Abstract
Formed by non-covalent interactions and not defined at genetic level, the assemblies of small molecules in biology are complicated and less explored. A common morphology of the supramolecular assemblies of small molecules is nanofibrils, which coincidentally resembles the nanofibrils formed by proteins such as prions. So these supramolecular assemblies are termed as prion-like nanofibrils of small molecules (PriSM). Emerging evidence from several unrelated fields over the past decade implies the significance of PriSM in biology and medicine. This perspective aims to highlight some recent advances of the research on PriSM. This paper starts with description of the intriguing similarities between PriSM and prions, discusses the paradoxical features of PriSM, introduces the methods for elucidating the biological functions of PriSM, illustrates several examples of beneficial aspects of PriSM, and finishes with the promises and current challenges in the research of PriSM. We anticipate that the research of PriSM will contribute to the fundamental understanding at the intersection of supramolecular chemistry and cell biology and ultimately lead to a new paradigm of molecular (or supramolecular) therapeutics for biomedicine.
Abbreviations
| PriSM | = | prion-like nanofibrils of small molecules |
| PrPSc | = | prions |
| PrPc | = | prion protein |
| PriLiM | = | prion-like mechanism |
| MHPB | = | molecular hydrogel protein binding |
Prions (PrPSc) refer to the pathogenic, misfolded proteins that cause a variety of human and animal neurodegenerative diseases when their accumulation reaches over a critical threshold. Because their etiology and pathogenesis involve the modification and self-propagation of the prion protein (PrPc), a constituent of normal mammalian cells, these neurodegenerative diseases are classified together as prion diseases.Citation1 Usually, the normal cellular PrPc transforms into pathogenic PrPSc through a posttranslational process, resulting in a high content of β-sheets, and the subsequent PrPSc acts as a template to turn PrPc into a nascent PrPSc.Citation1 Although the initial awareness of prions came from mammalian diseases, the studies on yeast prionsCitation2,3 have generated many profound insights for understanding cellular control of prion propagation, roles of prion, and mechanisms of prion formation. Although PrPSc is highly neurotoxic and often associated with diseases, PrPc is essential for cellular processes and exhibits beneficial functions (e.g., PrPc is neuroprotective).Citation4 Interestingly, prion-related mechanisms, namely, prion-like mechanisms (PriLiMs, a process involving the self-templating propagation of protein conformations)Citation5 can provide beneficial functions in nature (e.g.,, building stable structures, signal propagation, dynamic scaffolding of ribonucleoprotein granules, bet-hedging in microorganisms, etc.). Increased cases of prion-like proteins are identified to be non-pathogenic and even confer benefits to cells, such as the cytoplasmic polyadenylation element-binding protein,Citation6 the mitochondrial antiviral signaling protein,Citation7 the T-cell-restricted intracellular antigen 1,Citation8 etc. It is also believed that there are likely many more prion-like proteins to be identified in a variety of cellular processes. These emerging evidences, undoubtedly, indicate that prion-like proteins and PriLiMs are more fundamental processes for maintaining the well-being of cells than merely being pathogenic.
Formed by non-covalent interactions and not defined at genetic level, one most common morphological feature of prions or prion-like structures is the formation of nanoscale aggregates (most cases give nanofibrils that can be observed by electron microscopy). Intriguingly, supramolecular assemblies of small molecules in aqueous phases, also formed by non-covalent interactions and not defined at genetic level, usually exhibit the morphology of nanofibrils. Thus, these prion-like nanofibrils of small molecules are termed as PriSM.Citation9 PriSM, indeed, share certain physiochemical and biochemical characteristics of prions (e.g., growing by self-propagation, high content of β-sheet like superstructures, dramatically different behaviors between nanofibirls/aggregates and their constituents), but possess distinctive properties, including reversibility, tunability, and degradability. Reversibility and degradability confer the transient characters to PriSM, which is essential for the spatiotemporal control of functions. Being an inherent feature of small molecules, the tunability allows easy manipulation and regulation of PriSM at molecular level. These unique features of PriSM, which are absent from pathogenic prions, imply that it is feasible to engineer PriSM as a new class of molecular entities for beneficial biological functions.
Because non-covalent intermolecular interactions are the driving forces for the formation of PriSM, the development of supramolecular chemistry, in the past few decades, has provided well-laid molecular foundation for the exploration of PriSM in cell biology. However, since the emergent properties of supramolecular assemblies drastically differ from those of the individual molecules, the interactions between proteins and the supramolecular assemblies should differ from that of their monomeric constituents. But there is no general assay for identifying the proteins that interact with the assemblies of small molecules (e.g.,, PriSM). To address this key problem, we have developed a molecular hydrogel protein binding (MHPB) assay for discovering the protein targets of the supramolecular assemblies of small molecules.Citation10,11 We used the molecular hydrogels of small molecules (instead of the precipitates of the small molecules) because molecular hydrogels,Citation12,13 having high water content, closely resemble the crowded cellular environment. Most importantly, the nanometer sizes of PriSM in the molecular hydrogels are comparable to the sizes of protein complexes. Such finite sizes minimize the area of hydrophobic patches, and reduce protein denature caused by nonspecific interactions.Citation14 Thus, molecular hydrogels serve as an equivalent of PriSM in cellular environment for evaluating the binding of proteins to PriSM. For example, we used a small peptide derivative (1) () to form a hydrogel or precipitates by changing temperature or pH, respectively. The hydrogel of 1 consists of PriSM and water, and the precipitates of 1 contain mainly microparticles. After the pull down of proteins by incubating the hydrogel or the precipitates with cell lysates, gel electrophoresis indicates that, while the precipitates hardly bind proteins, the molecular hydrogel selectively binds proteins and results in major bands of proteins. Protein profile (LC-MS/MS) of the proteins pulled-down by the hydrogel and the precipitates reveals that MHPB assay is a more effective and reliable method for identifying the protein targets of PriSM than the use of precipitates of the small molecules.Citation11
Figure 1. PriSM of 1 selectively inhibit cancer cells. (A) The structure of a building block (1) of PriSM. (B) ThT binding assays of as-prepared solution of 1. The ThT emission starts to increase with the concentration of 1 in a linear manner above the concentration of 163 μg/mL. The inset is the 48 h viability (MTT assay) of HeLa cells treated with as-prepared 1. (C) MHPB assay: SDS-PAGE; major proteins from MS protein profiling; Western blot confirms tubulins as the primary targets of PriSM of 1, and PriSM of 1 interacting with multiple proteins (lanes: complete cell lysate (C), wash-off proteins (W3), proteins bound on the molecular nanofibers (MN)). (D) Confocal images of tubulin staining of HeLa cells treated with PriSM of 1 to confirm PriSM of 1 disrupting the elongation process of microtubules (scale bar = 10 µm). (E) PriSMs of 1 enter the cell by macropinocytosis, impede the cytoskeletal proteins, and result in apoptosis.
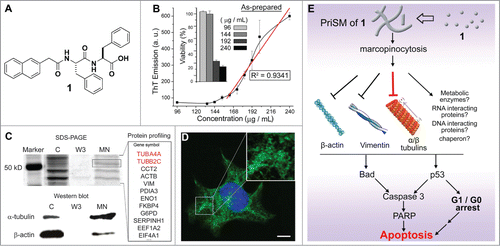
Recently, we have elucidated the biological functions of the PriSM of 1 and confirmed that PriSM are able to interact with multiple proteins and selectively inhibit cancer cells in cell culture, as well as xenograft tumor in animal models.Citation15,16 Experimental results indicate that 1 effectively inhibits the growth of cancer cells (e.g., HeLa) at or above the critical concentration (192 μg/mL). Thioflavin T (ThT, a fluorescence dye of β-sheet fibrils) binding assay confirms that 1 mainly exists as PriSM at or above the critical concentration (). Although the treatment of 1 above the critical concentration dramatically decreases the viability of HeLa cells, 1 remains innocuous to the cells below the critical concentration ( inset). Further studies demonstrate that the PriSM of 1 selectively inhibit different types of cancer cells (i.e., MES-SA, Capan-2, MCF-7, Hep G2, and T98G) of different tissue origins (i.e., uterus, pancreas, epithelial, liver, and brain), while remain largely innocuous to stromal cells (i.e., HS-5) and neuronal cells (i.e., PC12).Citation9 This selectivity is further verified by the co-culture of HS-5 and GFP-expressing HeLa cells (HeLa-GFP) (1:1 ratio) as the addition of PriSM of 1 kills most of HeLa-GFP cells, and leaves HS-5 cells alive.Citation9 Congo red staining of HeLa cells incubated with PriSM of 1 confirms that PriSM of 1 enter the cells, likely via macropinocytosis, and efficiently escape from lysosome to accumulate in cytosol. Using the MHPB assayCitation11, we analyzed 3 protein bands between 40 and 70 kDa (, lane MN) with protein profile after separating the protein targets of PriSM of 1 from cytosolic extract of HeLa cells. Protein profile (LC-MS/MS) reveals high protein coverage of the building block of cytoskeletal filaments (tubulins, vimentin, and actins) (, box). After using Western blot to confirm tubulins as the major targets of PriSM of 1, we further validated that PriSM of 1 disrupt the dynamics of microtubules by inducing cluster of short microtubules inside cells (), which causes mitochondria-dependent apoptosis. The knockdown of Tau protein, a protein that stabilizes microtubules, sensitizes PC12 cells to PriSM of 1, further proving the interaction between PriSM of 1 and tubulins.Citation9
These above results have provided new insights () for understanding the action of the PriSM of 1: (i) The monomeric molecules of 1 and PriSM of 1 behave drastically different—PriSM of 1 are cytotoxic, and monomeric 1 is innocuous; (ii) PriSM of 1 selectively accumulate in cancer cells via micropinocytosis; (iii) the accumulated PriSM of 1 inside cancer cells impede the dynamics of cytoskeletal filaments, thus inducing apoptosis. This work illustrates that PriSM interact with multiple proteins yet induce a primary phenotype (e.g., apoptosis), thus validating that PriSM acts as a new class of biofunctional entity to control the fate of cells.
While the selective accumulation of PriSM of 1 inside cells efficiently inhibits cancer cells over normal cells, the inhibition of cancer cells by selectively forming pericellular nanofibrils via dephosphorylation illustrated another example of PriSM for controlling the fate of cells.Citation16 During the study of enzymatic transformation and self-assembly of small molecules,Citation13 we unexpectedly observed the formation of PriSM of D-peptide derivatives in pericellular space of cancer cells (e.g., HeLa cells) due to the overexpress of ectophosphatases (e.g., Regan enzymeCitation17). Specifically, when incubating the HeLa cells with a D-peptide precursor (2, ) that is a substrate of tyrosine phosphatase, we found the formation of the hydrogel around the HeLa cells after the incubation of 2 with the cells for a relatively short time. Using Congo red, a dye for nanofibrils, together with TEM, we confirmed that the PriSM of 3 selectively form in the pericellular space of cancer cells because the red fluorescence of Congo red mainly appears in the pericellular space and DAPI, a dye for nucleolus cannot enter cell due to the pericellular hydrogel ().
Figure 2. PriSM formed in pericellular space for selectively inhibiting cancer cells. (A) The structures of a precursor (2) and the self-assembling small molecule (3). (B) 3D stacked z-scan fluorescent images of Congo red stained PriSM on HeLa cells incubated with 2 (400 μg/mL) (C) TEM images of the PriSM of 3 on the cells (scale bar = 100 nm). (D) Cell viabilities of HeLa treated by 2 or 3 at 200 µg/mL, or 2 (217 µg/mL)+L-Phe (54 µg/mL) for 48h. (E) ALPP-instructed formation of PriSM of 3 on cell surface inhibits the cancer cells, but 3, as the soluble monomers, is innocuous to cells. Adapted from Refs. Citation16 and Citation19. © 2014 John Wiley & Sons Inc. Reproduced by permission of John Wiley & Sons Inc. Permission to reuse must be obtained from the rightsholder.
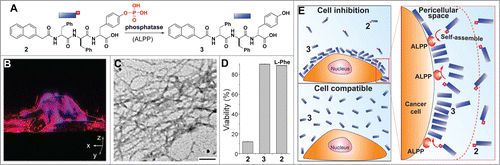
Since the phosphatases originated from the HeLa cells convert 2 to 3, one would expect that 2 and 3 inhibit the HeLa cells with the same activity after long incubation (e.g., 48h). However, the incubation of HeLa cells with 2 or 3 at the same concentration gives an apparently counterintuitive result: while 2 significantly inhibits the HeLa cells, 3 is innocuous to the cells. This result excludes the possibility that 3 inhibits the cells via a specific ligand-receptor interaction. Moreover, the addition of L-phenylalanine (L-Phe), an non-competitive inhibitor of placental alkaline phosphatase (ALPP),Citation18 stops the dephosphorylation and abrogates the inhibitory activity of 2, which confirms that ALPP, an ectoenzyme, dephosphorylates 2 to result in the PriSM of 3 on cell surface to inhibit the HeLa cells.Citation19 This result establishes that the ectophosphatase (i.e., ALPP) instructs the formation of pericellular PriSM of innocuous monomers (e.g., 3) for inhibiting cancer cells selectively, as illustrate in. Being innocuous in monomeric form, 3 would unlikely cause chronic toxicity since the PriSM of 3 would dissociate after the death of cancer cells. This observation, as the first example of enzyme-instructed formation of PriSM in pericellular spaces, not only demonstrates an unexpected yet fundamentally new mechanism to selectively inhibit cancer cells, but also represents a case of beneficial aspect of PriSM.
Not surprisingly, nature has already explored the merits of PriSM for important biological functions. For example, human α-defensin 6 (HD 6), a small antibacterial peptide highly expressed by secretory Paneth cells of small intestine, undergoes ordered self-assembly to form PriSM as nanonets that surround and entangle bacteria, thus providing protection against invasion by enteric bacterial pathogens.Citation20 Moreover, emerging evidences from several recent studies also highlight the promises and importance of PriSM. Walker et al. has demonstrated that ramoplanin (a glycopeptidic antibiotic against gram positive bacteria), in the presence of its receptor, forms PriSM that enhance binding to bacteria cell wall, which makes ramoplanin a very effective inhibitor against bacteria.Citation21 Several proposed mechanistic modelsCitation22 of the action of antimicrobial peptides (AMP) also imply the formation of PriSM of the AMPs. Maruyama et al. reported that the self-assembly of a peptide lipid, triggered by a cancer-related enzyme (matrix metalloproteinase-7, MMP-7), results in PriSM that selectively inhibit cancer cells over normal cells.Citation23 Pires and Ulijn et al. also reported that the PriSM formed by enzymatic dephosphorylation of aromatic carbohydrate amphiphile selectively inhibit osteosarcoma cells.Citation24
In addition to enzyme triggering,Citation13,25 ligand-receptor interaction is able to catalyze the formation of PriSM, as shown by a recent example that vancomycin catalyzes the formation of PriSM of a small D-Ala-D-Ala derivative to induce cell necroptosis.Citation26 One intriguing observation is that the presence of vancomycin is required to maintain the cytotoxicity of the PriSM of the D-Ala-D-Ala derivative.Citation26 Wells et al. reported that the PriSM of a small molecule cause apoptosis via multiple mechanisms (e.g., activating caspaseCitation27 and disrupting lysosomesCitation28). Besides inhibiting cells, PriSM also promote cell proliferation, and our recent studies show that the PriSM of a conjugate of nucleobase, amino acids, and carbohydrates, are able to mimic the multifarious functions of glycoproteins/proteoglycans and promote the proliferation of mouse embryonic stem cells and the development of zygotes into blastocysts of mouse.Citation29 Moreover, an L-rhamnose-based small molecule self-assembles to form PriSM, which, in contrast to the properties of monomeric L-rhamnose, suppress the immune response to antigens (e.g.,, phycoerythrin) in mice.Citation30 These results are consistent with the paradoxical nature of PriSM.
In summary, many seemingly unrelated studies indicate that the formation of PriSM is a rather general phenomenon in cell biology and biomedicine. However, the properties and functions of PriSM,Citation9,28,31,32 like prions and nonpathogenic protein aggregates,Citation33,34 are paradoxical. Thus, it is becoming increasingly important to recognize their existence and to explore the molecular science related to PriSM. In fact, the aggregates of small molecules (i.e., PriSM) contribute to 95% false positive in high-throughput screening (HTS),Citation35 implying the generality of PriSM. However, the elucidation of biological functions of PriSM remains difficult because i) promiscuous interactions between proteins and PriSM significantly depart from the well-established ligand-receptor dogma, and few techniques and methods are readily available for studying them; ii) PriSM behave dramatically different from their monomers, while researchers tend to emphasize the functions of the monomers, but overlook the self-assembled forms; iii) PriSM are not defined at genetic level, conventional biochemical and genetic methods are insufficient for controlling their formation. Fortunately, supramolecular chemistry has revealed considerable insights of non-covalent intermolecular interactions that may facilitate molecular scientists to study and to explore PriSM for biomedicine. However, since supramolecular chemistry, in the past, has been explored largely in non-aqueous phase,Citation36,37 it is necessary to explore and understand molecular self-assembly in waterCitation12,13,38,39 for formulating the guiding principle and establishing the starting point to study PriSM at the intersection of supramolecular chemistry and cell biology. One of the promising starting points, in our biased view, would be catalytic formation of PriSM in cellular environment. More importantly, by studying the biological functions and the cellular mechanism of PriSM, researchers will have an opportunity to take advantage of the promiscuity of PriSM for targeting redundancy that causes drug resistance in many diseases (e.g., cancerCitation40 and infectious diseasesCitation41). The paradigm-shift of assemblies of small molecules—from supramolecular chemistry into cell biology, undoubtedly, will lead to new discoveries in cell biologyCitation42 and contribute new knowledge to biomedicine.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was partially supported by NIH (CA142746), NSF MRSEC (DMR-1420382), and W. M. Keck Foundation. JZ is a Howard Hughes Medical Institute (HHMI) International Research Fellow.
REFERENCES
- Prusiner SB. Prions. Proc Natl Acad Sci U S A 1998; 95:13363–83; PMID:9811807; http://dx.doi.org/10.1073/pnas.95.23.13363
- Liebman SW, Chernoff YO. Prions in yeast. Genetics 2012; 191:1041–72; PMID:22879407; http://dx.doi.org/10.1534/genetics.111.137760
- Crow ET, Li L. Newly identified prions in budding yeast, and their possible functions. Semin Cell Dev Biol 2011; 22:452–9; PMID:21397710; http://dx.doi.org/10.1016/j.semcdb.2011.03.003
- Bounhar Y, Zhang Y, Goodyer CG, LeBlanc A. Prion protein protects human neurons against Bax-mediated apoptosis. J Biol Chem 2001; 276:39145–9; PMID:11522774; http://dx.doi.org/10.1074/jbc.C100443200
- Newby GA, Lindquist S. Blessings in disguise: biological benefits of prion-like mechanisms. Trends Cell Biol 2013; 23:251–9; PMID:23485338; http://dx.doi.org/10.1016/j.tcb.2013.01.007
- Si K, Lindquist S, Kandel ER. In Brief. Nat Rev Neurosci 2004; 5:81–2
- Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 2011; 146:448–61; PMID:21782231; http://dx.doi.org/10.1016/j.cell.2011.06.041
- Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell 2004; 15:5383–98; PMID:15371533; http://dx.doi.org/10.1091/mbc.E04-08-0715
- Kuang Y, Long MJ, Zhou J, Shi J, Gao Y, Xu C, Hedstrom L, Xu B. Prion-like nanofibrils of small molecules (PriSM) selectively inhibit cancer cells by impeding cytoskeleton dynamics. J Biol Chem 2014; 289:29208–18; PMID:25157102; http://dx.doi.org/10.1074/jbc.M114.600288
- Gao Y, Long MJ, Shi J, Hedstrom L, Xu B. Using supramolecular hydrogels to discover the interactions between proteins and molecular nanofibers of small molecules. Chem Commun (Camb) 2012; 48:8404–6; PMID:22801479; http://dx.doi.org/10.1039/c2cc33631f
- Kuang Y, Xu B. Disruption of the dynamics of microtubules and selective inhibition of glioblastoma cells by nanofibers of small hydrophobic molecules. Angew Chem Int Ed Engl 2013; 52:6944–8; PMID:23686848; http://dx.doi.org/10.1002/anie.201302658
- Estroff LA, Hamilton AD. Water gelation by small organic molecules. Chem Rev 2004; 104:1201–18; PMID:15008620; http://dx.doi.org/10.1021/cr0302049
- Yang Z, Liang G, Xu B. Enzymatic hydrogelation of small molecules. Acc Chem Res 2008; 41:315–26; PMID:18205323; http://dx.doi.org/10.1021/ar7001914
- Kiyonaka S, Sada K, Yoshimura I, Shinkai S, Kato N, Hamachi I. Semi-wet peptide/protein array using supramolecular hydrogel. Nat Mater 2004; 3:58–64; PMID:14661016; http://dx.doi.org/10.1038/nmat1034
- Kuang Y, Xu B. Disruption of the dynamics of microtubules and selective inhibition of glioblastoma cells by nanofibers of small hydrophobic molecules. Angew Chem Int Ed Engl 2013; 52:6944–8; PMID:23686848; http://dx.doi.org/10.1002/anie.201302658
- Kuang Y, Du X, Zhou J, Xu B. Supramolecular nanofibrils inhibit cancer progression in vitro and in vivo. Adv Healthc Mater 2014; 3:1217–21; PMID:24574174; http://dx.doi.org/10.1002/adhm.201300645
- Fishman WH, Inglis NR, Green S, Anstiss CL, Gosh NK, Reif AE, Rustigian R, Krant MJ, Stolbach LL. Immunology and biochemistry of Regan isoenzyme of alkaline phosphatase in human cancer. Nature 1968; 219:697–9; PMID:5691166; http://dx.doi.org/10.1038/219697a0
- Fernley HN, Walker PG. Inhibition of alkaline phosphatase by L-phenylalanine. Biochem J 1970; 116:543–4; PMID:5435696
- Shi J, Du X, Yuan D, Zhou J, Zhou N, Huang Y, Xu B. D-amino acids modulate the cellular response of enzymatic-instructed supramolecular nanofibers of small peptides. Biomacromolecules 2014; 15:3559–68; PMID:25230147; http://dx.doi.org/10.1021/bm5010355
- Chu H, Pazgier M, Jung G, Nuccio SP, Castillo PA, de Jong MF, Winter MG, Winter SE, Wehkamp J, Shen B, et al. Human α-defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science 2012; 337:477–81; PMID:22722251; http://dx.doi.org/10.1126/science.1218831
- Lo MC, Men H, Branstrom A, Helm J, Yao N, Goldman R, Walker S. A new mechanism of action proposed for ramoplanin. J Am Chem Soc 2000; 122:3540–1; http://dx.doi.org/10.1021/ja000182x
- Yang L, Harroun TA, Weiss TM, Ding L, Huang HW. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys J 2001; 81:1475–85; PMID:11509361; http://dx.doi.org/10.1016/S0006-3495(01)75802-X
- Tanaka A, Fukuoka Y, Morimoto Y, Honjo T, Koda D, Goto M, Maruyama T. Cancer-cell death induced by the intracellular self-assembly of an enzyme-responsive supramolecular gelator. J Am Chem Soc 2015; 137:770–75; PMID:25521540
- Pires RA. Controlling Cancer Cell Fate using Localized Biocatalytic Self-Assembly of an Aromatic Carbohydrate Amphiphile. J Am Chem Soc 2015; 137:579–79; PMID:25539667
- Yang ZM, Gu HW, Fu DG, Gao P, Lam JK, Xu B. Enzymatic formation of supramolecular hydrogels. Adv Mater 2004; 16:1440–4; http://dx.doi.org/10.1002/adma.200400340
- Shi J, Du X, Huang Y, Zhou J, Yuan D, Wu D, Zhang Y, Haburcak R, Epstein IR, Xu B. Ligand-Receptor Interaction Catalyzes the Aggregation of Small Molecules To Induce Cell Necroptosis. J Am Chem Soc 2015; 137:26–9; PMID:25522243; http://dx.doi.org/10.1021/ja5100417
- Zorn JA, Wille H, Wolan DW, Wells JA. Self-Assembling Small Molecules Form Nanofibrils That Bind Procaspase-3 To Promote Activation. J Am Chem Soc 2011; 133:19630–3; PMID:22066605; http://dx.doi.org/10.1021/ja208350u
- Julien O, Kampmann M, Bassik MC, Zorn JA, Venditto VJ, Shimbo K, Agard NJ, Shimada K, Rheingold AL, Stockwell BR, et al. Unraveling the mechanism of cell death induced by chemical fibrils. Nat Chem Biol 2014; 10:969–76; PMID:25262416; http://dx.doi.org/10.1038/nchembio.1639
- Du X, Zhou J, Guvench O, Sangiorgi FO, Li X, Zhou N, Xu B. Supramolecular assemblies of a conjugate of nucleobase, amino acids, and saccharide act as agonists for proliferation of embryonic stem cells and development of zygotes. Bioconjug Chem 2014; 25:1031–5; PMID:24798034; http://dx.doi.org/10.1021/bc500187m
- Zhao F, Heesters BA, Chiu I, Gao Y, Shi J, Zhou N, Carroll MC, Xu B. L-Rhamnose-containing supramolecular nanofibrils as potential immunosuppressive materials. Org Biomol Chem 2014; 12:6816–9; PMID:25078446; http://dx.doi.org/10.1039/C4OB01362J
- Xing B, Yu CW, Chow KH, Ho PL, Fu D, Xu B. Hydrophobic interaction and hydrogen bonding cooperatively confer a vancomycin hydrogel: a potential candidate for biomaterials. J Am Chem Soc 2002; 124:14846–7; PMID:12475316; http://dx.doi.org/10.1021/ja028539f
- Newcomb CJ, Sur S, Ortony JH, Lee OS, Matson JB, Boekhoven J, Yu JM, Schatz GC, Stupp SI. Cell death versus cell survival instructed by supramolecular cohesion of nanostructures. Nat Commun 2014; 5:3321; PMID:24531236; http://dx.doi.org/10.1038/ncomms4321
- Svensson M, Hakansson A, Mossberg AK, Linse S, Svanborg C. Conversion of α-lactalbumin to a protein inducing apoptosis. Proc Natl Acad Sci U S A 2000; 97:4221–6; PMID:10760289; http://dx.doi.org/10.1073/pnas.97.8.4221
- Chiti F, Dobson CM. Amyloid formation by globular proteins under native conditions. Nat Chem Biol 2009; 5:15–22; PMID:19088715; http://dx.doi.org/10.1038/nchembio.131
- McGovern SL, Caselli E, Grigorieff N, Shoichet BK. A common mechanism underlying promiscuous inhibitors from virtual and high-throughput screening. J Med Chem 2002; 45:1712–22; PMID:11931626; http://dx.doi.org/10.1021/jm010533y
- Macdonald JC, Whitesides GM. Solid-State Structures of Hydrogen-Bonded Tapes Based on Cyclic Secondary Diamides. Chem Rev 1994; 94:2383–420; http://dx.doi.org/10.1021/cr00032a007
- Lehn J-M. From Molecular to Supramolecular Chemistry. Supramolecular Chemistry: Wiley-VCH Verlag GmbH & Co. KGaA 2006:1–9.
- Sievers SA, Karanicolas J, Chang HW, Zhao A, Jiang L, Zirafi O, Stevens JT, Munch J, Baker D, Eisenberg D. Structure-based design of non-natural amino-acid inhibitors of amyloid fibril formation. Nature 2011; 475:96–100; PMID:21677644; http://dx.doi.org/10.1038/nature10154
- Liang C, Ni R, Smith JE, Childers WS, Mehta AK, Lynn DG. Kinetic intermediates in amyloid assembly. J Am Chem Soc 2014; 136:15146–9; PMID:25313920; http://dx.doi.org/10.1021/ja508621b
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100:57–70; PMID:10647931; http://dx.doi.org/10.1016/S0092-8674(00)81683-9
- Cooper MA, Shlaes D. Fix the antibiotics pipeline. Nature 2011; 472:32; PMID:21475175; http://dx.doi.org/10.1038/472032a
- Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, et al. Cell-free Formation of RNA Granules: Low Complexity Sequence Domains Form Dynamic Fibers within Hydrogels. Cell 2012; 149:753–67; PMID:22579281; http://dx.doi.org/10.1016/j.cell.2012.04.017
