this population is currently based on multidisciplinary clinical assessment involving observations, informant interviews and/or screening case notes (Day, Citation1985; Duggan et al., Citation1996; Evenhuis, Citation1990; Määttä et al., Citation2006; Margallo-Lana et al., Citation2007; Reid & Aungle, Citation1974; Sauna-Aho et al., Citation2018). Improving the diagnostic procedures requires developing a dedicated dementia screening instrument specifically adapted to dementia symptoms observed in people with SPI(M)D. However, literature on dementia in this population is scarce (Wissing, Ulgiati et al., Citation2022). Therefore, previous studies have identified dementia symptoms in this population by practice-based observation in order to develop a dedicated instrument (Dekker, Wissing et al., Citation2021; Wissing, Fokkens et al., Citation2022). In addition, whereas existing instruments as a whole are considered unsuitable to diagnose dementia in people with SPI(M)D, specific items within those instruments may still be applicable for this population. Therefore, this study aimed to identify applicable items for people with SPI(M)D in already existing dementia screening instruments available for people with ID.
Methods
Study Consortium
This study is part of the research project “Practice-based questions about dementia in people with severe/profound intellectual (and multiple) disabilities” (Dekker, Wissing et al., Citation2021; Wissing, Fokkens et al., Citation2022, Wissing, Ulgiati et al., Citation2022), a collaborative effort of Hanze University of Applied Sciences, University of Groningen and University Medical Center Groningen (UMCG) with four care institutions throughout The Netherlands (Alliade, ’s Heeren Loo, Ipse de Bruggen, Royal Dutch Visio). These care institutions are representative for the Dutch intellectual disability care sector given the high number of people with SPI(M)D for whom they provide diagnostic work-up, treatments and deliver care.
Study Design
In this explorative study, applicable items for people with SPI(M)D were identified within dementia screening instruments available for people with ID. Four instruments frequently used in The Netherlands were examined 1) adapted Dutch version of the Dementia Scale for Down Syndrome (DSVH; Maaskant & Hoekman, Citation2011), 2) Behavioral and Psychological Symptoms of Dementia in Down Syndrome evaluation scale version II (BPSD-DS II; Dekker, Ulgiati et al., Citation2021; Dekker et al., Citation2018), 3) original Dutch Dementia Questionnaire for persons with Mental Retardation (DVZ; Evenhuis et al., Citation1998) and 4) Social competence Rating scale for people with ID (SRZ; Kraijer et al., Citation2004).
These four instruments are not only in The Netherlands, but also internationally, recommended and widely used to screen for dementia in people with ID. For instance, a recent review of Zeilinger et al. (Citation2022) recommended the usage of the BPSD-DS II and DLD (in Dutch: DVZ). The DLD is one of the most frequently used instrument for dementia assessment in people with ID (among others: Burt et al., Citation2005; Coppus, Citation2017; Coppus et al., Citation2006, Citation2008, Citation2009, Citation2012; Deb & Braganza, Citation1999; Dekker, Coppus et al., Citation2015; Hoekman & Maaskant, Citation2002; Kirk et al., Citation2006; Koran et al., Citation2014; Lott et al., Citation2012; Mccarron et al., Citation2014; Prasher, Citation1997; Rösner et al., Citation2021; Shultz et al., Citation2004; Silverman et al., Citation2004; Startin, Hamburg et al., Citation2016; Walker et al., Citation2015; Zigman et al., Citation2004). Moreover, many studies reported the usage of the Dementia Scale for Down Syndrome (DSDS; in Dutch adapted as DSVH) as instrument to aid diagnosing dementia in people with ID (among others: Burt et al., Citation2005; Deb & Braganza, Citation1999; Devenny et al., Citation2000; Huxley et al., Citation2000; Krinsky-McHale et al., Citation2002; Shultz et al., Citation2004; Temple et al., Citation2001). Additionally, various studies have applied the SRZ as part of their dementia screening procedure (Blok et al., Citation2017; Coppus, Citation2017; Coppus et al., Citation2006, Citation2008, Citation2009, Citation2012; Dekker, Coppuset al., Citation2015; De Knegt et al., Citation2013, Citation2016). Other internationally recommended and widely used dementia screening that – in 2021 – were not (yet) translated/validated/available in Dutch were not examined.
To evaluate whether items in those instruments may be applicable for people with SPI(M)D, it is of essence that people with SPI(M)D are able to display these items at baseline, i.e., the highest level of functioning before decline/dementia occurs. After all, to aid the diagnostis of dementia, identification of change (decline) is essential. The selected four dementia screening instruments in our study were, therefore, completed by conducting interviews with informants of people with SPI(M)D without dementia. For each specific item informants were asked whether that item was applicable for the individual with SPI(M)D. If an item was considered to be not applicable, informants should provide one or more reasons why that item was not applicable.
Dementia Screening Instruments
DSVH (DSDS)
The DSVH is an adapted, Dutch version of the DSDS, developed in Canada by Gedye (Citation1995) to aid diagnosing dementia in people with ID. Information about behavioral changes in relation to persons’ cognitive and activity of daily living (ADL) skills are gathered by interviewing informants. The questions of the original DSDS were translated and studied in 121 persons with ID in The Netherlands (Maaskant & Hoekman, Citation2011). Similarly to the DSDS, the DSVH contains a total of 60 items, however the order of items is different. The 60 items are divided into three categories indicating the stage of dementia. Each item is scored as either “present,“ “absent,” “characteristic” or “not applicable.” Characteristic indicates that behavior has been present throughout the adult life, whereas present refers to newly developed behavior.
BPSD-DS II
The BPSD-DS II is a recently developed evaluation scale to identify behavioral and psychological symptoms of dementia in people with DS (Dekker, Ulgiati et al., Citation2021; Dekker et al., Citation2018). After initial development, the scale was first studied in 281 people with DS (Dekker et al., Citation2018). Based on results obtained in this study and clinical experiences, the scale was optimized. The optimized scale was subsequently studied in 524 individuals with DS (Dekker, Ulgiati et al., Citation2021). The BPSD-DS II consists of 52 items divided into 11 sections, namely anxious, irritable, obstinate, restless & stereotypic, aggressive, apathetic behavior, depressive, psychotic, disinhibited, eating & drinking behavior and sleeping problems. For every item in the scale, frequency (five-point scale) and severity (four-point scale) are scored for two periods of time, i.e., last 6 months and typical/characteristic behavior before deterioration occurred, subsequently resulting in a frequency change or severity change score.
DVZ (DMR/DLD)
The DVZ is originally developed in The Netherlands to screen for signs of dementia over time in people with ID (Evenhuis, Citation1992). Internationally, this dementia screening instrument is known as the DMR and was later renamed as DLD. It encompasses a total of 50 items divided into cognitive skills, i.e., short-term memory, long-term memory and spatial and temporal orientation, and social skills, i.e., speech, practical skills, mood, activity and interest, and behavioral disturbance. Items can be scored as either “normally yes,” “sometimes” or “normally no”.
SRZ
The SRZ is designed to screen for a decline in social competences over time (Kraijer et al., Citation2004). It consists of 31 items, which covers aspects regarding ADL skills, effective use of language, social skills and the ability to define and execute tasks. Each item has four answer options, ranging from less to more able to deal with themselves, other people and everyday situations.
Ethics and Consent
The Medical Ethical Committee of the UMCG decided that the Dutch Medical Research Human Subjects Act did not apply to this study (METc 2019/198). The study was registered in the UMCG Research Register (no. 201,900,193) and conducted in accordance with the UMCG Research Code and the EU General Data Protection Regulation. Legal representatives of people with SPI(M)D provided written informed consent for evaluation of item applicability in the DSVH, BPSD-DS II, DVZ and SRZ and processing/analyzing coded data for this study.
Study Population
Eligible participants were identified within the four participating care institutions based on the following inclusion and exclusion criteria; inclusion criteria: presence of severe/profound ID established according to (medical) records and clinical judgment, aged 25 years to 50 years, stable functioning, thus no changes relative to a persons’ typical/characteristic functioning, exclusion criteria: mild or moderate ID, (suspected) dementia, functional decline (according to the judgment of involved ID psychologist), long-term admission to hospital in the past 6 months, bedridden or in terminal care, absence of at least one informant able to describe the persons’ typical/characteristic functioning. Recent life events, e.g., moving home or death of a family member, having long-term impact on the persons’ functioning (according to clinical judgment) also led to exclusion of an individual. People were eligible to participate regardless of the presence of DS or other disabilities such as visual or motor impairments. Given that people with DS have an extremely high genetic risk of developing dementia due to Alzheimer’s disease (Ballard et al., Citation2016) it was made sure that at least 25% of the participants had a phenotypical diagnosis of DS. After selection, information letters with informed consent forms were sent to legal representatives of eligible participants.
Data Collection
A data collection form was constructed in REDCap (Harris et al., Citation2009), hosted within the secured network of the UMCG. Firstly, demographic data were gathered about age, sex, living situation, attending day care, presence of a syndrome, formal diagnosis of autism spectrum disorder, IQ, social-emotional functioning, verbal communication skills, gross and fine motor function. Gross motor function was according to the judgment of involved ID psychologist categorized into one of the five levels of the Gross Motor Function Classification System (GMFCS): Level I, can walk without limitations; Level II, walk with limitations; Level III, walk with assistive mobility device; Level IV, walking ability severely limited even with assistive devices, use of power wheelchair; Level V, transported by manual wheelchair (Palisano et al., Citation1997). Similarly, fine motor function was categorized according to the Manual Ability Classification System (MACS) levels: Level I, handles objects easily and successfully; Level II, handles most objects but with somewhat reduced quality and/or speed of achievement; Level III, handles objects with difficulty, needs help to prepare and/or modify activities; Level IV, handles a limited selection of easily managed objects in adapted situations; V, does not handle objects and has severely limited ability to perform even simple actions (Eliasson et al., Citation2006). Secondly, data were gathered about the presence of (un)treated comorbidities associated with dementia like symptoms for which the list (part A) in the BPSD-DS II was used (Dekker, Ulgiati et al., Citation2021).
Next, the DSVH, BPSD-DS II, DVZ and SRZ were administered in this sequence. The order of items within these instruments was maintained. The sum of all items of the four instruments was 193 items. For the BPSD-DS II, only the frequency of typical/characteristic behavior was considered, given that in this study the individuals with SPI(M)D had no dementia, i.e., no deterioration in behavior was expected. Regardless of the instrument, for every individual item, the answer option “not applicable” was added, if that was not already a possible answer. Not applicable was defined as follows: an individual could impossibly demonstrate the skill/behavior represented in the item, meaning that the skill/behavior cannot occur. Informants were subsequently asked why they answered “not applicable.” They could select one or multiple predefined reasons or provide an alternative reason (open answer). Predefined reasons – different depending on the item – based on characteristics of the SPI(M)D group (Nakken & Vlaskamp, Citation2007) were limited intellectual functioning, limited verbal communication, limited motor functioning, hearing problems, vision problems, ADL dependency complemented with the options limited social-emotional functioning, wheelchair dependent, restrictive measures, and incontinence.
Interviewers
The four instruments were completed by conducting online interviews with informants in Microsoft Teams (due to COVID-19-measures) according to a procedural protocol drawn up in advance. Each interview was performed by an experienced interviewer, such as an ID psychologist (behavioral therapists who studied psychology or special needs education (in Dutch: orthopedagogiek)) or psychological assistant working at the care institutions part of the study consortium. For reasons of uniformity, all interviewers received instructions about the procedure and digital system and were able to practice with system in advance. Interviewers adhered to the procedural protocol and sequence of items. In total, seven ID psychologists and five psychological assistants alternately conducted the interviews. To improve understanding of items, interviewers shared their screen so that informants could also read items and item explanations. Moreover, a researcher (MBGW), unacquainted with the individuals with SPI(M)D, was present at each interview to explain the procedure, provide technical assistance, made sure that answers were provided by informants, and keep track of the provided answers (parallel completion of the data collection form) to check afterward for compliance with instructions and protocol. The interviewer and the informant(s) could not see which answer option this researcher selected. Overall, the interviews lasted 60 to 195 minutes.
Informants
Interviews were conducted with at least one key informant of the person with SPI(M)D, such as caregivers working in day-care center/residential facilities or family members. Beforehand, interviewers checked whether informant(s) were able to provide an accurate description of the typical/characteristic functioning. In the case of multiple informants, they were interviewed in a single session. Prior to the interview, informants received information about the procedure by e-mail. Interviews were conducted in absence of the person with SPI(M)D to facilitate honest answering. In line with the procedural protocol, each interview started with welcoming informants, the researcher (MBGW) introduced the topic, checked if an informed consent form was signed, and explained the procedure and confidentiality. Subsequently, the interviewer ran through the demographic information which was on forehand filled out by the interviewer based on information in (medical) records of the individual. Thereafter, in total, 193 questions about item applicability of DSVH, BPSD-DS II, DVZ and SRZ were asked. Prior to each instrument the scoring system of the instrument was explained to the informants. If necessary, interviewers provided clarification of items and reminded informants to give short and succinct answers. Furthermore, if there was disagreement between informants, the interviewer made sure that consensus was reached during the interview.
Data Analysis
Firstly, each completed interview was checked for inclusion/exclusion criteria and compared with the data collection form filled out by the researcher (MBGW). Provided answers were corrected according to the protocol if 1) not applicable was unjustifiably scored, the individual was able to show the skill/behavior, or 2) an item was unjustifiably considered to be applicable, the individual could impossibly demonstrate the skill/behavior. The data were analyzed using SPSS Statistics version 25 (IBM Corp). Standard descriptive statistics were used to present results. For each item, the percentage of “not applicable” responses was calculated. If one or more times an item was considered to be not applicable, the percentage of a provided “not applicable” reason was calculated with respect to the “not applicable” score.
To structure the broad range of items, all 193 items were divided according to five domains in line with dementia diagnostic criteria (American Psychiatric Association, Citation2013; McKhann et al., Citation2011; World Health Organization, Citation2018) and literature (Dekker, Ulgiati et al., Citation2021; Ries, Citation2018; Strydom et al., Citation2010) covering the following: cognitive functioning, ADL, behavioral and psychological functioning, motor functioning, and medical comorbidities. To further improve interpretation, items within each domain were further categorized. Cognitive categories consisted of cognitive functions affected by Alzheimer’s disease (Alzheimer’s Association, Citation2022): memory, orientation in time, orientation in place, understanding visual images/spatial relationships, language skills, losing objects, person recognition complemented with a category other cognitive functions. ADL comprised items of the Barthel Index (Mahoney & Barthel, Citation1965): feeding, dressing grooming/bathing, transfers, toilet use and two instrumental ADL: housework and shopping. Behavioral and psychological categories were defined in accordance with the BPSD-DS II scale (Dekker, Ulgiati et al., Citation2021): anxious behavior, sleeping problems, irritable behavior, obstinate behavior, restless/stereotypical behavior, aggressive behavior, apathetic behavior, depressive behavior, psychotic behavior, disinhibited behavior and eating/drinking behavior. The motor domain contained motor skills: walking, balance/fall frequency, movement speed/quality and fine motor skills (Ries, Citation2018). The last domain focused on medical comorbidities (Strydom et al., Citation2010), namely epilepsy, incontinence complemented with a category other medical comorbidities. Within each category, the calculated percentages of “not applicable” responses were ordered from lowest to highest and subsequently divided into four quartiles, namely 0–25% meaning applicable, 26–50% meaning somewhat applicable, 51–75% meaning hardly applicable and 76–100% meaning not at all applicable.
Lastly, additional analyses were performed for items focusing on verbal communication and gross motor function. In the focus group study of Dekker, Wissing et al. (Citation2021) participants already indicated that symptoms like decline in speech and ability to walk cannot be recognized in persons who are non-verbal/entirely dependent on a wheelchair. Moreover, results of the study of (Wissing, Fokkens et al., Citation2022) indeed showed that the observation of particular symptoms depended on whether individuals had verbal communication or walking skills at baseline. Therefore, for each verbal item, the percentage of “not applicable” responses were calculated separately for people with and without verbal communication skills. Similarly, for gross motor items, the percentages of “not applicable” responses were calculated for people with (i.e., GMFCS level I, II and III) and without (i.e., GMFCS level IV, V) independent walking skills. These percentages were also ordered and subsequently divided into four quartiles.
Results
Legal representatives of 99 identified eligible participants received an information letter with informed consent form. Legal representatives of 46 people with SPI(M)D provided written informed consent, 9 did not provide consent and 44 did not respond. Before planning the interviews, legal representatives of two individuals withdrew their consent without providing a reason. Moreover, four persons were after checking (medical) records and clinical judgment excluded because they had a moderate ID (n = 3) or unstable functioning (n = 1).
presents demographic data of the 40 participants. None of these participants had (suspected) dementia, and their functioning was stable, i.e., major life events as well as (un)treated comorbidities did not – according to clinical judgment – result in evident changes of the persons’ functioning. For none of them an IQ score was determined and reported in their (medical) records. In more than half of the study population the ID was of genetic origin: 11 individuals had DS and another 11 had other genetic syndromes, namely Rett syndrome (n = 2), Fragile X-syndrome (n = 1), Angelman syndrome (n = 1), Cri du chat syndrome (n = 1), Kleefstra syndrome (n = 1), Edwards syndrome (n = 1), Turner syndrome (n = 1), Wolf-Hirschhorn syndrome (n = 1), abnormal X chromosome: 46, Y, dup (X) (p22.31 p22.33) (n = 2).
Table 1. Participants’ and informants’ characteristics.
The 40 interviews were conducted with key informants: in 47.5% of cases one informant was interviewed, in 35.0% two informants and in 17.5% three informants. Key informants were caregivers (54.4%), family members (44.1%) or legal representatives without being a family member (1.5%). shows the informants’ characteristics.
Applicability of Items
The 193 items (sum of all items of the four instruments) were completed for all 40 participants. During the data check, 117 of the total 7720 provided answers (1.5%) were corrected in accordance with the protocol. Of these 117 items, 63 were unjustifiably scored as “not applicable,” whereas 54 were unjustifiably considered to be applicable. display the calculated percentages of “not applicable” responses for cognitive, ADL, behavioral and psychological, motor and medical comorbidities items, respectively.
Table 2. Applicability of items about cognitive functioning for people with SPI(M)D.
Table 3. Applicability of items about ADL for people with SPI(M)D.
Table 4. Applicability of items about behavioral and psychological functioning for people with SPI(M)D.
Table 5. Applicability of items about motor functioning for people with SPI(M)D.
Table 6. Applicability of items about medical comorbidities for people with SPI(M)D.
Cognitive Items
In total, 70 items about cognitive functioning were identified within the four existing dementia screening instruments. As shown in , the percentages of “not applicable” responses of 25 items fell inside the first quartile (0–25%), meaning that these items were considered to be applicable. Applicable items were identified within different cognitive functioning categories, namely memory (7 items), orientation in place (5), person recognition (3), orientation in time (2), responsiveness (2), understanding visual images/spatial relationships (1), losing objects (1) and other cognitive functions (4), i.e., knowing what to do with objects, attention for the task, expressing wishes and using objects correctly. Only for the category language skills there were no items which fell inside the first quartile. Moreover, 11 items, such as knowing your age/the year, fell inside the fourth quartile (76–100%), and were thus not at all applicable. Not only for these 11 items but also for the other cognitive items, the two most provided reasons why items were not applicable were limited intellectual and verbal functioning.
ADL Items
The applicability of the 25 identified ADL items is presented in . In total, five items were found to be applicable given that these items fell inside the first quartile (0–25%). Most of the applicable ADL items focused on feeding (3 items): use of cutlery, everyday support or extensive assistance with eating. The remaining applicable items were items regarding making transfers (1) and doing housework (1). For the categories dressing, grooming/bathing, toilet use and shopping, none of the identified items fell inside the first quartile. Limited intellectual functioning and ADL dependency were within the ADL domain the two most provided reasons why items were not applicable.
Behavioral and Psychological Items
presents the applicability of the 81 items categorized within the behavioral and psychological domain. What stands out is that almost three-fourths of the items fell inside the first quartile (0–25%). Accordingly, applicable items were found within 10 of the total 11 behavioral and psychological categories. The apathetic behavior category comprised the most applicable items (13 items). Moreover, applicable items were found for depressive behavior (9), sleeping problems (7), obstinate behavior (6), anxious behavior (5), irritable behavior (5), restless/stereotypic behavior (5), eating/drinking behavior (5), aggressive behavior (4) and disinhibited behavior (1). For psychotic behavior, items fell either in the third (51–75%) or fourth quartile (76–100%), and thus no applicable items were identified within this category. For the behavioral and psychological domain, a variety of reasons why items were not applicable – depending on the item – were provided.
Motor Items
As shown in , the percentages of “not applicable” responses of all six identified motor items fell inside the first quartile (0–25%). The balance/fall frequency and movement speed/quality category each consisted of two applicable items, namely loss of balance, sitting down and slowness of movements, slow/clumsy movements, respectively. Moreover, the other two items were motor items regarding walking and fine motor skills. For the few individuals for whom a motor item was not applicable, the main reasons provided were limited motor functioning and wheelchair dependent.
Medical Comorbidities Items
Eleven items about medical comorbidities were identified in the dementia screening instruments. In , it is apparent that all three items about epilepsy as well as the two items in the category other medical comorbidities fell inside the first quartile (0–25%) and were thus considered to be applicable. In contrast, six items about incontinence were hardly or not at all applicable, primarily because of pre-existing incontinence.
Verbal Communication Items
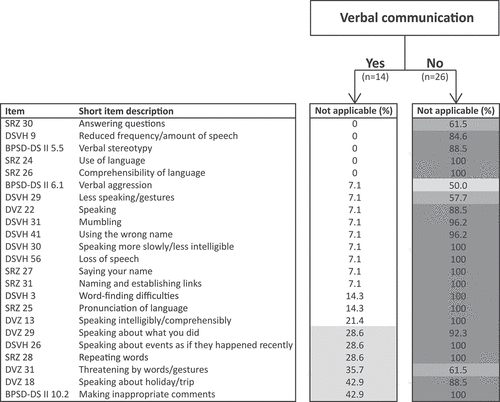
To compare differences in applicability of verbal items for people with and without verbal communication skills at baseline, the percentages of “not applicable” responses were for verbal items calculated for each subgroup. The study population was divided on the basis of verbal communication skills at baseline: among the 40 participants, 14 had verbal communication skills, whereas 26 had never acquired such skills. displays an overview of the percentages of “not applicable” responses – separately for each subgroup – for the 23 identified verbal items. The initial analysis revealed that none of the 23 items fell inside the first quartile (0–25%; ). However, additional analysis in the subgroups showed that 17 of these items were applicable for those with verbal communication skills at baseline. The remaining six verbal items fell for the verbal communication subgroup within the second quartile (26–50%), meaning that these items were considered to be somewhat applicable. In contrast, in the subgroup without verbal communication skills at baseline, 19 items were not at all applicable, and 3 were hardly applicable. Only the item focusing on verbal aggression was somewhat applicable within this subgroup. Evidently, applicability of verbal-related items depended on pre-existing verbal communication skills.
Figure 1. Applicability of verbal items for people with SPI(M)D with and without verbal communication skills. The not applicable percentages within each subgroup were divided into four quartiles, namely 0–25%, 26–50%, 51–75% and 76–100%. 0–25% are white meaning applicable, 26–50% are light gray meaning somewhat applicable, 51–75% are middle gray meaning hardly applicable and 76–100% are dark gray meaning not at all applicable. Abbreviations: BPSD-DS II, Behavioral and Psychological Symptoms of Dementia in Down Syndrome evaluation scale version II; DSVH, adapted Dutch version of the Dementia Scale for Down Syndrome; DVZ, original Dutch Dementia Questionnaire for persons with mental retardation; SRZ, Social competence Rating scale for people with intellectual disabilities.
Gross Motor Function Items
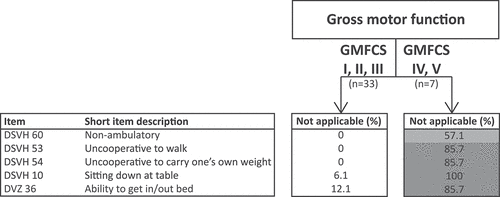
Additional analysis was also performed for items about gross motor function. Among the 40 participants, 33 had independent walking skills (i.e., GMFCS I, II, III) and 7 had not acquired walking skills (i.e., GMFCS IV, V). In total, five gross motor function items were identified. What stands out in is that all five items fell inside the first quartile (0–25%) for those able to independently walk and thus can consider to be applicable for this subgroup. Conversely, for those not able to independently walk, one item was hardly applicable, whereas the remaining four items were not at all applicable. Evidently, applicability of items about gross motor function depended on the abilityto walk independently.
Figure 2. Applicability of gross motor function items for people with SPI(M)D with (i.e., GMFCS level I, II and III) and without (i.e., GMFCS level IV, V) independent walking skills. The not applicable percentages within each subgroup were divided into four quartiles, namely 0–25%, 26–50%, 51–75% and 76–100%. 0–25% are white meaning applicable, 26–50% are light gray meaning somewhat applicable, 51–75% are middle gray meaning hardly applicable and 76–100% are dark gray meaning not at all applicable. Gross Motor Function Classification System (GMFCS) levels: Level I, can walk without limitations; Level II, walk with limitations; Level III, walk with assistive mobility device; Level IV, walking ability severely limited even with assistive devices, use of power wheelchair; Level V, transported by manual wheelchair. Abbreviations: DSVH, adapted Dutch version of the Dementia Scale for Down Syndrome; DVZ, original Dutch Dementia Questionnaire for persons with mental retardation.
Discussion
In this study, applicable items in existing dementia screening instruments, namely DSVH, BPSD-DS II, DVZ and SRZ, were identified by interviewing key informants of people with SPI(M)D. Our results demonstrated that 101 of the total 193 items can be considered as applicable for individuals with SPI(M)D. Almost two-third of the applicable items focused on behavioral and psychological functioning, namely apathetic (13 items), depressive (9), sleeping problems (7), obstinate (6), anxious (5), irritable (5), restless/stereotypic (5), eating/drinking (5), aggressive (4) and disinhibited behavior (1). Moreover, among the 101 applicable items, 25 items focused on cognitive functioning, i.e., memory (7 items), orientation in place (5), person recognition (3), orientation in time (2), responsiveness (2), understanding visual images/spatial relationships (1), losing objects (1) and other cognitive functions (4). The remaining applicable items were items regarding motor functioning (6), ADL (5) and medical comorbidities (5). Additional analyses revealed that among 23 verbal communication items, 17 were applicable for individuals with verbal communication skills at baseline, but not if a person had never acquired such skills. Similarly, five items concerning gross motor function were found to be only applicable for those able to independently walk (GMFCS levels I, II, III) at baseline.
To diagnose dementia in people with SPI(M)D it is of essence to identify changes (decline). The results of this study indicate which skills/behavior people with SPI(M)D could potentially display before decline/dementia. If someone, at baseline (without decline) is able to show such skills/behavior, these may be of use in the context of dementia as informants may observe changes. Previous studies have focused on identifying observable dementia symptoms in this population (Dekker, Wissing et al., Citation2021; Wissing, Fokkens et al., Citation2022 Wissing, Ulgiati et al., Citation2022). Hereafter, we conceptualize our findings about item applicability with reported observable dementia symptoms in previous studies, separately for the five domains: cognitive functioning, ADL, behavioral and psychological functioning, motor functioning and medical comorbidities (American Psychiatric Association, Citation2013; Dekker, Ulgiati et al., Citation2021; McKhann et al., Citation2011; Ries, Citation2018; Strydom et al., Citation2010; World Health Organization, Citation2018).
Cognitive Functioning
One of the characteristics of the SPI(M)D population is that their cognitive functioning is limited resulting from their underlying ID (American Psychiatric Association, Citation2013; Nakken & Vlaskamp, Citation2007). Therefore, in clinical practice, it is commonly believed that it would be very hard to identify applicable cognitive items, because those with more severe ID may be unable to display cognitive skills (Startin, Rodger et al., Citation2016). Despite low levels of baseline cognitive functioning, still 25 items focusing on cognitive functioning turned out to be applicable. This is consistent with three SPI(M)D dementia studies that showed that it is possible to observe cognitive dementia symptoms in individuals with SPI(M)D (Dekker, Wissing et al., Citation2021; Wissing, Fokkens et al., Citation2022, Wissing, Ulgiati et al., Citation2022). Cognitive symptoms like memory loss, disorientation in place and language problems were in those with more severe ID particularly observed in different contexts, e.g., ADL, communication, leisure activities (Benejam, Citation2009; Dekker, Wissing et al., Citation2021). Initial analysis in this study revealed indeed applicable items focusing on memory and orientation in place but not on language skills. This finding could be attributed to limited or even absent verbal communication skills (Nakken & Vlaskamp, Citation2007; Nieuwenhuis-Mark, Citation2009; Oliver & Kalsy, Citation2005). Additional analysis revealed that in total 17 items – 15 cognitive and 2 behavioral and psychological items – about verbal communication were applicable for those with verbal communication skills but not for those without such skills. As already addressed in the studies of Dekker, Wissing et al. (Citation2021) and Wissing, Fokkens et al. (Citation2022), observing alterations in language depends on whether at baseline someone has developed such skills.
ADL
Individuals with SPI(M)D often need high levels of support to perform ADL. They might hardly have developed specific skills and therefore are (fully) dependent on others for daily tasks (Dekker, Wissing et al., Citation2021; Nakken & Vlaskamp, Citation2007). However, in the study of Wissing, Fokkens et al. (Citation2022(), interviewees stressed that despite required assistance, in almost all individuals with SPI(M)D and dementia, they had observed a decline in eating/drinking skills. In line with that, most applicable items about ADL were identified within the feeding category (3 items). Moreover, applicable items were found focusing on transfers (1) and housework (1), whereas no applicable items were identified for the categories dressing, toilet use, grooming/bathing and shopping. Items within these four categories are thus not applicable for the total SPI(M)D population. In contrast, it was previously reported that dementia symptoms like deterioration in the ability to dress or use the toilet were observed in individuals with SPI(M)D (Dekker, Wissing et al., Citation2021; Wissing, Fokkens et al., Citation2022). This may be explained by the fact that also persons with SPI(M)D are able to perform small tasks within a larger activity, for example, by putting their arm in the sleeve during dressing. Even performing such small sub-tasks can deteriorate, and were therefore named in previous studies (Dekker, Wissing et al., Citation2021; Wissing, Fokkens et al., Citation2022). It is thus important to develop items specifically regarding performing the sub-tasks within larger tasks according to experiences in practice.
Behavioral and Psychological Functioning
To identify behavioral changes over time one should disentangle behavioral alterations from characteristic/typical behavior of an individual (Dekker, Strydom et al., Citation2015). Our results showed that people with SPI(M)D could at baseline display behavior represented in 60 behavioral and psychological items. Such items should be used to screen for dementia in people with SPI(M)D. Behavioral and psychological symptoms of dementia are namely observed in all types of dementia (Finkel, Citation2000) and also prominent in people with DS (Dekker, Strydom et al., Citation2015; Dekker, Ulgiati et al., Citation2021; Dekker et al., Citation2018). Moreover, they are frequently observed dementia symptoms in people with SPI(M)D (Dekker, Wissing et al., Citation2021; Wissing, Fokkens et al., Citation2022, Wissing, Ulgiati et al., Citation2022). In fact, behavioral and psychological changes related to dementia are more notable than alterations in cognitive functioning (Ball, Holland, Hon et al., Citation2006, Ball et al., Citation2008; Engelborghs et al., Citation2005; Nelson et al., Citation2001), certainly in those with SPI(M)D (Wissing, Fokkens et al., Citation2022). In the SPI(M)D population, particularly dementia symptoms like increased irritability, anxiety, apathy and decreased eating/drinking behavior were frequently observed, whereas psychotic symptoms seem less prevalent (Dekker, Wissing et al., Citation2021; Wissing, Fokkens et al., Citation2022). In this study, items focusing on psychotic behavior were either hardly or not at all applicable, mainly because of limited intellectual functioning and verbal communication. Previous studies indeed noted that recognizing psychotic symptoms is particularly complex in those with limited verbal communication skills, because they are hardly able to self-report the inner experiences hallucinations and/or delusions (Cooper & Smiley, Citation2007; Moss et al., Citation1993; Temple & Konstantareas, Citation2005).
Motor Functioning
Many people with SPI(M)D have to some extent limitations in motor functioning (Houwen et al., Citation2014; Nakken & Vlaskamp, Citation2007). However, our results demonstrated that despite pre-existing motor problems, all motor items, namely balance/fall frequency (2 items), movement speed/quality (2), fine motor skills (1) and walking (1) were applicable for persons with SPI(M)D. This may seem contradictory, but every individual is – despite limitations in motor functioning – to a certain extent able to move (parts of) their body. Consequently, motor changes can also be observed in individuals with SPI(M)D, for example, decreased movement speed and/or quality. Such motor changes might be related to dementia given that a decline in motor functioning was recognized in individuals with dementia, not only in the general population (Ries, Citation2018) but also in the SPI(M)D population (Dekker, Wissing et al., Citation2021; Wissing, Fokkens et al., Citation2022i, Wissing, Ulgiati et al., Citation2022). Moreover, a decline in walking skills in people with SPI(M)D and dementia was only observed in individuals who were able to walk at baseline (Dekker et al. Citation2021; Wissing, Fokkens et al. Citation2022). Indeed, our additional analysis of gross motor function showed that five items about gross motor function, including the motor item about walking, were only applicable for those able to independently walk at baseline (GMFCS levels I, II, III).
Medical Comorbidities
People with SPI(M)D frequently experience physical health problems such as vision problems, epilepsy, constipation and incontinence (Nakken & Vlaskamp, Citation2007; Van Timmeren et al., Citation2017). Particularly, the onset of epilepsy and incontinence are medical comorbidities related to dementia not only in the general (Kurrle et al., Citation2012) and DS population (Aller-Alvarez et al., Citation2017; Strydom et al., Citation2010) but also in the SPI(M)D population (Dekker, Wissing et al., Citation2021; Wissing, Fokkens et al., Citation2022, Wissing, Ulgiati et al., Citation2022). Our results demonstrated that items focusing on epilepsy were applicable for all 40 individuals with SPI(M)D, and thus could be used for this population. Conversely, no applicable items focusing on incontinence were identified, whereas previous studies have shown increased incontinence in people with SPI(M)D and dementia (Dekker, Wissing et al., Citation2021; Wissing, Fokkens et al., Citation2022, Wissing, Ulgiati et al., Citation2022). Not identifying applicable items for incontinence is likely to be related to individuals being incontinent at baseline. In fact, the study of Van Timmeren et al. (Citation2016) found a prevalence rate for incontinence of 56% for people with SPI(M)D.
Study Strengths
Existing dementia screening instruments for people with ID as a whole were found to be unsuitable for people with SPI(M)D (Elliott-King et al., Citation2016; Evenhuis, Citation1990; Hon et al., Citation1999; Margallo-Lana et al., Citation2007). To the best of our knowledge, this study is the first showing that specific items within existing lists are applicable to screen for dementia in individuals with SPI(M)D. Another strength of this study is that we took into account the heterogeneity of the SPI(M)D population. We included persons with either a severe or a profound ID and various underlying causes, including DS. We took into account the high genetic risk of developing dementia for people with DS (Ballard et al., Citation2016), by making sure that at least one-fourth of the total participants had DS. Moreover, we considered the variety of verbal communication and gross motor skills in people with SPI(M)D. Additional analyses allowed to refine results in relation to the presence or absence of these skills.
Study Limitations
Relatively, a large number of legal representatives, which received an information letter with informed consent form, did either not respond or did not provide consent. This might be explained by the fact that they might not see the added value of filling out dementia screening instruments when the functioning of the person is stable and their relative does not (yet) have dementia. When the information was further clarified, either face-to-face or by a phone call, legal representatives were more willing to provide informed consent. Due to practical difficulties, this was not done within every care institution. Moreover, there are no standardized tests applicable for a valid estimation of the level of ID (Nakken & Vlaskamp, Citation2007). Therefore, the categorization of severe ID (60%) and profound ID (40%) is based on clinical judgment. There seems to be a slight underrepresentation of those with the most severe ID. As a consequence, items having not applicable percentages around the threshold of quartiles could potentially have been attributed to another quartile when more individuals with profound ID were included. Another possible limitation is the fact that some interviewers were involved in the diagnostic work-up/care for the individual with SPI(M)D. To minimize risk of bias, an independent researcher, unacquainted with the individuals with SPI(M)D, made sure that answers were provided by informants (not the interviewer). Moreover, although care institutions in The Netherlands provide care/support in a variety of residential facilities, ranging from smaller assisted living facilities in communities to larger, specialized locations, we cannot rule out a potential effect of living situation of individuals on the scoring. Lastly, we only identified applicable items in dementia screening instruments for which a translated/validated Dutch version was available, and thus not for internationally used instruments such as the CAMDEX-DS (Ball, Holland, Huppert et al., Citation2006). Nevertheless, the four selected instruments are internationally recommended and widely used to screen for dementia in people with ID (Zeilinger et al., Citation2013).
Future Implications
Timely recognizing and diagnosing dementia in people with SPI(M)D is a major challenge. Today, a clinical diagnosis of dementia in individuals with SPI(M)D is purely based on observations, interviewing informants and/or screening case notes (Day, Citation1985; Duggan et al., Citation1996; Evenhuis, Citation1990; Määttä et al., Citation2006; Margallo-Lana et al., Citation2007; Reid & Aungle, Citation1974; Sauna-Aho et al., Citation2018). Existing dementia screening instruments as a whole are namely unsuitable for this population. This primarily relates to the pre-existing disabilities, which make that not all items within instruments can be scored. In this study, we have shown which skills/behavior individuals with SPI(M)D may – despite pre-existing disabilities – display before decline/dementia. Based on these results, it cannot yet be determined whether applicable items are indeed relevant to screen for dementia symptoms in those with SPI(M)D. Further research is required to establish whether persons with SPI(M)D and dementia indeed show alterations in applicable items. Previous studies already demonstrated which dementia symptoms could potentially be observed in those with SPI(M)D (Dekker, Wissing et al., Citation2021; Wissing, Fokkens et al., Citation2022, Wissing, Ulgiati et al., Citation2022). The authors stress that both aspects: 1) identified applicable items in existing dementia instruments available for people with ID and 2) identified practice-based observation of dementia symptoms in the SPI(M)D population should form the basis for developing a novel dementia screening instrument dedicated to people with SPI(M)D. Moreover, such an instrument should, differently from direct neuropsychological tests, not only focus on a decline in cognitive functioning. Instead, also the ADL, behavioral and psychological, motor and medical comorbidities domains should be included, because in those with SPI(M)D a decline in cognitive functioning will be observable in all other domains (Benejam, Citation2009; Dekker, Wissing et al., Citation2021). Additionally, such an instrument should contain a statement that symptoms could be caused by – often treatable – conditions such as depression, delirium, vision or hearing problems, hypothyroidism, sleep apnea or vitamin B12 deficiency, which should be ruled out as much as possible before diagnosing dementia (Moriconi et al., Citation2015; Scott & Barrett, Citation2007).
Conclusion
This study provided an overview of applicable items for people with SPI(M)D in existing dementia screening instruments available for people with ID. Among 193 items, 101 were found to be applicable for individuals with SPI(M)D. Most applicable items were identified within the behavioral and psychological domain (60 items), followed by cognitive (25), motor (6), ADL (5) and medical comorbidities (5) domains. Moreover, 17 items focusing on verbal communication skills and 5 about gross motor function were specifically found to be applicable for individuals with verbal/walking skills at baseline. The inventory of applicable items together with the findings of observable dementia symptoms in people with SPI(M)D (Dekker, Wissing et al., Citation2021; Wissing, Fokkens et al., Citation2022, Wissing, Ulgiati et al., Citation2022) are key elements for developing a new dementia screening instrument dedicated to people with SPI(M)D. Developing a new instrument is essential to be able to timely identify dementia and prevent (too) late diagnosis or no diagnosis at all. This allows to early respond to the person’s changing wishes and needs in order to maintain quality of life in people with SPI(M)D and dementia.
Acknowledgments
The authors wish to thank all caregivers and family members of persons with SPI(M)D for participating as informants in the interviews. Moreover, we would like to give special thanks to all staff members who contributed to the project (per care institution): Roelie Fopma, Nienke Stap, Karen van Huizen, Aurora Ulgiati (Alliade), Liesbeth van Dam, Mireille Meeuwsen, Sjoerd Bos (‘s Heeren Loo), José Nicolaas, Mieke Schippers, Lyanne Hassefras, Kimberly Hard, Stefan van Dijk (Ipse de Bruggen), Joke ter Maat, Anne Beenakkers and Marja Brouwer (Royal Dutch Visio). This project is a collaborative effort of Alliade, ‘s Heeren Loo, Ipse de Bruggen, Royal Dutch Visio, Hanze University of Applied Sciences, University of Groningen and UMCG.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aller-Alvarez, J. S., Menéndez-González, M., Ribacoba-Montero, R., Salvado, M., Vega, V., Suárez-Moro, R., Sueiras, M., Toledo, M., Salas-Puig, J., & Álvarez-Sabin, J. (2017). Myoclonic epilepsy in down syndrome and Alzheimer disease. Neurología (English Edition), 32(2), 69–73. https://doi.org/10.1016/j.nrleng.2014.12.019
- Alzheimer’s Association. (2022). 2022 Alzheimer’s disease facts and figures. Alzheimer’s and Dementia, 18(4), 700–789. https://doi.org/10.1002/alz.12638
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (5th ed.). Arlington, VA (USA): American Psychiatric Association Publishing. https://doi.org/10.1176/appi.books.9780890425596
- Ball, S. L., Holland, A. J., Hon, J., Huppert, F. A., Treppner, P., & Watson, P. C. (2006). Personality and behaviour changes mark the early stages of Alzheimer’s disease in adults with Down’s syndrome: Findings from a prospective population-based study. International Journal of Geriatric Psychiatry, 21(7), 661–673. https://doi.org/10.1002/gps.1545
- Ball, S. L., Holland, A. J., Huppert, F. A., Treppner, P., & Dodd, K. (2006). CAMDEX-DS: The Cambridge examination for mental disorders of older people with Down’s Syndrome and others with intellectual disabilities. Cambridge University Press.
- Ball, S. L., Holland, A. J., Huppert, F. A., Treppner, P., Watson, P., & Hon, J. (2004). The modified CAMDEX informant interview is a valid and reliable tool for use in the diagnosis of dementia in adults with Down’s syndrome. Journal of Intellectual Disability Research, 48(6), 611–620. https://doi.org/10.1111/j.1365-2788.2004.00630.x
- Ball, S. L., Holland, A. J., Treppner, P., Watson, P. C., & Huppert, F. A. (2008). Executive dysfunction and its association with personality and behaviour changes in the development of Alzheimer’s disease in adults with Down syndrome and mild to moderate learning disabilities. British Journal of Clinical Psychology, 47(1), 1–29. https://doi.org/10.1348/014466507X230967
- Ballard, C., Mobley, W., Hardy, J., Williams, G., & Corbett, A. (2016). Dementia in Down’s syndrome. The Lancet Neurology, 15(6), 622–636. https://doi.org/10.1016/S1474-4422(16)00063-6
- Benejam, B. (2009). Dementia symptoms in Down syndrome. SD-DS International Medical Journal on down Syndrome, 13(2), 18–21. https://doi.org/10.1016/S2171-9748(09)70018-2
- Bittles, A. H., & Glasson, E. J. (2004). Clinical, social, and ethical implications of changing life expectancy in Down syndrome. Developmental Medicine and Child Neurology, 46(4), 282–286. https://doi.org/10.1111/j.1469-8749.2004.tb00483.x
- Blok, J. B., Scheirs, J. G. M., & Thijm, N. S. (2017). Personality and behavioural changes do not precede memory problems as possible signs of dementia in ageing people with Down syndrome. International Journal of Geriatric Psychiatry, 32(12), 1257–1263. https://doi.org/10.1002/gps.4606
- Burt, D. B., Primeaux-Hart, S., Loveland, K. A., Cleveland, L. A., Lewis, K. R., Lesser, J., & Pearson, P. L. (2005). Tests and medical conditions associated with dementia diagnosis. Journal of Policy and Practice in Intellectual Disabilities, 2(1), 47–56. https://doi.org/10.1111/j.1741-1130.2005.00007.x
- Cooper, S. A., & Smiley, E. (2007). The prevalence, incidence and factors predictive of mental ill-health in adults with profound intellectual disabilities. Prospective Study. Journal of Applied Research in Intellectual Disabilities, 20(6), 505–509. https://doi.org/10.1111/j.1468-3148.2007.00403.x
- Coppus, A. M. W. (2013). People with intellectual disability: What do we know about adulthood and life expectancy? Developmental Disabilities Research Reviews, 18(1), 6–16. https://doi.org/10.1002/ddrr.1123
- Coppus, A. M. W. (2017). Comparing generational differences in persons with Down Syndrome. Journal of Policy and Practice in Intellectual Disabilities, 14(2), 118–123. https://doi.org/10.1111/jppi.12214
- Coppus, A. M. W., Evenhuis, H. M., Verberne, G. J., Visser, F. E., Oostra, B. A., Eikelenboom, P., Van Gool, W. A., Janssens, A. C. J. W., & Van Duijn, C. M. (2008). Survival in elderly persons with down syndrome. Journal of the American Geriatrics Society, 56(12), 2311–2316. https://doi.org/10.1111/j.1532-5415.2008.01999.x
- Coppus, A. M. W., Evenhuis, H., Verberne, G. J., Visser, F., Van Gool, P., Eikelenboom, P., & Van Duijin, C. (2006). Dementia and mortality in persons with Down’s syndrome. Journal of Intellectual Disability Research, 50(10), 768–777. https://doi.org/10.1111/j.1365-2788.2006.00842.x
- Coppus, A. M. W., Fekkes, D., Verhoeven, W. M. A., Evenhuis, H. M., & van Duijn, C. M. (2009). Neopterin and the risk of dementia in persons with Down syndrome. Neuroscience Letters, 458(2), 60–64. https://doi.org/10.1016/j.neulet.2009.04.020
- Coppus, A. M. W., Schuur, M., Vergeer, J., Janssens, A. C. J. W., Oostra, B. A., Verbeek, M. M., & van Duijn, C. M. (2012). Plasma β amyloid and the risk of Alzheimer’s disease in Down syndrome. Neurobiology of Aging, 33(9), 1988–1994. https://doi.org/10.1016/j.neurobiolaging.2011.08.007
- Day, K. (1985). Psychiatric disorder in the middle-aged and elderly mentally handicapped. British Journal of Psychiatry, 147(6), 660–667. https://doi.org/10.1192/bjp.147.6.660
- De Knegt, N. C., Evenhuis, H. M., Lobbezoo, F., Schuengel, C., & Scherder, E. J. A. (2013). Does format matter for comprehension of a facial affective scale and a numeric scale for pain by adults with Down syndrome? Research in Developmental Disabilities, 34(10), 3442–3448. https://doi.org/10.1016/j.ridd.2013.07.016
- De Knegt, N. C., Schuengel, C., Lobbezoo, F., Visscher, C. M., Evenhuis, H. M., Boel, J. A., & Scherder, E. J. A. (2016). Comprehension of pictograms for pain quality and pain affect in adults with Down syndrome. Journal of Intellectual and Developmental Disability, 41(3), 222–232. https://doi.org/10.3109/13668250.2016.1176129
- Deb, S., & Braganza, J. (1999). Comparison of rating scales for the diagnosis of dementia in adults with Down’s syndrome. Journal of Intellectual Disability Research, 43 (Pt 5), 400–407. https://doi.org/10.1046/j.1365-2788.1999.043005400.x
- Dekker, A. D., Coppus, A. M. W., Vermeiren, Y., Aerts, T., Van Duijn, C. M., Kremer, B. P., Naudé, P. J. W., Van Dam, D., & De Deyn, P. P. (2015). Serum MHPG strongly predicts conversion to Alzheimer’s disease in behaviorally characterized subjects with down syndrome. Journal of Alzheimer’s Disease, 43(3), 871–891. https://doi.org/10.3233/JAD-140783
- Dekker, A. D., Sacco, S., Carfi, A., Benejam, B., Vermeiren, Y., Beugelsdijk, G., Schippers, M., Hassefras, L., Eleveld, J., Grefelman, S., Fopma, R., Bomer-Veenboer, M., Boti, M., Oosterling, G. D. E., Scholten, E., Tollenaere, M., Van Goethem, G., zu Eulenburg, C., Coppus, A. M. W., De Deyn, P. P. (2018). The Behavioral and Psychological Symptoms of Dementia in Down Syndrome (BPSD-DS) Scale: Comprehensive assessment of psychopathology in Down Syndrome. Journal of Alzheimer’s Disease, 63(5), 797–820. https://doi.org/10.3233/JAD-170920
- Dekker, A. D., Strydom, A., Coppus, A. M. W., Nizetic, D., Vermeiren, Y., Naudé, P. J. W., Van Dam, D., Potier, M. C., Fortea, J., & De Deyn, P. P. (2015). Behavioural and psychological symptoms of dementia in Down syndrome: Early indicators of clinical Alzheimer’s disease? Cortex, 73, 36–61. https://doi.org/10.1016/j.cortex.2015.07.032
- Dekker, A. D., Ulgiati, A. M., Groen, H., Boxelaar, V. A., Sacco, S., Falquero, S., Carfi, A., Di Paola, A., Benejam, B., Valldeneu, S., Fopma, R., Oosterik, M., Hermelink, M., Beugelsdijk, G., Schippers, M., Henstra, H., Scholten-Kuiper, M., Willink-Vos, J., de Ruiter, L., … , De Deyn, P.P. (2021). The Behavioral and Psychological Symptoms of Dementia in Down Syndrome scale (BPSD-DS II): Optimization and further validation. Journal of Alzheimer’s Disease, 81(4), 1505–1527. https://doi.org/10.3233/JAD-201427
- Dekker, A. D., Wissing, M. B. G., Ulgiati, A. M., Bijl, B., van Gool, G., Groen, M. R., Grootendorst, E. S., van der Wal, I. A., Hobbelen, J. S. M., De Deyn, P. P., & Waninge, A. (2021). Dementia in people with severe or profound intellectual (and multiple) disabilities: Focus group research into relevance, symptoms and training needs. Journal of Applied Research in Intellectual Disabilities, 34(6), 1602–1617. https://doi.org/10.1111/jar.12912
- Devenny, D. A., Krinsky-McHale, S. J., Sersen, G., & Silverman, W. P. (2000). Sequence of cognitive decline in dementia in adults with Down’s syndrome. Journal of Intellectual Disability Research, 44(6), 654–665. https://doi.org/10.1111/j.1365-2788.2000.00305.x
- Duggan, L., Lewis, M., & Morgan, J. (1996). Behavioural changes in people with learning disability and dementia: A descriptive study. Journal of Intellectual Disability Research, 40(4), 311–321. https://doi.org/10.1111/j.1365-2788.1996.tb00636.x
- Eliasson, A. C., Krumlinde-Sundholm, L., Rösblad, B., Beckung, E., Arner, M., Öhrvall, A. M., & Rosenbaum, P. (2006). The Manual Ability Classification System (MACS) for children with cerebral palsy: Scale development and evidence of validity and reliability. Developmental Medicine and Child Neurology, 48(7), 549–554. https://doi.org/10.1017/S0012162206001162
- Elliott-King, J., Shaw, S., Bandelow, S., Devshi, R., Kassam, S., & Hogervorst, E. (2016). A critical literature review of the effectiveness of various instruments in the diagnosis of dementia in adults with intellectual disabilities. Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring, 4, 126–148. https://doi.org/10.1016/j.dadm.2016.06.002
- Engelborghs, S., Maertens, K., Nagels, G., Vloeberghs, E., Mariën, P., Symons, A., Ketels, V., Estercam, S., Somers, N., & De Deyn, P. P. (2005). Neuropsychiatric symptoms of dementia: Cross-sectional analysis from a prospective, longitudinal Belgian study. International Journal of Geriatric Psychiatry, 20(11), 1028–1037. https://doi.org/10.1002/gps.1395
- Esbensen, A. J., Hooper, S. R., Fidler, D., Hartley, S. L., Edgin, J., Liogier d’Ardhuy, X., Capone, G., Conners, F. A., Mervis, C. B., Abbeduto, L., Rafii, M. S., Krinsky-Mchale, S. J., Urv, T., Group, O. M. W., D’Ardhuy, X. L., Capone, G., Conners, F. A., Mervis, C. B., Abbeduto, L., & Weir, S. (2017). Outcome measures for clinical trials in Down syndrome. American Journal on Intellectual and Developmental Disabilities, 122(3), 247–281. https://doi.org/10.1352/1944-7558-122.3.247
- Evans, E., Bhardwaj, A., Brodaty, H., Sachdev, P., Draper, B., & Trollor, J. N. (2013). Dementia in people with intellectual disability: Insights and challenges in epidemiological research with an at-risk population. International Review of Psychiatry, 25(6), 755–763. https://doi.org/10.3109/09540261.2013.866938
- Evenhuis, H. M. (1990). The natural history of dementia in Down’s Syndrome. Archives of Neurology, 47(3), 263–267. https://doi.org/10.1001/archneur.1990.00530030029011
- Evenhuis, H. M. (1992). Evaluation of a screening instrument for dementia in ageing mentally retarded persons. Journal of Intellectual Disability Research, 36(4), 337–347. https://doi.org/10.1111/j.1365-2788.1992.tb00532.x
- Evenhuis, H. M., Kengen, M. M. F., & Eurlings, H. A. L. (1998). Dementie Vragenlijst voor Verstandelijk Gehandicapten (DVZ). Handleiding. Pearson Assessment and Information B.V.
- Evenhuis, H. M., Kengen, M. F., & Eurlings, H. A. L. (2006). Dementia questionnaire for people with intellectual disabilities manual (second edition). Harcourt Test Publishers.
- Finkel, S. I. (2000). Introduction to behavioural and psychological symptoms of dementia (BPSD). International Journal of Geriatric Psychiatry, 15(1), 2–4. https://.doi.org/10.1002/ (SICI)1099-1166(200004)15:1+3.0.CO;2-3.
- Fletcher, R. J., Barnhill, J., McCarthy, J., & Strydom, A. (2016). From DSM to DM-ID. Journal of Mental Health Research in Intellectual Disabilities, 9(3), 189–204. https://doi.org/10.1080/19315864.2016.1185324
- Gedye, A. (1995). Dementia scale for Down Syndrome: manual. http://www.gedye.ca
- Harris, P. A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., & Conde, J. G. (2009). Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. https://doi.org/10.1016/j.jbi.2008.08.010
- Hoekman, J., & Maaskant, M. A. (2002). Comparison of instruments for the diagnosis of dementia in individuals with intellectual disability. Journal of Intellectual and Developmental Disability, 27(4), 296–309. https://doi.org/10.1080/1366825021000029339
- Hon, J., Huppert, F. A., Holland, A. J., & Watson, P. (1999). Neuropsychological assessment of older adults with Down’s syndrome: An epidemiological study using the Cambridge Cognitive Examination (CAMCOG). British Journal of Clinical Psychology, 38(2), 155–165. https://doi.org/10.1348/014466599162719
- Houwen, S., van der Putten, A., & Vlaskamp, C. (2014). A systematic review of the effects of motor interventions to improve motor, cognitive, and/or social functioning in people with severe or profound intellectual disabilities. Research in Developmental Disabilities, 35(9), 2093–2116. https://doi.org/10.1016/j.ridd.2014.05.006
- Huxley, A., Prasher, V. P., & Haque, M. S. (2000). The dementia scale for Down’s syndrome. Journal of Intellectual Disability Research, 44(6), 697–698. https://doi.org/10.1111/j.1365-2788.2000.00295.x
- Keller, S. M., Janicki, M. P., & Esralew, L. (2016). Dementia: Screening, evaluation, diagnosis and management. In I. L. Rubin, J. Merrick, D. E. Greydanus, & D. R. Patel (Eds.), Health care for people with intellectual and developmental disabilities across the lifespan (pp. 1449–1463). Springer International Publishing. https://doi.org/10.1007/978-3-319-18096-0_116
- Kirk, L. J., Hick, R., & Laraway, A. (2006). Assessing dementia in people with learning disabilities. Journal of Intellectual Disabilities, 10(4), 357–364. https://doi.org/10.1177/1744629506070053
- Koran, M. E. I., Hohman, T. J., Edwards, C. M., Vega, J. N., Pryweller, J. R., Slosky, L. E., Crockett, G., De Rey, L. V., Meda, S. A., Dankner, N., Avery, S. N., Blackford, J. U., Dykens, E. M., & Thornton-Wells, T. A. (2014). Differences in age-related effects on brain volume in Down syndrome as compared to Williams syndrome and typical development. Journal of Neurodevelopmental Disorders, 6(1), 1–11. https://doi.org/10.1186/1866-1955-6-8
- Kraijer, D. W., Kema, G. N., & Bildt, A. A. D. (2004). Sociale Redzaamheidsschalen, SRZ/SRZI, Handleiding. Pearson Assessment and Information B.V.
- Krinsky-McHale, S. J., Devenny, D. A., & Silverman, W. P. (2002). Changes in explicit memory associated with early dementia in adults with Down’s syndrome. Journal of Intellectual Disability Research, 46(3), 198–208. https://doi.org/10.1046/j.1365-2788.2002.00365.x
- Kurrle, S., Hogarth, R., & Brodaty, H. (2012). Physical comorbidities of dementia. Cambridge University Press. https://doi.org/10.1007/978-981-10-0370-7_14-1
- Lott, I. T., Doran, E., Nguyen, V. Q., Tournay, A., Movsesyan, N., & Gillen, D. L. (2012). Down syndrome and dementia: Seizures and cognitive decline. Journal of Alzheimer’s Disease, 29(1), 177–185. https://doi.org/10.3233/JAD-2012-111613
- Maaskant, M. A., & Hoekman, J. (2011). Dementieschaal voor mensen met een verstandelijke handicap, DSVH, Handleiding. Bohn Stafleu van Loghum.
- Määttä, T., Tervo-Määttä, T., Taanila, A., Kaski, M., & Livanainen, M. (2006). Mental health, behaviour and intellectual abilities of people with Down syndrome. Down’s Syndrome, Research and Practice, 11(1), 37–43. https://doi.org/10.3104/reports.313
- Mahoney, F. I., & Barthel, D. (1965). Functional evaluation: The Barthel Index. Maryland State Med Journal, 14, 56–61.
- Margallo-Lana, M. L., Moore, P. B., Kay, D. W. K., Perry, R. H., Reid, B. E., Berney, T. P., & Tyrer, S. P. (2007). Fifteen-year follow-up of 92 hospitalized adults with Down’s syndrome: Incidence of cognitive decline, its relationship to age and neuropathology. Journal of Intellectual Disability Research, 51(6), 463–477. https://doi.org/10.1111/j.1365-2788.2006.00902.x
- Mccarron, M., Mccallion, P., Reilly, E., & Mulryan, N. (2014). A prospective 14-year longitudinal follow-up of dementia in persons with Down syndrome. Journal of Intellectual Disability Research, 58(1), 61–70. https://doi.org/10.1111/jir.12074
- McKenzie, K., Metcalfe, D., & Murray, G. (2018). A review of measures used in the screening, assessment and diagnosis of dementia in people with an intellectual disability. Journal of Applied Research in Intellectual Disabilities, 31(5), 725–742. https://doi.org/10.1111/jar.12441
- McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Kawas, C. H., Klunk, W. E., Koroshetz, W. J., Manly, J. J., Mayeux, R., Mohs, R. C., Morris, J. C., Rossor, M. N., Scheltens, P., Carrillo, M. C., Thies, B., Weintraub, S., & Phelps, C. H. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 263–269. https://doi.org/10.1016/j.jalz.2011.03.005
- Moriconi C, Schlamb C and Harrison B. (2015). Down Syndrome and Dementia: Guide to Identification, Screening, and Management. The Journal for Nurse Practitioners, 11(8), 812–818. 10.1016/j.nurpra.2015.05.015
- Moss, S., Patel, P., Prosser, H., Goldberg, D., Simpson, N., Rowe, S., & Lucchino, R. (1993). Psychiatric morbidity in older people with moderate and severe learning disability. I: Development and reliability of the patient interview (PAS-ADD). British Journal of Psychiatry, 163(4), 471–480. https://doi.org/10.1192/bjp.163.4.471
- Nakken, H., & Vlaskamp, C. (2007). A need for a taxonomy for profound intellectual and multiple disabilities. Journal of Policy and Practice in Intellectual Disabilities, 4(2), 83–87. https://doi.org/10.1111/j.1741-1130.2007.00104.x
- Nelson, L. D., Orme, D., Osann, K., & Lott, I. T. (2001). Neurological changes and emotional functioning in adults with down syndrome. Journal of Intellectual Disability Research, 45(5), 450–456. https://doi.org/10.1046/j.1365-2788.2001.00379.x
- Nieuwenhuis-Mark, R. E. (2009). Diagnosing Alzheimer’s dementia in Down syndrome: Problems and possible solutions. Research in Developmental Disabilities, 30(5), 827–838. https://doi.org/10.1016/j.ridd.2009.01.010
- Oliver, C., & Kalsy, S. (2005). The assessment of dementia in people with intellectual disabilities: Key assessment instruments. In J. Hogg & A. Langa (Eds.), Assessing adults with intellectual disabilities: A service providers guide (pp. 98–107). BPS Blackwell.
- Palisano, R., Rosenbaum, P., Walter, S., Russell, D., Wood, E., & Galuppi, B. (1997). Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental Medicine and Child Neurology, 39(4), 214–223. https://doi.org/10.1111/j.1469-8749.1997.tb07414.x
- Prasher, V. P. (1997). Dementia questionnaire for persons with mental retardation (DMR): Modified criteria for adults with Down’s syndrome. Journal of Applied Research in Intellectual Disabilities, 10(1), 54–60. https://doi.org/10.1111/j.1468-3148.1997.tb00006.x
- Prasher, V. P. (2009). Neuropsychological Assessments of Dementia in down Syndrome and Intellectual Disabilities. https://doi.org/10.1007/978-3-319-61720-6
- Reid, A. H., & Aungle, P. G. (1974). Dementia in ageing mental defectives: A Clinical psychiatric study. Journal of Mental Deficiency Research, 18(1), 15–23. https://doi.org/10.1111/j.1365-2788.1974.tb01214.x
- Ries, J. D. (2018). Rehabilitation for individuals with dementia: Facilitating success. Current Geriatrics Reports, 7(1), 59–70. https://doi.org/10.1007/s13670-018-0237-1
- Rösner, P., Berger, J., Tarasova, D., Birkner, J., Kaiser, H., Diefenbacher, A., & Sappok, T. (2021). Assessment of dementia in a clinical sample of persons with intellectual disability. Journal of Applied Research in Intellectual Disabilities, 34(6), 1618–1629. https://doi.org/10.1111/jar.12913
- Salmon, D. P., & Bondi, M. W. (2009). Neuropsychological assessment of dementia. Annual Review of Psychology, 60(1), 257–282. https://doi.org/10.1146/annurev.psych.57.102904.190024
- Sauna-Aho, O., Bjelogrlic-Laakso, N., Siren, A., & Arvio, M. (2018). Signs indicating dementia in down, Williams and Fragile X syndromes. Molecular Genetics and Genomic Medicine, 6(5), 855–860. https://doi.org/10.1002/mgg3.430
- Scott K R and Barrett A M. (2007). Dementia syndromes: evaluation and treatment. Expert Review of Neurotherapeutics, 7(4), 407–422. 10.1586/14737175.7.4.407
- Shultz, J., Aman, M., Kelbley, T., Wallace, C. L. C., Burt, D. B., Primeaux-Hart, S., Loveland, K., Thorpe, L., Bogos, E. S., Timon, J., Patti, P., & Tsiouris, J. (2004). Evaluation of screening tools for dementia in older adults with mental retardation. American Journal on Mental Retardation, 109(2), 98–110. https://doi.org/10.1352/0895-8017(2004)109<98:EOSTFD>2.0.CO;2
- Silverman, W., Schupf, N., Zigman, W., Devenny, D., Miezejeski, C., Schubert, R., & Ryan, R. (2004). Dementia in adults, with mental retardation: Assessment at a single point in time. American Journal on Mental Retardation, 109(2), 111–125+194. https://doi.org/10.1352/0895-8017(2004)109<111:DIAWMR>2.0.CO;2
- Startin, C. M., Hamburg, S., Hithersay, R., Davies, A., Rodger, E., Aggarwal, N., Al-Janabi, T., & Strydom, A. (2016). The LonDownS adult cognitive assessment to study cognitive abilities and decline in Down syndrome. Wellcome Open Research, 1–11. https://doi.org/10.12688/wellcomeopenres.9961.1
- Startin, C. M., Rodger, E., Fodor-Wynne, L., Hamburg, S., & Strydom, A. (2016). Developing an informant questionnaire for cognitive abilities in down syndrome: The Cognitive Scale for Down Syndrome (CS-DS). PLoS ONE, 11(5), e0154596. https://doi.org/10.1371/journal.pone.0154596
- Strydom, A., Shooshtari, S., Lee, L., Raykar, V., Torr, J., Tsiouris, J., Jokinen, N., Courtenay, K., Bass, N., Sinnema, M., & Maaskant, M. (2010). Dementia in older adults with intellectual disabilities - epidemiology, presentation, and diagnosis. Journal of Policy and Practice in Intellectual Disabilities, 7(2), 96–110. https://doi.org/10.1111/j.1741-1130.2010.00253.x
- Temple, V., Jozsvai, E., Konstantareas, M. M., & Hewitt, T. A. (2001). Alzheimer dementia in Down’s syndrome: The relevance of cognitive ability. Journal of Intellectual Disability Research, 45(1), 47–55. https://doi.org/10.1111/j.1365-2788.2001.00299.x
- Temple, V., & Konstantareas, M. M. (2005). A comparison of the behavioural and emotional characteristics of Alzheimer’s dementia in individuals with and without Down Syndrome. Canadian Journal on Aging/La Revue Canadienne Du Vieillissement, 24(2), 179–189. https://doi.org/10.1353/cja.2005.0071
- van Timmeren, E. A., van der Putten, A. A. J., van Schrojenstein Lantman-de Valk, H. M. J., van der Schans, C. P., & Waninge, A. (2016). Prevalence of reported physical health problems in people with severe or profound intellectual and motor disabilities: A cross-sectional study of medical records and care plans. Journal of Intellectual Disability Research, 60(11), 1109–1118. https://doi.org/10.1111/jir.12298
- van Timmeren, E. A., van der Schans, C. P., van der Putten, A. A. J., Krijnen, W. P., Steenbergen, H. A., van Schrojenstein Lantman-de Valk, H. M. J., & Waninge, A. (2017). Physical health issues in adults with severe or profound intellectual and motor disabilities: A systematic review of cross-sectional studies. Journal of Intellectual Disability Research, 61(1), 30–49. https://doi.org/10.1111/jir.12296
- Walker, B., Macbryer, S., Jones, A., & Law, J. (2015). Interinformant agreement of the dementia questionnaire for people with learning disabilities. British Journal of Learning Disabilities, 43(3), 227–233. https://doi.org/10.1111/bld.12102
- Wissing, M. B. G., Fokkens, A. S., Dijkstra, R., Hobbelen, J. S. M., van der Putten, A. A. J., De Deyn, P. P., Waninge, A., & Dekker, A. D. (2022). Dementia in people with severe/profound intellectual (and multiple) disabilities: Practice-Based observations of symptoms. Journal of Mental Health Research in Intellectual Disabilities. https://doi.org/10.1080/19315864.2022.2061092
- Wissing, M. B. G., Ulgiati, A. M., Hobbelen, J. S. M., De Deyn, P. P., Waninge, A., & Dekker, A. D. (2022). The neglected puzzle of dementia in people with severe/profound intellectual disabilities : A systematic literature review of observable symptoms. Journal of Applied Research in Intellectual Disabilities 35(1), 24–45. https://doi.org/10.1111/jar.12920
- World Health Organization. (2018). International statistical classification of diseases and related health problems, 11th revision (ICD-11). https://icd.who.int/browse11/l-m/en
- Zeilinger, E. L., Stiehl, K. A. M., & Weber, G. (2013). A systematic review on assessment instruments for dementia in persons with intellectual disabilities. Research in Developmental Disabilities, 34(11), 3962–3977. https://doi.org/10.1016/j.ridd.2013.08.013
- Zeilinger, E. L., Zrnic Novakovic, I., Komenda, S., Franken, F., Sobisch, M., Mayer, A.-M., Neumann, L. C., Loosli, S. V., Hoare, S., & Pietschnig, J. (2022). Informant-based assessment instruments for dementia in people with intellectual disability: A systematic review and standardised evaluation. Research in Developmental Disabilities, 121(December 2021), 104148. https://doi.org/10.1016/j.ridd.2021.104148
- Zigman, W. B., Schupf, N., Devenny, D. A., Miezejeski, C., Ryan, R., Urv, T. K., Schubert, R., & Silverman, W. (2004). Incidence and prevalence of dementia in elderly adults with mental retardation without down syndrome. American Journal on Mental Retardation, 109(2), 126–141. https://doi.org/10.1352/0895-8017(2004)109<126:IAPODI>2.0.CO;2
