ABSTRACT
Prions cause fatal neurodegenerative diseases in humans and animals and can be transmitted zoonotically. Chronic wasting disease (CWD) is a highly transmissible prion disease of wild deer and elk that affects cervids over extensive regions of the United States and Canada. The risk of cross-species CWD transmission has been experimentally evaluated in a wide array of mammals, including non-human primates and mouse models expressing human cellular prion protein. Here we review the determinants of cross-species CWD transmission, and propose a model that may explain a structural barrier for CWD transmission to humans.
CHRONIC WASTING DISEASE OF CERVIDS
Chronic wasting disease (CWD) is the only known prion disorder affecting free-ranging wildlife, including deer, elk, and moose, and has spread extensively throughout North America, occurring in 23 US states and 2 Canadian provinces.Citation1,2 CWD prions are highly infectious and readily transmitted among cervids, leading to remarkably high prevalences that can exceed 90% in captive deer.Citation3 Humans, wildlife, and domestic species such as cattle and sheep are likely exposed to CWD through consumption of prion-infected animals or grazing on prion-contaminated pastures.
Within an individual animal, CWD prions are extraordinarily widespread and accumulate in neural and non-neural tissues and body fluids, including brain and spinal cord fat, pancreas, adrenal gland, heart, peripheral nerves, lymph nodes, saliva, blood, and skeletal muscle, many of which are ingested by humans and other animals.Citation4-9 Venison consumption is common; more than 60% of Americans have eaten deer or elk meat,Citation10 and known human exposures to CWD-infected venison have occurred in New YorkCitation11 and in Wisconsin, where hundreds of people have eaten CWD-infected cervids (E. Belay, personal commun). As CWD-infected animals gain access to new areas through migration or animal transport, human and animal exposure to CWD prions will likely increase. Here we review species susceptibility to CWD infection as well as new models to study CWD species barriers.
CROSS-SPECIES CWD PRION TRANSMISSION
Prions transmitted into a different species typically result in few infections and prolonged incubation periods due to a transmission barrier.Citation12 Transmission barriers are caused by amino acid sequence differences between the host cellular prion protein, PrPC, and the misfolded, aggregated conformation, PrPSc.Citation13-15 The conformation of PrPSc also plays a role in species barriers. For example, CWD prions are not transmissible to mice expressing human PrPC, yet are efficiently transmitted to mice expressing cervid PrPC, demonstrating the importance of amino acid sequence in susceptibility.Citation16,17 Interestingly, transgenic mice expressing human PrPC are more susceptible to human sporadic Creutzfeldt-Jakob disease (sCJD) prions than to variant CJD (vCJD) prions.Citation18 vCJD prions transmit readily to wild type mice, indicating that PrPSc conformation also impacts prion transmission.Citation18 Bank vole PrPC has been touted as a universal acceptor as it is efficiently converted by diverse human and animal prions despite sequence differences between bank vole PrPC and the infectious PrPSc.Citation19-21
Many species have been experimentally exposed to CWD prions by intracerebral or oral routes of inoculation, including rodents, mustelids, felids, and ruminants. Oral inoculation with CWD led to prion disease in cervids and squirrel monkeys, whereas 5 additional species resisted oral CWD challenge ().Citation22-24 However, intracerebral CWD inoculation caused prion infection in voles, hamsters, ferrets, sheep, cats, mink, and cattle, with variable attack rates.Citation25-33 Wild type mice and raccoons resisted CWD prion infection ().Citation16,34,Citation35 An extensive study of CWD susceptibility in transgenic mice expressing ovine and bovine PrPC revealed no mice with prion disease, supporting the strong barrier to CWD infection observed in sheep and cattle.Citation36
TABLE 1. Species susceptibility to CWD infection following experimental exposure via the intracerebral (IC) or oral (PO) routes of exposure.
The ability of CWD prions to convert PrPC from 12 mammalian species was evaluated in vitro by protein misfolding cyclic amplification (PMCA).Citation37 Efficient CWD conversion strongly correlated with Prnp encoding PrPC with an asparagine (N) at position 170, similar to cervid PrP. CWD converted PrPC from 5 of 5 species having N170 (Syrian, Chinese, and Armenian hamsters, prairie vole, Peromyscus mouse), but only 1 of 7 species having serine (S) at position 170 (ferret).Citation37 CWD did not convert PrPC from mink, Mus mouse, cat, coyote, macaque, or transgenic mice expressing human PrPC, suggesting a lower risk of CWD infection for species having a Prnp gene encoding S170. Interestingly, prions from CWD-infected prairie voles (N170) converted PrPC from several species that express S170 (coyotes, cats and mink), consistent with PrPSc conformation playing an important role in conversion.Citation37 Collectively, these studies suggest that few species would be orally susceptible to CWD following prion ingestion, and that an asparagine at position 170 of PrPC is a risk factor for CWD infection.
ASSESSING PRIMATE SUSCEPTIBILITY TO CWD INFECTION
To gain insight into human susceptibility to CWD, squirrel monkeys and cynomolgus macaques were challenged with CWD prions and showed surprising results, in that squirrel monkeys were highly susceptible to CWD by either intracerebralCitation22 or oral exposure routes,Citation22,23 whereas macaques resisted CWD prion infection, even after intracerebral injection.Citation23 A comparison of the PrP amino acid sequences of the squirrel monkey and macaque shows that both primates express S170, in contrast to the N170 expressed by deer. However, 2 intriguing amino acid differences in the N-terminus (positions 100 and 108) of squirrel monkeys and macaques may impact the CWD barrier. Nevertheless, the underlying structural mechanism that explains the profound differences in CWD susceptibility remains unresolved, and the CWD susceptibility of squirrel monkeys is not likely predictive for that of humans.
To further assess human susceptibility to CWD, 4 laboratories performed an intracerebral CWD challenge of transgenic mice expressing human PrP.Citation17,36,Citation38,39 Mice either overexpressed or expressed endogenous levels of human PrP. All human codon 129 polymorphisms were represented (129MM, VV, or MV). Mice invariably resisted deer and elk CWD infection, as not a single animal developed clinical disease or PrPSc deposits in the brain, suggesting a strong barrier for CWD conversion of human PrP. This result is consistent with in vitro conversion experiments, which also indicate a strong barrier for CWD conversion of human PrPC.Citation40,41
STRUCTURAL DETERMINANTS OF CWD SUSCEPTIBILITY
The structural underpinnings of PrP sequence differences associated with prion resistance are unclear, however recent findings from our lab and others have revealed the importance of key interacting segments for CWD conversion. Mammalian PrPC consists of approximately 210 amino acids, with an unstructured N-terminus and a globular C-terminal domain composed of 3 α-helices and a short anti-parallel β-sheet.Citation42 One region of structural diversity is the β2-α2 loop (residues 165–175), which shows either a disordered, or a well-defined conformation by NMR spectroscopy.Citation43 For example, elk and bank vole PrPC show a well-defined loop, whereas human and mouse PrPC show a disordered loop.Citation42-46 To determine how the β2-α2 loop conformation impacts species barriers, mice were engineered to express mouse PrPC with the elk β2-α2 loop, which required the S170N and N174T substitutions. These two substitutions change the loop from disordered (mouse) to well-defined (elk).Citation43 The resulting Tg(MoPrPS170N,N174T) mice were highly susceptible to CWD infection compared to mice expressing wild type mouse PrPC.Citation47 Further studies utilizing mice with a well-defined loop due to a different substitution, D167S, showed that the loop conformation had no effect on CWD susceptibility,Citation48 as the mice had identical barriers as WT mice. Taken together, these results suggest that the 165–175 sequence similarity between cervid and host PrP, and not the secondary structure, governs CWD susceptibility.
Tamguney et al. generated an extensive series of transgenic mice expressing chimeric elk/mouse PrPC sequences to determine the key residues involved in CWD conversion, with a focus on the C-terminus of PrP from 170 to 220 (human numbering)Citation49 as prior studies had shown the importance of this region.Citation50 Mice that expressed elk/mouse chimeric PrPC having the mouse β2-α2 loop sequence (170S, 170N) showed barriers to CWD infection with attack rates ranging from 0–57%. In contrast, 6 of 7 lines having the elk N170 residue showed attack rates of 100%, further illustrating the importance of the loop segment for conversion of CWD.Citation49
INVESTIGATING THE CWD-HUMAN SPECIES BARRIER
The elk β2-α2 loop promoted CWD conversion of mouse PrP, suggesting that the loop sequence may serve as a gatekeeper for CWD conversion in the context of mammalian PrPC. To investigate this possibility, transgenic mice expressing human PrP with the elk β2-α2 loop sequence [Tg(HuPrPelk166-174)] were exposed to deer and elk CWD prions. As previously observed, mice expressing human PrP [Tg(HuPrP) mice] resisted CWD infection. However, [Tg(HuPrPelk166-174)] mice expressing human/elk chimeric PrP were highly susceptible to CWD prions. Further passage of CWD-infected Tg(HuPrPelk166-174) brain transmitted the prion infection to all Tg(HuPrPelk166-174) mice, yet to only 1 of 17 Tg(HuPrP) mice, indicating a significant barrier for prion transmission from Tg(HuPrPelk166-174) to Tg(HuPrP) mice, even though the PrP sequences differed by only 4 amino acid residues (). Interestingly, the elk β2-α2 loop sequence in human PrP created a barrier to sCJD infection, as the Tg(HuPrPelk166-174) mice were infected with human CJD prions after a moderate delay as compared to the Tg(HuPrP) mice.
FIGURE 1. Investigating the structural determinants of the CWD-human transmission barrier. The human and elk β2-α2 loop amino acid sequences differ at 4 positions: 166, 168, 170, and 174 (top). Transgenic mice expressing full-length human PrPC (blue) or human PrPC with the elk β2-α2 loop (red) were inoculated intracerebrally with CWD prions. Although mice expressing human PrPC did not develop disease, mice expressing the human-elk loop PrPC [Tg(HuPrPelk166-174)] were susceptible to CWD infection (83%). Inoculation of brain from a CWD-infected Tg(HuPrPelk166-174) mouse into additional transgenic mice transmitted the disease to all Tg(HuPrPelk166-174) mice, but to only 1 of 17 Tg(HuPrP) mice.
![FIGURE 1. Investigating the structural determinants of the CWD-human transmission barrier. The human and elk β2-α2 loop amino acid sequences differ at 4 positions: 166, 168, 170, and 174 (top). Transgenic mice expressing full-length human PrPC (blue) or human PrPC with the elk β2-α2 loop (red) were inoculated intracerebrally with CWD prions. Although mice expressing human PrPC did not develop disease, mice expressing the human-elk loop PrPC [Tg(HuPrPelk166-174)] were susceptible to CWD infection (83%). Inoculation of brain from a CWD-infected Tg(HuPrPelk166-174) mouse into additional transgenic mice transmitted the disease to all Tg(HuPrPelk166-174) mice, but to only 1 of 17 Tg(HuPrP) mice.](/cms/asset/3180df98-b9f3-41a6-af4b-d16c74af5792/kprn_a_1118603_f0001_c.gif)
STERIC ZIPPER MODELS MAY EXPLAIN SPECIES BARRIERS
Solving the molecular mechanism underlying cross-species prion conversion has been challenging due to the lack of high resolution structures for prions. Sequence similarity between PrPC and PrPSc facilitates cross-species prion conversion, suggesting that the packing of amino acid side chains may play an important role in determining susceptibility to prion conversion (). A potential mechanism for PrPC conversion is suggested by high resolution crystallography of microcrystals formed from amyloidogenic segments of fibril-forming proteins. The microcrystals are composed of β-sheets arranged parallel to the fibril axis, with complementary side chains from adjacent sheets interdigitating to form a dry “steric zipper” interface.Citation51
FIGURE 2. Structural models of elk and human side chain packing within the β2-α2 loop may explain CWD transmission barriers. Atomic space-filling models of amino acid side chains within the β2-α2 loop of PrP were modeled as a parallel β-sheet.Citation53 In this model, the CWD PrPSc and cervid PrPC (top pair) interdigitate in a steric zipper. In contrast, the CWD PrPSc and human PrPC (bottom pair) interaction generates a steric clash (blue rectangle) and a cavity (arrow) that would be incompatible with zipper formation and may explain why CWD does not convert human PrPC. Amino acids common to both cervids and humans are yellow; human-specific residues are green.
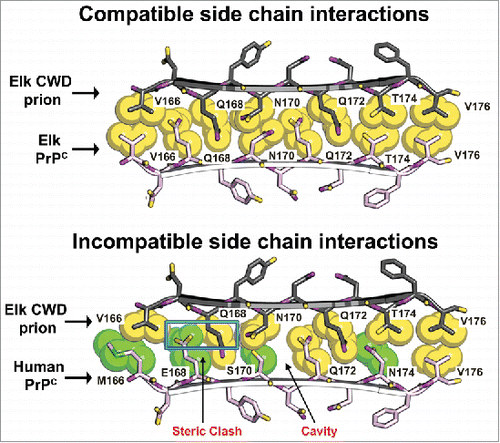
Several isolated PrP segments form microcrystals with steric zipper interfaces, including residues 170–175 from the β2-α2 loop. Interestingly, the 170–175 segment (SNQNNF in humans and mice, NNQNTF in deer and elk) forms distinct steric zipper structures in humans and mice as compared to deer and elk. These differences in zipper structures offer an explanation for how a few human residues can inhibit conversion by CWD. Modeling the human and elk 165–175 segment reveals poor interdigitation of the human and cervid amino acid side chains at positions 168 and 170 that leads to gaps and steric clashes expected to destabilize the zipper (). Thus, PrPC and PrPSc side chain interactions at the 165–175 segment may inhibit the stable incorporation of the PrPC monomer into a growing fibril. One assumption in this model is that the interacting 165–175 segment is exposed in both PrPC and PrPSc.
In support of the steric zipper model for CWD conversion, in vitro conversion experiments using human PrPC with elk substitutions revealed that human PrPC with the elk E168Q and S170N substitutions was converted as efficiently as full length cervid PrPC.Citation53 Surprisingly, human PrPC with the elk E168Q, S170N, and N174T substitutions was converted poorly, revealing that the human N174 residue had bolstered CWD conversion. These findings indicate that in some cases, PrP sequence mismatches between the infectious prion and the host PrPC promote cross-species conversion. These results also suggest a basis for the high susceptibility of voles to CWD infection, as the bank vole PrP sequence includes Q168, N170, and N174.
CONCLUSIONS
CWD has spread rapidly within the United States over the past decade. With the increased exposure of wildlife and other species to CWD, predicting prion infection risk has become more important and will enable a more targeted species surveillance as well as management of potential CWD reservoirs, such as wild voles. Utilizing a structural model of PrPC-PrPSc interaction may facilitate those predictions.
Although the secondary structure of the β2-α2 loop varies depending on the sequence of the loop or the tightly interacting third helix, the secondary structure has not correlated with susceptibility to CWD prion conversion.Citation48,52,Citation53 Instead, the amino acid sequence of the β2-α2 loop has an important role in promoting CWD conversion of PrPC from other species. However, as the ferret and the squirrel monkey are highly susceptible to CWD infection, and neither has a β2-α2 loop that matches elk, it is clear that other PrP segments also interact during CWD conversion. Additionally, how segments interact in the context of full length PrP, as well as how potential hetero-zippers that could be accommodated in the new models of PrPSc structure should be considered. Future studies to define PrPC : PrPSc interaction sites will help to refine the list of species most at risk for CWD infection.
ABBREVIATIONS
| CWD | = | chronic wasting disease |
| PrPC | = | cellular prion protein |
| PrPSc | = | misfolded, aggregated prion protein |
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Funding
This work was supported by NIH grant NS069566 and NS076896 (CJS) and 1 K01 0D019919–01 (TDK).
REFERENCES
- Williams ES, Young S. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis 1980; 16:89-98; PMID:7373730; http://dx.doi.org/10.7589/0090-3558-16.1.89.
- Williams ES, Young S. Spongiform encephalopathy of Rocky Mountain elk. J Wildl Dis 1982; 18:465-71.
- Williams ES, Young S. Spongiform encephalopathies in Cervidae. Rev Sci Tech 1992; 11:551-67; PMID:1617203.
- Race B, Meade-White K, Race R, Chesebro B. Prion infectivity in fat of deer with chronic wasting disease. J Virol 2009; 83:9608-10; PMID:19570855; http://dx.doi.org/10.1128/JVI.01127-09.
- Sigurdson CJ, Spraker TR, Miller MW, Oesch B, Hoover EA. PrP(CWD) in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J Gen Virol 2001; 82:2327-34; PMID:11562526; http://dx.doi.org/10.1099/0022-1317-82-10-2327.
- Sigurdson CJ, Barillas-Mury C, Miller MW, Oesch B, van Keulen LJ, Langeveld JP, Hoover EA. PrP(CWD) lymphoid cell targets in early and advanced chronic wasting disease of mule deer. J Gen Virol 2002; 83:2617-28; PMID:12237446; http://dx.doi.org/10.1099/0022-1317-83-10-2617.
- Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes-Klug J, Seelig DM, et al. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 2006; 314:133-6; http://dx.doi.org/10.1126/science.1132661.
- Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, Hoover EA, Telling GC. Prions in skeletal muscles of deer with chronic wasting disease. Science 2006; 311:1117; http://dx.doi.org/10.1126/science.1122864.
- Jewell JE, Brown J, Kreeger T, Williams ES. Prion protein in cardiac muscle of elk (Cervus elaphus nelsoni) and white-tailed deer (Odocoileus virginianus) infected with chronic wasting disease. J Gen Virol 2006; 87:3443-50; PMID:17030881; http://dx.doi.org/10.1099/vir.0.81777-0.
- Abrams JY, Maddox RA, Harvey AR, Schonberger LB, Belay ED. Travel history, hunting, and venison consumption related to prion disease exposure, 2006-2007 FoodNet Population Survey. J Am Diet Assoc 2011; 111:858-63; PMID:21616198; http://dx.doi.org/10.1016/j.jada.2011.03.015.
- Garruto RM, Reiber C, Alfonso MP, Gastrich H, Needham K, Sunderman S, Walker S, Weeks J, Derosa N, Faisst E, et al. Risk behaviors in a rural community with a known point-source exposure to chronic wasting disease. Environ Health 2008; 7:31; PMID:18577220; http://dx.doi.org/10.1186/1476-069X-7-31.
- Kimberlin RH, Cole S, Walker CA. Transmissible mink encephalopathy (TME) in Chinese hamsters: identification of two strains of TME and comparisons with scrapie. Neuropathol Appl Neurobiol 1986; 12:197-206; PMID:2940469; http://dx.doi.org/10.1111/j.1365-2990.1986.tb00050.x.
- Barron RM, Thomson V, Jamieson E, Melton DW, Ironside J, Will R, Manson JC. Changing a single amino acid in the N-terminus of murine PrP alters TSE incubation time across three species barriers. EMBO J 2001; 20:5070-8; PMID:11566872; http://dx.doi.org/10.1093/emboj/20.18.5070.
- Carlson GA, Westaway D, DeArmond SJ, Peterson Torchia M, Prusiner SB. Primary structure of prion protein may modify scrapie isolate properties. Proc Natl Acad Sci U S A 1989; 86:7475-9; PMID: 2798418; http://dx.doi.org/10.1073/pnas.86.19.7475.
- Scott M, Foster D, Mirenda C, Serban D, Coufal F, Waelchli M, Torchia M, Groth D, Carlson G, DeArmond SJ, et al. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell 1989; 59:847-57; PMID:2574076; http://dx.doi.org/10.1016/0092-8674(89)90608-9.
- Browning SR, Mason GL, Seward T, Green M, Eliason GA, Mathiason C, Miller MW, Williams ES, Hoover E, Telling GC. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J Virol 2004; 78:13345-50; PMID:15542685; http://dx.doi.org/10.1128/JVI.78.23.13345-13350.2004.
- Kong Q, Huang S, Zou W, Vanegas D, Wang M, Wu D, Yuan J, Zheng M, Bai H, Deng H, et al. Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models. J Neurosci 2005; 25:7944-9; PMID:16135751; http://dx.doi.org/10.1523/JNEUROSCI.2467-05.2005.
- Hill AF, Desbruslais M, Joiner S, Sidle KC, Gowland I, Collinge J, Doey LJ, Lantos P. The same prion strain causes vCJD and BSE [letter] Nature 1997; 389:448-50; PMID:9333232; http://dx.doi.org/10.1038/38925.
- Di Bari MA, Nonno R, Castilla J, D'Agostino C, Pirisinu L, Riccardi G, Conte M, Richt J, Kunkle R, Langeveld J, et al. Chronic wasting disease in bank voles: characterisation of the shortest incubation time model for prion diseases. PLoS Pathog 2013; 9:e1003219; PMID:23505374; http://dx.doi.org/10.1371/journal.ppat.1003219.
- Nonno R, Bari MA, Cardone F, Vaccari G, Fazzi P, Dell'omo G, Cartoni C, Ingrosso L, Boyle A, Galeno R, et al. Efficient transmission and characterization of Creutzfeldt-Jakob disease strains in bank voles. PLoS Pathog 2006; 2:e12; PMID: 16518470; http://dx.doi.org/10.1371/journal.ppat.0020012.
- Watts JC, Giles K, Patel S, Oehler A, DeArmond SJ, Prusiner SB. Evidence that bank vole PrP is a universal acceptor for prions. PLoS Pathog 2014; 10:e1003990; PMID:24699458; http://dx.doi.org/10.1371/journal.ppat.1003990.
- Marsh RF, Kincaid AE, Bessen RA, Bartz JC. Interspecies transmission of chronic wasting disease prions to squirrel monkeys (Saimiri sciureus). J Virol 2005; 79:13794-6; PMID:16227298; http://dx.doi.org/10.1128/JVI.79.21.13794-13796.2005.
- Race B, Meade-White KD, Miller MW, Barbian KD, Rubenstein R, LaFauci G, Cervenakova L, Favara C, Gardner D, Long D, et al. Susceptibilities of nonhuman primates to chronic wasting disease. Emerg Infect Dis 2009; 15:1366-76; PMID: 19788803; http://dx.doi.org/10.3201/eid1509.090253.
- Race B, Meade-White KD, Phillips K, Striebel J, Race R, Chesebro B. Chronic wasting disease agents in nonhuman primates. Emerg Infect Dis 2014; 20:833-7; PMID:24751215; http://dx.doi.org/10.3201/eid2005.130778.
- Hamir AN, Miller JM, Kunkle RA, Hall SM, Richt JA. Susceptibility of cattle to first-passage intracerebral inoculation with chronic wasting disease agent from white-tailed deer. Vet Pathol 2007; 44:487-93; PMID:17606510; http://dx.doi.org/10.1354/vp.44-4-487.
- Hamir AN, Kunkle RA, Cutlip RC, Miller JM, O'Rourke KI, Williams ES, Miller MW, Stack MJ, Chaplin MJ, Richt JA. Experimental transmission of chronic wasting disease agent from mule deer to cattle by the intracerebral route. J Vet Diagn Invest 2005; 17:276-81; PMID:15945388; http://dx.doi.org/10.1177/104063870501700313.
- Hamir AN, Kunkle RA, Cutlip RC, Miller JM, Williams ES, Richt JA. Transmission of chronic wasting disease of mule deer to Suffolk sheep following intracerebral inoculation. J Vet Diagn Invest 2006; 18:558-65; PMID:17121083; http://dx.doi.org/10.1177/104063870601800606.
- Hamir AN, Kunkle RA, Miller JM, Greenlee JJ, Richt JA. Experimental second passage of chronic wasting disease (CWD(mule deer)) agent to cattle. J Comp Pathol 2006; 134:63-9; PMID:16423572; http://dx.doi.org/10.1016/j.jcpa.2005.07.001.
- Heisey DM, Mickelsen NA, Schneider JR, Johnson CJ, Johnson CJ, Langenberg JA, Bochsler PN, Keane DP, Barr DJ. Chronic wasting disease (CWD) susceptibility of several North American rodents that are sympatric with cervid CWD epidemics. J Virol 2010; 84:210-5; PMID:19828611; http://dx.doi.org/10.1128/JVI.00560-09.
- Kurt TD, Seelig DM, Schneider JR, Johnson CJ, Telling GC, Heisey DM, Hoover EA. Alteration of the chronic wasting disease species barrier by in vitro prion amplification. J Virol 2011; 85:8528-37; PMID:21697475; http://dx.doi.org/10.1128/JVI.00809-11.
- Bartz JC, Marsh RF, McKenzie DI, Aiken JM. The host range of chronic wasting disease is altered on passage in ferrets. Virology 1998; 251:297-301; PMID:9837794; http://dx.doi.org/10.1006/viro.1998.9427.
- Sigurdson CJ, Mathiason CK, Perrott MR, Eliason GA, Spraker TR, Glatzel M, Manco G, Bartz JC, Miller MW, Hoover EA. Experimental chronic wasting disease (CWD) in the ferret. J Comp Pathol 2008; 138:189-96; PMID:18387626; http://dx.doi.org/10.1016/j.jcpa.2008.01.004.
- Raymond GJ, Raymond LD, Meade-White KD, Hughson AG, Favara C, Gardner D, Williams ES, Miller MW, Race RE, Caughey B. Transmission and adaptation of chronic wasting disease to hamsters and transgenic mice: evidence for strains. J Virol 2007; 81:4305-14; PMID:17287284; http://dx.doi.org/10.1128/JVI.02474-06.
- Hamir AN, Miller JM, Cutlip RC, Stack MJ, Chaplin MJ, Jenny AL, Williams ES. Experimental inoculation of scrapie and chronic wasting disease agents in raccoons (Procyon lotor). Vet Rec 2003; 153:121-3; PMID: 12918830; http://dx.doi.org/10.1136/vr.153.4.121.
- Harrington RD, Baszler TV, O'Rourke KI, Schneider DA, Spraker TR, Liggitt HD, Knowles DP. A species barrier limits transmission of chronic wasting disease to mink (Mustela vison). J Gen Virol 2008; 89:1086-96; PMID:18343853; http://dx.doi.org/10.1099/vir.0.83422-0.
- Tamguney G, Giles K, Bouzamondo-Bernstein E, Bosque PJ, Miller MW, Safar J, Dearmond SJ, Prusiner SB. Transmission of elk and deer prions to transgenic mice. J Virol 2006; 80:9104-14; PMID: 16940522; http://dx.doi.org/10.1128/JVI.00098-06.
- Kurt TD, Telling GT, Zabel MD, Hoover EA. Trans-species amplification of PrPCWD and correlation with rigid loop 170N. Virology 2009; 387:235-43; PMID:19269662; http://dx.doi.org/10.1016/j.virol.2009.02.025.
- Sandberg MK, Al-Doujaily H, Sigurdson CJ, Glatzel M, O'Malley C, Powell C, Asante EA, Linehan JM, Brandner S, Wadsworth JD, et al. Chronic wasting disease prions are not transmissible to transgenic mice overexpressing human prion protein. J Gen Virol 2010; 91:2651-7; PMID:20610667; http://dx.doi.org/10.1099/vir.0.024380-0.
- Wilson R, Plinston C, Hunter N, Casalone C, Corona C, Tagliavini F, Suardi S, Ruggerone M, Moda F, Graziano S, et al. Chronic wasting disease and atypical forms of bovine spongiform encephalopathy and scrapie are not transmissible to mice expressing wild-type levels of human prion protein. J Gen Virol 2012; 93:1624-9; PMID:22495232; http://dx.doi.org/10.1099/vir.0.042507-0.
- Raymond GJ, Bossers A, Raymond LD, O'Rourke KI, McHolland LE, Bryant PK, 3rd, Miller MW, Williams ES, Smits M, Caughey B. Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J 2000; 19:4425-30; PMID:10970836; http://dx.doi.org/10.1093/emboj/19.17.4425.
- Kurt TD, Telling GC, Zabel MD, Hoover EA. Trans-species amplification of PrP(CWD) and correlation with rigid loop 170N. Virology 2009; 387:235-43; PMID:19269662; http://dx.doi.org/10.1016/j.virol.2009.02.025.
- Riek R, Hornemann S, Wider G, Billeter M, Glockshuber R, Wüthrich K. NMR structure of the mouse prion protein domain PrP(121-321). Nature 1996; 382:180-2; PMID:8700211; http://dx.doi.org/10.1038/382180a0.
- Gossert AD, Bonjour S, Lysek DA, Fiorito F, Wüthrich K. Prion protein NMR structures of elk and of mouse/elk hybrids. Proc Natl Acad Sci U S A 2005; 102:646-50; PMID:15647363; http://dx.doi.org/10.1073/pnas.0409008102.
- Perez DR, Damberger FF, Wüthrich K. Horse prion protein NMR structure and comparisons with related variants of the mouse prion protein. J Mol Biol 2010; 400:121-8; PMID:20460128; http://dx.doi.org/10.1016/j.jmb.2010.04.066.
- Zahn R, Liu A, Lührs T, Riek R, von Schroetter C, Lopez Garcia F, Billeter M, Calzolai L, Wider G, Wüthrich K. NMR solution structure of the human prion protein. Proc Natl Acad Sci U S A 2000; 97:145-50; PMID:10618385; http://dx.doi.org/10.1073/pnas.97.1.145.
- Christen B, Perez DR, Hornemann S, Wüthrich K. NMR structure of the bank vole prion protein at 20°C contains a structured loop of residues 165-171. J Mol Biol 2008; 383:306-12; PMID:18773909; http://dx.doi.org/10.1016/j.jmb.2008.08.045.
- Sigurdson CJ, Nilsson KP, Hornemann S, Manco G, Fernandez-Borges N, Schwarz P, Castilla J, Wüthrich K, Aguzzi A. A molecular switch controls interspecies prion disease transmission in mice. J Clin Invest 2010; 120:2590-9; PMID:20551516; http://dx.doi.org/10.1172/JCI42051.
- Bett C, Fernandez-Borges N, Kurt TD, Lucero M, Nilsson KP, Castilla J, Sigurdson CJ. Structure of the beta2-alpha2 loop and interspecies prion transmission. FASEB J 2012; 26(7):2868-76; PMID:22490928.
- Tamguney G, Giles K, Oehler A, Johnson NL, DeArmond SJ, Prusiner SB. Chimeric elk/mouse prion proteins in transgenic mice. J Gen Virol 2013; 94:443-52; PMID:23100369; http://dx.doi.org/10.1099/vir.0.045989-0.
- Telling GC, Scott M, Mastrianni J, Gabizon R, Torchia M, Cohen FE, DeArmond SJ, Prusiner SB. Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell 1995; 83:79-90; PMID:7553876; http://dx.doi.org/10.1016/0092-8674(95)90236-8.
- Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, Grothe R, Eisenberg D. Structure of the cross-β spine of amyloid-like fibrils. Nature 2005; 435:773-8; PMID:15944695; http://dx.doi.org/10.1038/nature03680.
- Kurt TD, Jiang L, Bett C, Eisenberg D, Sigurdson CJ. A proposed mechanism for the promotion of prion conversion involving a strictly conserved tyrosine residue in the beta2-alpha2 loop of PrPC. J Biol Chem 2014; 289:10660-7; PMID:24596090; http://dx.doi.org/10.1074/jbc.M114.549030.
- Kurt TD, Jiang L, Fernandez-Borges N, Bett C, Liu J, Yang T, Spraker TR, Castilla J, Eisenberg D, Kong Q, et al. Human prion protein sequence elements impede cross-species chronic wasting disease transmission. J Clin Invest 2015; 125(4): 1485–1496; PMID:25705888; http://dx.doi.org/10.1172/JCI79408
- Hamir AN, Gidlewski T, Spraker TR, Miller JM, Creekmore L, Crocheck M, Cline T, O'Rourke KI. Preliminary observations of genetic susceptibility of elk (Cervus elaphus nelsoni) to chronic wasting disease by experimental oral inoculation. J Vet Diagn Invest 2006; 18:110-4; PMID:16566268; http://dx.doi.org/10.1177/104063870601800118.
- O'Rourke KI, Spraker TR, Zhuang D, Greenlee JJ, Gidlewski TE, Hamir AN. Elk with a long incubation prion disease phenotype have a unique PrPd profile. Neuroreport 2007; 18:1935-8; PMID:18007190; http://dx.doi.org/10.1097/WNR.0b013e3282f1ca2f.
- Hamir AN, Greenlee JJ, Nicholson EM, Kunkle RA, Richt JA, Miller JM, Hall M. Experimental transmission of chronic wasting disease (CWD) from elk and white-tailed deer to fallow deer by intracerebral route: final report. Can J Vet Res 2011; 75:152-6; PMID:21731188.
- Fox KA, Jewell JE, Williams ES, Miller MW. Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). J Gen Virol 2006; 87:3451-61; PMID:17030882; http://dx.doi.org/10.1099/vir.0.81999-0.
- Hamir AN, Richt JA, Miller JM, Kunkle RA, Hall SM, Nicholson EM, O'Rourke KI, Greenlee JJ, Williams ES. Experimental transmission of chronic wasting disease (CWD) of elk (Cervus elaphus nelsoni), white-tailed deer (Odocoileus virginianus), and mule deer (Odocoileus hemionus hemionus) to white-tailed deer by intracerebral route. Vet Pathol 2008; 45:297-306; PMID:18487485; http://dx.doi.org/10.1354/vp.45-3-297.
- Mitchell GB, Sigurdson CJ, O'Rourke KI, Algire J, Harrington NP, Walther I, Spraker TR, Balachandran A. Experimental oral transmission of chronic wasting disease to reindeer (Rangifer tarandus tarandus). PloS One 2012; 7:e39055; PMID:22723928; http://dx.doi.org/10.1371/journal.pone.0039055.
- Nalls AV, McNulty E, Powers J, Seelig DM, Hoover C, Haley NJ, Hayes-Klug J, Anderson K, Stewart P, Goldmann W, et al. Mother to offspring transmission of chronic wasting disease in reeves' muntjac deer. PloS One 2013; 8:e71844; PMID:23977159; http://dx.doi.org/10.1371/journal.pone.0071844.
- Balachandran A, Harrington NP, Algire J, Soutyrine A, Spraker TR, Jeffrey M, Gonzalez L, O'Rourke KI. Experimental oral transmission of chronic wasting disease to red deer (Cervus elaphus elaphus): early detection and late stage distribution of protease-resistant prion protein. Can Vet J 2010; 51:169-78; PMID:20436863.
- Kreeger TJ, Montgomery DL, Jewell JE, Schultz W, Williams ES. Oral transmission of chronic wasting disease in captive Shira's moose. J Wildl Dis 2006; 42:640-5; PMID:17092895; http://dx.doi.org/10.7589/0090-3558-42.3.640.
- Mathiason CK, Nalls AV, Seelig DM, Kraft SL, Carnes K, Anderson KR, Hayes-Klug J, Hoover EA. Susceptibility of domestic cats to chronic wasting disease. J Virol 2013; 87:1947-56; PMID: 23236066; http://dx.doi.org/10.1128/JVI.02592-12.
- Sigurdson CJ, Manco G, Schwarz P, Liberski P, Hoover EA, Hornemann S, Polymenidou M, Miller MW, Glatzel M, Aguzzi A. Strain fidelity of chronic wasting disease upon murine adaptation. J Virol 2006; 80:12303-11; PMID:17020952; http://dx.doi.org/10.1128/JVI.01120-06.
