Abstract
Although Korea had a national surveillance system for Creutzfeldt-Jakob disease (CJD), it was mainly dependent on attending physician's reports. Thus, little prospective data about the epidemiology, characteristics, and final diagnoses of suspected patients were available. We have established a nationwide network for the active surveillance of patients with suspected CJD. When the requested cerebrospinal fluid (CSF) samples tested positive for 14-3-3 protein, we investigated the clinical characteristics of the corresponding patients and followed them until their final diagnoses were confirmed. A total of 218 samples were requested for CSF assays from May 2010 to August 2012, and 106 (48.6%) were positive for 14-3-3 protein. In 89 patients with complete clinical data, 38 (42.7%) were diagnosed with probable CJD and the estimated annual occurrence of CJD was 16.3 persons-per-year. The most common diagnoses of the remainder were central nervous system infection and any-cause encephalopathy. Non-CJD subjects showed worse initial consciousness levels than CJD patients. This preliminary study showed that the number of reported cases of CJD and the true positivity rates of CSF 14-3-3 protein assays were both low in Korea. An active surveillance system is urgently needed to provide the latest nationwide epidemiological data of CJD.
Abbreviations
| CSF | = | cerebrospinal fluid |
| CJD | = | Creutzfeldt-Jakob disease |
| K-CDC | = | Korean Center for Disease Control |
| WHO | = | World Health Organization |
Introduction
Creutzfeldt-Jakob disease (CJD) surveillance systems were constructed in European countries in the early 1990sCitation1 and in China,Citation2 Japan,Citation3 and ArgentinaCitation4 in the late 1990s. Those systems supervise case registrations, conduct biospecimen analyses, and actively investigate and follow the clinical information of suspected patients as well as manage nationwide epidemiological data.Citation1
However, our national surveillance system was dependent on attending physicians' reports and did not involve active investigations of suspected patients. As a result, little epidemiological data and clinical information are available for patients suspected of having human prion disease in Korea.Citation5 In a previous report, 121 cases were reported to have probable or definite CJD during 2001–2004. However, the diagnoses of CJD were mostly based on cerebrospinal fluid (CSF) 14-3-3 protein assays, and few subjects underwent biopsies or autopsies.Citation5 Furthermore, since 2005, there have been no follow-up reports.
14-3-3 protein in the CSF has been widely accepted as a surrogate marker of CJD.Citation6–9 However, the clinical usefulness of this assay was thought to be variable according to pretest probability, and its use has been suggested to be warranted only in patients with a high suspicion of CJD.Citation7 The findings of previous studies that have shown a high sensitivity and specificity of 14-3-3 protein in CSF might have been the result of active and efficient surveillance systems that were able to rule out other causes of rapidly progressive dementia based on clinical background.Citation10
Our research is preparatory work for an active surveillance system for CJD in Korea. We aimed to estimate the annual occurrence of domestic CJD cases and the positivity rates of CSF 14-3-3 protein assays among suspected patients. In addition, we investigated the confirmed diagnoses of non-CJD patients with positive CSF 14-3-3 protein assay results.
Results
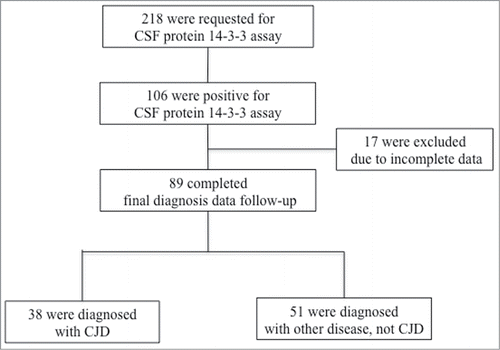
A total of 218 samples were requested to undergo CSF 14-3-3 protein assays during the 28 months of the study. Among them, 106 (48.6%) were positive for 14-3-3 protein in the CSF. We reached consensus for the final confirmed diagnoses of 89 patients at the end of the follow-up or at the patients' death. The remaining 17 subjects were excluded due to the lack of detailed clinical information ().
Among the 89 patients who were initially suspected of having CJD and who had positive CSF 14-3-3 protein assays, the steering committee diagnosed 38 (42.7%) with probable CJD according to the WHO criteria based on the clinical and laboratory test results after long-term follow-up and repeated tests.Citation11 The estimated annual occurrence of CJD was 16.3 persons-per-year (38 cases in 28 months). Among the probable CJD patients who had given consent for genetic testing (n = 14), all patients had polymorphism of PRNP at codon 129 with Met/Met in line with the previous report which described that majority of patients had codon 129 with Met/Met in Korea.Citation12
The confirmed diagnoses of the remaining 51 (57.3%) patients are summarized in . Central nervous system infections and any-cause encephalopathy were the most common diagnoses.
TABLE 1. The confirmed diagnosis of non-CJD subjects
For the subjects who were enrolled since 2010 and whose clinical and laboratory test results were available, the non-CJD subjects showed worse initial consciousness levels compared to the CJD patients. () Other clinical manifestations did not show any significant differences between the groups. In the non-CJD subjects, electroencephalography revealed a slowing rather than a typical periodic wave, and magnetic resonance imaging was also uncharacteristic.
TABLE 2. Clinical characteristics of probable CJD and non-CJD subjects
Discussion
In this preliminary study, we estimated the annual occurrence of domestic CJD cases and the positivity rates of CSF 14-3-3 protein assays among patients who were suspected of having CJD. We also described the final confirmed diagnoses of Korean non-CJD subjects with CSF 14-3-3 protein.
Annual Occurrence Rates of CJD in Korea and Worldwide
Estimates of the worldwide incidence of CJD have been 1.0–1.5 per million in previous reports.Citation2,11,13 Thus, there might be 50 to 75 cases that newly develop annually in Korea.Citation11,14 However, in our results, the estimated annual occurrence rate was only a quarter of that estimate.
In the Japanese study, the annual incidence rate was 0.49 cases per million population for males and 0.68 for females.Citation15 In Taiwan, the overall annual incidence rate was 0.55 cases per million.Citation16 Our estimated annual occurrence of 0.33 cases per million is still low, however there was a tendency that the annual incidence rates of Asian countries were lower than those of European countries or worldwide incidences. Although, our surveillance program might not be perfectly recognized all the corresponding clinical cases, we suggest that our results are still reportable and comparable to Asian epidemiological data.
Explanations for the Low Annual Occurrence Rate in Korea
Several possible explanations for the low annual occurrence rate have been suggested. Besides the differences due to geographical location and ethnicity, there might be a lack of motivation among attending physicians to report these cases, even though CJD is one of the officially designated communicable diseases and there is a fine for undeclared cases. Because it took over 2 weeks to obtain the final reports of the CSF 14-3-3 protein assays for the requested samples, we could not access the patients' clinical data without timely notification of the attending physicians during those periods. As a result, we could not complete all of the data collection for some of the patients.
Misdiagnosis might be another reason for the low occurrence rate. A recent survey has revealed that the awareness of and preparedness for prion disease among neurologists are limited, and about 44% of neurologists reported that they were not confident in diagnosing CJD.Citation14 Considering that any diseases that cause acute neuronal loss or damage, such as meningoencephalitis, malignancy, and cerebrovascular diseases, could increase CSF 14-3-3 protein levels, there could be a considerable number of misdiagnoses unless there is an appropriate clinical background.Citation8,10
Based on these assumptions, a considerable number of patients with CJD might still be underdiagnosed in Korea. Thus, an active surveillance system, which supervises case registrations and actively investigates and follows the clinical information of suspected patients, may be beneficial to improve the practical aspects of the detection rate. In addition, continuing education about prion disease for primary physicians and neurologists should also be considered.
Furthermore, the current diagnostic criteria of the WHO and National CJD Surveillance Unit are mainly useful for the clinical diagnoses of patients whose symptoms are evident enough to be detected.Citation17 Patients with CJD have shown negative results on CSF 14-3-3 protein assays in the early stages of the disease.Citation18 Thus, considering that the purpose of surveillance is to detect patients in the early phase of illness, new criteria with high sensitivity are needed for the early detection of suspected cases.
Relatively Low CSF 14-3-3 Positivity Rates
The total number of 218 samples was tested for CSF 14-3-3 and 48.6% revealed a positive CSF 14-3-3 signal in the Western blot. The positivity rate of CSF 14-3-3 was relatively low compared to other studies, which reported that the sensitivity and specificity of CSF 14-3-3 for CJD was between 80 and 90%.Citation19–22
Actually, there might be some discrepancies in our clinical situation with those previous studies. For the establishment of active surveillance network, we have encouraged participating physicians to request CSF 14-3-3 analysis for those who had been doubted with CJD if there were any possibilities. In other words, we permitted less convincing cases for CSF 14-3-3 analysis not to lose any true cases. On the contrary, the sensitivities and specificities of the previous studies were based on the clinically well-characterized cases.
Thus, we have used the term, “positivity rates” rather than “sensitivity.” Actually, the sensitivities of CSF 14-3-3 protein analysis has been estimated from 75% to 90.9% according to the clinical stages of well-characterized CJD cases with EEG and DWI changes in our single-hospital based research. [unpublished data]
Confusing Diagnoses with Probable CJD
In our results, less than 50% of the patients who were positive for CSF 14-3-3 protein were finally diagnosed with probable CJD. The most confusing diagnoses were central nervous system infections and encephalopathy. The rapid progression of cognitive deficits, impairments of consciousness, and the lack of any lateralizing signs could mislead the diagnosis of prion diseases.Citation13 The initial consciousness levels consisted of stupor or drowsiness for non-CJD patients, which was in contrast to the alertness observed in most CJD subjects. Although our analysis was not adequately powered, one should note that rapid progressive cognitive decline with an initial obscuration of consciousness might reflect causes other than CJD.
Autopsy should be performed to confirm a definite CJD diagnosis, however data on this are entirely lacking in our study. Due to social convention, autopsy is still conducted in very few instances in Korea. Spouses and children are reluctant to autopsy due to Confucian ideas that preservation one's body wholly is one of important aspects in filial duty. To overcome these obstacles, we had planned to follow up patients until their death and investigate the best possible diagnosis with the clinical characteristics and other laboratory findings such as DWI and EEG.
Conclusion
In conclusion, this preliminary study showed that the number of reported cases of CJD and the positivity rates of CSF 14-3-3 protein assays were both low, and these findings may improve the understanding of the pitfalls of cases with false-positive CSF 14-3-3 protein assays. An active surveillance system is urgently needed in Korea in order to provide regular nationwide epidemiological data updates and to estimate accurate incidences and prevalence of CJD.
Materials and Methods
Groundwork for Setting up the Korean CJD Surveillance Network (KCJDSN)
In January 2010, the preparatory work for the KCJDSN was initiated by the Korean Center for Disease Control (K-CDC), the Seoul National University Bundang Hospital, the Seoul National University Boramae Hospital, the Bobath Memorial Hospital, the Jungang University Hospital, and the Korean National Institute of Health. Neurology specialists of each hospital made up the steering committee of the KCJDSN. The local institutional review board approved this study, and all subjects submitted informed consent forms.
Surveillance Pipeline
When physicians requested CSF 14-3-3 protein assays for patients with suspected CJD from the K-CDC and the assay results were positive, this information was relayed to the steering committee. The members of the steering committee visited the corresponding hospitals and gathered medical histories and clinical information through patient or caregiver interviews. The committee members met with the attending physicians and discussed the patients' conditions at admission, their progress, and diagnosis. Medical records and neuroimaging data were also reviewed. The committee followed all cases until any of the following circumstances were observed: 1) a confirmed diagnosis of CJD, 2) another confirmed diagnosis, such as encephalopathy or meningoencephalitis, in non-CJD cases, or 3) death without any evidence of diseases other than CJD or non-CJD human prionopathy.
Laboratory and Neuroimaging Evaluations
We analyzed the blood samples with routine laboratory evaluations of vitamin B12, folate, thyroid function, venereal disease, and homocysteine levels and the CSF samples with routine examinations of cell counts, chemistry, culture, and 14-3-3 protein. We also investigated the polymorphism of PRNP at codon 129 for those who had given consent for genetic testing. During admission, electroencephalography was conducted at least once. Neuroimaging evaluations included brain computed tomography, magnetic resonance imaging including diffusion-weighted imaging.
CSF 14-3-3 Protein Assay
14-3-3 protein levels were analyzed in the CSF with western blotting. Detailed protocol was described in the supplementary materials.
Diagnostic Criteria for Patients with Suspected CJD
When all members of the steering committee were in agreement, we determined the diagnosis of a case as CJD according to the World Health Organization (WHO) diagnostic criteria for probable sporadic CJD.Citation11 The 4 diagnostic criteria were 1) progressive dementia; 2) at least 2 out of the following 4 clinical features: myoclonus, visual or cerebellar disturbance, pyramidal/extrapyramidal dysfunction, or akinetic mutism; 3) atypical electroencephalogram recordings during an illness of any duration and/or a positive CSF 14-3-3 protein assay with a clinical duration to death of less than 2 years; and 4) routine investigations that did not suggest any alternative diagnosis.
Merging with the Previous Database in 2006
We conducted a similar small pilot study in 2006 [unpublished]. Although detailed clinical data were absent, we had final diagnoses for the suspected patients. Thus, we merged those databases with those of the current study for further analysis (Table S1).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental_Material.docx
Download MS Word (20.2 KB)Funding
This study was supported by a grant from the Korea Center for Diseases Control; Ministry of Health, Welfare, and Family Affairs; Republic of Korea (2010-E53001-00, 2011-E21004-00).
REFERENCES
- Brandel J-P, Salomon D, Capek I, Vaillant V, Alpérovitch A. Epidemiological surveillance of Creutzfeldt-Jakob in France. Rev Neurol (Paris) 2009; 165:684–93; PMID:19467685; http://dx.doi.org/10.1016/j.neurol.2009.04.006
- Gao C, Shi Q, Tian C, Chen C, Han J, Zhou W, Zhang BY, Jiang HY, Zhang J, Dong XP. The epidemiological, clinical, and laboratory features of sporadic Creutzfeldt-Jakob disease patients in China: surveillance data from 2006 to 2010. PLoS One 2011; 6:e24231; PMID:21904617; http://dx.doi.org/10.1371/journal.pone.0024231
- Sanjo N, Mizusawa H. Prion disease - the characteristics and diagnostic points in Japan. Clin Neurol 2010; 50:287–300; PMID:20535976
- Begue C, Martinetto H, Schultz M, Rojas E, Romero C, D'Giano C, Sevlever G, Somoza M, Taratuto AL. Creutzfeldt-Jakob disease surveillance in Argentina, 1997–2008. Neuroepidemiology 2011; 37:193–202; PMID:22067221; http://dx.doi.org/10.1159/000331907
- Choi SI JB, Kim YS. Development of policy and strategy for the control of Creutzfeldt-Jakob Disease in Korea. Korean J Epidemiol 2005; 27:81–9
- Hsich G, Kenney K, Gibbs CJ, Lee KH, Harrington MG. The 14-3-3 brain protein in cerebrospinal fluid as a marker for transmissible spongiform encephalopathies. N Eng J Med 1996; 335:924–30; PMID:8782499; http://dx.doi.org/10.1056/NEJM199609263351303
- Muayqil T, Gronseth G, Camicioli R. Evidence-based guideline: diagnostic accuracy of CSF 14-3-3 protein in sporadic Creutzfeldt-Jakob disease: report of the guideline development subcommittee of the American Academy of Neurology. Neurology 2012; 79:1499–506; PMID:22993290; http://dx.doi.org/10.1212/WNL.0b013e31826d5fc3
- Lemstra AW, van Meegen MT, Vreyling JP, Meijerink PH, Jansen GH, Bulk S, Baas F, van Gool WA. 14-3-3 testing in diagnosing Creutzfeldt-Jakob disease: a prospective study in 112 patients. Neurology 2000; 55:514–6; PMID:10953182; http://dx.doi.org/10.1212/WNL.55.4.514
- Zerr I, Bodemer M, Gefeller O, Otto M, Poser S, Wiltfang J, Windl O, Kretzschmar HA, Weber T. Detection of 14-3-3 protein in the cerebrospinal fluid supports the diagnosis of Creutzfeldt-Jakob disease. Ann Neurol 1998; 43:32–40; PMID:9450766; http://dx.doi.org/10.1002/ana.410430109
- Green AJ. Use of 14-3-3 in the diagnosis of Creutzfeldt-Jakob disease. Biochem Soc Trans 2002; 30(4):382–86; PMID:12196099; http://dx.doi.org/10.1042/BST0300382
- Global surveillance, diagnosis and therapy of human transmissible spongiform encephalopathies: report of a WHO consultation. Geneva, Switzerland: WHO; 1998.
- Jeong BH, Lee KH, Kim NH, Jin JK, Kim JI, Carp RI, Kim YS. Association of sporadic Creutzfeldt-Jakob disease with homozygous genotypes at PRNP codons 129 and 219 in the Korean population. Neurogenetics 2005; 6:229–32; PMID:16217673; http://dx.doi.org/10.1007/s10048-005-0016-y
- Nozaki I, Hamaguchi T, Sanjo N, Noguchi-Shinohara M, Sakai K, Nakamura Y, Sato T, Kitamoto T, Mizusawa H, Moriwaka F et al. Prospective 10-year surveillance of human prion diseases in Japan. Brain 2010; 133:3043–57; PMID:20855418; http://dx.doi.org/10.1093/brain/awq216
- Jang J-W, Park YH, Lim JS, Park SC, Cheong H-K, Kim JE, Kim SY. Neurologists' awareness and preparedness on prion diseases in Korea. Dement Neurocognitive Disord 2013; 12:9–20; http://dx.doi.org/10.12779/dnd.2013.12.1.9
- Nakamura Y, Yanagawa H, Hoshi K, Yoshino H, Urata J, Sato T. Incidence rate of Creutzfeldt-Jakob disease in Japan. Int J Epidemiol 1999; 28:130–4; PMID:10195677; http://dx.doi.org/10.1093/ije/28.1.130
- Lu CJ, Sun Y, Chen SS. Incidence of Creutzfeldt-Jakob disease in Taiwan: a prospective 10-year surveillance. Eur J Epidemiol 2010; 25:341–7; PMID:20333444; http://dx.doi.org/10.1007/s10654-010-9446-4
- Heath CA, Cooper SA, Murray K, Lowman A, Henry C, MacLeod MA, Stewart G, Zeidler M, McKenzie JM, Knight RS, et al. Diagnosing variant Creutzfeldt-Jakob disease: a retrospective analysis of the first 150 cases in the UK. J Neurol Neurosurg Psychiatry 2011; 82:646–51; PMID:21172857; http://dx.doi.org/10.1136/jnnp.2010.232264
- Geschwind MD, Martindale J, Miller D, DeArmond SJ, Uyehara-Lock J, Gaskin D, Kramer JH, Barbaro NM, Miller BL. Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol 2003; 60:813–6; PMID:12810484; http://dx.doi.org/10.1001/archneur.60.6.813
- Baldeiras IE, Ribeiro MH, Pacheco P, Machado A, Santana I, Cunha L, Oliveira CR. Diagnostic value of CSF protein profile in a Portuguese population of sCJD patients. J Neurol 2009; 256:1540–50; PMID:19418113; http://dx.doi.org/10.1007/s00415-009-5160-0
- Matsui Y, Satoh K, Miyazaki T, Shirabe S, Atarashi R, Mutsukura K, Satoh A, Kataoka Y, Nishida N. High sensitivity of an ELISA kit for detection of the gamma-isoform of 14-3-3 proteins: usefulness in laboratory diagnosis of human prion disease. BMC Neurol 2011; 11:120; PMID:21970675; http://dx.doi.org/10.1186/1471-2377-11-120
- Collins SJ, Sanchez-Juan P, Masters CL, Klug GM, van Duijn C, Poleggi A, Pocchiari M, Almonti S, Cuadrado-Corrales N, de Pedro-Cuesta J, et al. Determinants of diagnostic investigation sensitivities across the clinical spectrum of sporadic Creutzfeldt-Jakob disease. Brain 2006; 129:2278–87; PMID:16816392; http://dx.doi.org/10.1093/brain/awl159
- Green AJ, Ramljak S, Muller WE, Knight RS, Schroder HC. 14-3-3 in the cerebrospinal fluid of patients with variant and sporadic Creutzfeldt-Jakob disease measured using capture assay able to detect low levels of 14-3-3 protein. Neurosci Lett 2002; 324:57–60; PMID:11983294; http://dx.doi.org/10.1016/S0304-3940(02)00172-6