ABSTRACT
The cellular prion protein PrPc plays important roles in proliferation, cell death and survival, differentiation and adhesion. The participation of PrPc in tumor growth and metastasis was pointed out, but the underlying mechanisms were not deciphered completely. In the constantly renewing intestinal epithelium, our group demonstrated a dual localization of PrPc, which is targeted to cell-cell junctions in interaction with Src kinase and desmosomal proteins in differentiated enterocytes, but is predominantly nuclear in dividing cells. While the role of PrPc in the dynamics of intercellular junctions was confirmed in other biological systems, we unraveled its function in the nucleus only recently. We identified several nuclear PrPc partners, which comprise γ-catenin, one of its desmosomal partners, β-catenin and TCF7L2, the main effectors of the canonical Wnt pathway, and YAP, one effector of the Hippo pathway. PrPc up-regulates the activity of the β-catenin/TCF7L2 complex and its invalidation impairs the proliferation of intestinal progenitors. We discuss how PrPc could participate to oncogenic processes through its interaction with Wnt and Hippo pathway effectors, which are controlled by cell-cell junctions and Src family kinases and dysregulated during tumorigenesis. This highlights new potential mechanisms that connect PrPc expression and subcellular redistribution to cancer.
INTRODUCTION
Beside its central role in transmissible spongiform encephalopathies, through a structural conversion into a pathogenic conformer,Citation1 the cellular prion protein (PrPc), which is encoded by the Prnp gene and expressed in a wide range of tissues and cell types, was shown to contribute to the regulation of many basic biological processes, such as cell proliferation, differentiation, survival and adhesion.Citation2 Despite absence of any severe phenotype, thorough analyses of Prnp knockout mice demonstrated that PrPc participates to several specific functions in specialized tissues, such as neuroprotection, synaptic activity, olfaction, immune response, epithelial and endothelial barriers.Citation3,4 The molecular mechanisms underlying the large repertoire of PrPc functions is far from being totally elucidated but the identification of multiple interactors began to provide more comprehensive knowledge.
PrPc was described to be produced mostly as a glycosylphosphatidyl inositol (GPI)-anchored glycoprotein associated to lipid raft microdomains of the plasma membrane, where it forms a complex with several protein partners, i.e. secreted proteins, integral transmembrane proteins and peripheral membrane proteins including signaling proteins of the Src family kinases (SFK).Citation5,6 Thus, the ability of PrPc to modulate cell signaling was proposed to mediate some of its biological effects.Citation2,3
Using the constantly renewing intestinal epithelium as a model, we demonstrated a dual localization of PrPc: i) in differentiated enterocytes, plasma membrane PrPc is addressed specifically toward sites of cell-cell contacts, and more precisely at desmosomes,Citation6-8 whereas ii) in dividing cells, it is targeted to the nucleus.Citation7,9
Intercellular adhesion is able to control cell fate, especially cell proliferation. Such a process relies, in particular, on the ability of junctional complexes to sequester proteins that are otherwise able to enter the nucleus and act as transcriptional cofactors. This paradigm has been extensively illustrated by analyzing the relationships between cadherin adhesion complexes and β-catenin, the main effector of the canonical Wnt pathway.Citation10 Our recent results, which identified the nuclear partners of PrPc, highlight its role in signaling complexes that contribute to a coordinated regulation of proliferation and cell-cell adhesion through a nucleo-junctional interplay, thus participating to epithelium homeostasis.Citation9 We will discuss how these observations in tumoral and non-tumoral intestinal epithelial cells can shed a new light on recent data that identified PrPc as a potential important player in tumor biology in a large range of tissues.Citation11,12
PRPc IS A COMPONENT OF DESMOSOMES IN INTESTINAL EPITHELIUM AND IS NECESSARY FOR PROPER DYNAMIC OF CELL-CELL JUNCTIONS IN SEVERAL EPITHELIAL CELL TYPES
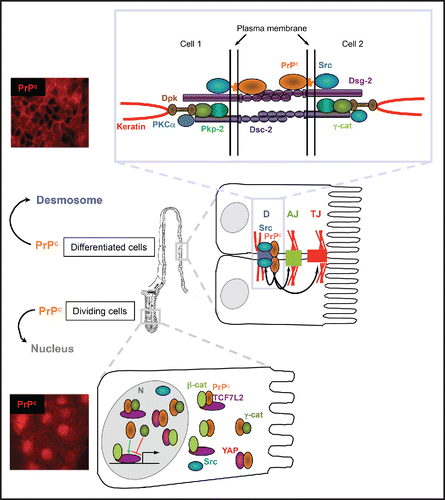
Cell-cell junctions are multiprotein complexes. New components and partner proteins are being identified continually. Our team demonstrated the presence of PrPc in intercellular junctions of differentiated intestinal cells, in raft-like microdomains, within a protein complex including Src kinase,Citation6 and components of desmosomes: desmoglein 2, γ-catenin (also known as plakoglobin), plakophilin 2A and desmoplakin ().Citation7 This junctional localization is also found in intestine in vivo, in epithelial cells of villi and at the top of crypts.Citation7
FIGURE 1. Dual localization of PrPc in intestinal epithelial cells and associated functions. In differentiated epithelial cells of the intestinal villi, as well as in confluent Caco-2/TC7 enterocytes, PrPc is addressed to desmosomes, where it interacts with several desmosomal proteins and with Src tyrosine kinase. PrPc, which contributes to the organization of desmosomes (D) and also of adherens junctions (AJ) and tight junctions (TJ) (black arrows), is involved in the global intestinal barrier function.Citation4 The mechanisms underlying the dialog between desmosomes and the other cell-cell junctions through PrPc and its molecular partners remain to be determined. The Src kinase, which is a well-known regulator of cell-cell junctions, could mediate PrPc effects, as recently shown for adherens junctions in zebrafish embryos.Citation40 In proliferative cells of the intestinal crypts and in proliferative Caco-2/TC7 cells, Src is present in the nucleus (N) along with PrPc, which interacts with plakoglobin (γ-catenin, γ-cat), with the effectors of the canonical Wnt pathway β-catenin and TCF7L2 (previously known as TCF4) and with the effector of Hippo pathway YAP. PrPc stimulates the transcriptional activity of the β-catenin/TCF7L2 complex (green arrow), which is decreased by γ-catenin (red arrow). Our experiments of co-immunoprecipitation and proximity ligation assay have suggested multiple combinations of protein complexes in both cytoplasmic and nuclear compartments,Citation9 whose detailed compositions and outcome remain to be specified. YAP/β-catenin interaction was also shown to activate transcription through factors other than TCF,Citation29 which are not represented on this scheme, and it is still unknown whether and how PrPc modulate the activity of these complexes as well.
Desmosomes are known for their mechano-resistance properties. They have been studied mainly in skin and cardiac muscle and in pathologies of these tissues, but much less is known about desmosomes in intestine, which is highly subjected to mechanical stress. The molecular organization of these complexes is very similar to that of adherens junctions. They are composed of 2 cadherin family proteins with single transmembrane domains, desmoglein and desmocollin, whose cytoplasmic domains bind 2 proteins of the catenin family, γ-catenin and plakophilin. The engagement of cell-cell contacts leads to the recruitment of a plakin family protein, desmoplakin, which links to intermediate filaments belonging to the keratin family in epithelial cells (for review see ref. Citation13).
Invalidation of PrPc in enterocytes resulted in a notable alteration in the junctional targeting of its desmosomal partners and of Src kinase, which were more diffusely distributed in the cytoplasm. The length of desmosomes was shorter in the intestinal epithelium of PrPc knockout mice than in wild type mice, small desmosomes being considered less mature.Citation7 In addition, in both cell and mouse models of PrPc invalidation, we showed global defects in the organization of cell-cell junctions and in intestinal barrier function.Citation8 Mislocalization of components of adherens junctions, e.g. E-cadherin, and of tight junctions, e.g., occludin, ZO-1 and tricellulin, accompanied that of desmosomal proteins. There was no modification of the global levels of the different proteins, suggesting that PrPc contributes to mechanisms that control the targeting and/or the turnover of proteins at intercellular junctions and that it is required for the proper assembly or the stability not only of desmosomes, but also of adherens and tight junctions.
The role of PrPc in intercellular adhesion is not restricted to intestinal epithelial cells. A similar localization at cell-cell contacts can be observed in keratinocytes (unpublished data). PrPc implication in the regulation of cell-cell adhesion, including E-cadherin-mediated adhesion, which was demonstrated in other human epithelial cell lines,Citation14 is evolutionarily conserved; this was shown by loss of embryonic cell adhesion upon PrP-1 knockdown in zebrafish, this phenotype being rescued by the zebrafish paralog PrP-2 and by mouse PrPc.Citation15 Moreover, PrP morphant embryos displayed deficient morphogenetic cell movements leading to gastrulation arrest, indicating that PrPc participates not only to junction stability, but also to their remodeling during dynamic rearrangements. Accordingly, PrP-2 was also shown necessary for the collective migration during lateral line sensory system development in zebrafish embryo; E-cadherin and β-catenin were mislocalized upon PrP-2 decreased expression.Citation16 In endothelial cells of the hemato-encephalic barrier, PrPc seems to be involved in junction remodeling upon extravasation of leucocytes and in endothelial cell migration.Citation17,18 PrPc-deficient mammary epithelial cells fail to address properly E-cadherin at cell-cell contacts, but, interestingly, they were at the same time unable to rearrange adherens junctions and to accomplish epithelial-mesenchymal transition in response to TGF-β.Citation19
Therefore, accumulating evidences in different biological systems and organisms converge to identify PrPc as an important regulator of the dynamic assembly/disassembly of several junctional complexes involved in cell-cell adhesion.
A POOL OF PRPc IS TARGETED TO THE NUCLEUS OF PROLIFERATIVE CELLS AND IS A PARTNER OF THE WNT PATHWAY IN INTESTINAL EPITHELIAL CELLS
In proliferating intestinal epithelial cells, i.e., in the intestinal crypts in vivo or in dividing human Caco-2/TC7 enterocytes in culture, we demonstrated that a PrPc pool was present in the nucleus.Citation7 Although a nuclear localization of the prion protein had been reported in few studies, in particular in a human promyelocytic leukemia cell lineCitation20 and in neuronal cells for both the protease-resistant PrP form in prion infected cellsCitation21 and the normal PrPc in non-infected cells,Citation22 the role of this particular pool was not elucidated. An association of nuclear PrPc with histone H3 in neurones and β cells of the endocrine pancreas suggested a possible role of PrPc in transcriptional regulation,Citation23 but this hypothesis was not demonstrated definitely until our recent study in enterocytes.Citation9
Through a proteomic approach, we identified γ-catenin, a component of desmosomes in differentiated cells, as a nuclear PrPc partner in proliferating intestinal epithelial cells. γ-catenin is a protein of the catenin family displaying a dual junctional and nuclear localization,Citation24 as its counterpart β-catenin. Whereas junctional γ-catenin is mainly associated with desmosomal cadherins, junctional β-catenin is exclusively localized at adherens junctions where it interacts with E-cadherin cytodomain. β-catenin was extensively studied because it is the central effector of the canonical Wnt pathway.Citation25 In the absence of Wnt stimulation, a cytoplasmic pool of β-catenin is phosphorylated by a destruction complex and addressed to the proteasome for degradation. In response to Wnt activation, the destruction complex is disrupted, and the stabilized cytoplasmic β-catenin is targeted to the nucleus where it acts as a co-activator of the transcription factors of the TCF/LEF family to regulate the expression of many target genes, which drive cell proliferation in intestinal crypts.Citation25,26 We showed that PrPc interacts, in cytoplasm and nucleus, not only with γ-catenin but also with β-catenin and TCF7L2 (previously known as TCF4), the main member of the TCF/LEF family in intestine. Furthermore, we demonstrated that PrPc stimulates the transcriptional activity of the β-catenin /TCF7L2 complex, whereas γ-catenin, which is known to interact with TCF/LEF as well, decreases it ().Citation9
Dysregulation of Wnt signaling was proposed to contribute to the pathogenesis of several neurodegenerative disorders and it must be noticed that Wnt/β-catenin signaling was shown impaired in the brain of mice infected with scrapie agents.Citation27 This raises the question of the respective impacts of the physiological and pathogenic forms of the prion protein on this pathway.
We showed that nuclear PrPc interacts also with YAP (Yes-associated protein),Citation9 which is a regulator of organ size and of progenitor cell proliferation. YAP and TAZ (transcriptional co-activator with PDZ-binding motif) are the major downstream effectors of the Hippo pathway (For review see ref. Citation28). YAP abundance and nuclear localization are negatively regulated by the Hippo kinase cascade, which is activated by the integration of multiple upstream signals including cell-cell contacts. The Hippo pathway is closely inter-linked with the Wnt pathway through multiple and complex mechanisms: cytoplasmic YAP and TAZ could function as inhibitors of Wnt signaling, whereas nuclear YAP and TAZ cooperate with β-catenin for the transcriptional activation of several target genes.Citation29 Interaction of PrPc with YAP in the cytoplasm and the nucleus is another mechanism through which PrPc may modulate the activity of Wnt effectors and the expression of their target genes. The outcome of the multiple combinations of protein complexes involving PrPc and components of the Wnt and Hippo pathways, whose composition probably differs between cytoplasm and nucleus,Citation9 remain to be deciphered, but clearly, the role of PrPc in transcriptional regulation is not restrained to its interaction with the β-catenin/TCF7L2 complex and may involve other co-regulators ().
PRPc INTERACTION WITH JUNCTIONAL AND NUCLEAR PARTNERS: A ROLE IN EPITHELIUM HOMEOSTASIS AND CANCER?
PrPc levels are increased in several cancer types including gastricCitation30 and colorectal cancers;Citation31 its augmented expression has been associated with adenoma-to-carcinoma progressionCitation32 and with high-grade tumors and poor prognosis of patients.Citation33 Based on such observations, PrPc was proposed to play a role on cancer development, tumor progression and response to therapy. The mechanisms by which PrPc mediated these effects have been unraveled in only few studies, which focused exclusively on its interaction with partners on the plasma membrane (For reviews see refs. Citation11, 12). We propose that PrPc must now be considered as an actor in oncogenic processes also through its role in the dynamic of cell-cell junctions and its dual junctional and nuclear localization leading to its capability to modulate the transcriptional activity of Wnt and potentially Hippo effectors. Both pathways are modulated by cell contacts and are deregulated at high frequency in many human cancers.Citation25,29
Proliferation of constantly renewing intestinal epithelial cells depends on the activation of the Wnt pathway. Moreover, activating Wnt pathway mutations are frequent and early events in colorectal cancer and have been described in a variety of other tumors.Citation25 In agreement with a role of PrPc in the activation of the Wnt effectors, we observed an alteration of nuclear β-catenin localization in intestinal crypts from PrPc knockout mice and an impairment of growth and survival of intestinal organoids derived from these mice.Citation9 This could explain the shortening of the villi that we described previously in PrPc knockout mice.Citation7 It must be determined whether the stimulation of proliferation by PrPc, which was described in gastric cancer cells,Citation34 or in colorectal cancer stem cells,Citation33 is linked to an increased nuclear localization of PrPc combined with an enhanced activity of Wnt effectors.
It was shown recently that Prnp transcription increased considerably during epithelial-mesenchymal transition (EMT).Citation19 EMT is a key regulator of metastasis in some cancers, during which cells change their epithelial properties to adopt a more mesenchymal and invasive phenotype (for review see ref. Citation35). PrPc contributes to metastatic capacity of colorectal cancer stem cells positive for the membrane glycoprotein receptor for hyaluronan CD44.Citation33 Moreover, PrPc interacts with CD44 and both proteins enhance the ability of migration and invasion of breast cancer cells.Citation36 Surprisingly, in this latter study, a predominant nuclear localization of PrPc in the breast tumor samples was observed, which, as emphasized by the authors, needed further investigations. In that context, it is interesting to note that CD44 is a target gene of Wnt effectors, the β-catenin/TCF complex,Citation37 that we demonstrated activated by nuclear PrPc. Mehrabian and colleagues showed that stable PrPc-deficient mammary epithelial cells failed to complete EMT in response to TGF-β.Citation19 This was linked to a lack of polysialylation of neural cell adhesion molecule 1 (NCAM1) caused by a perturbed transcription of the polysialyltransferase ST8SIA2 gene. Interestingly, they demonstrated that PrPc regulates the transcription of the polysialyltransferase ST8SIA2 gene through a β-catenin-dependent mechanism.Citation19 It thus appears that the links between PrPc and EMT could implicate not only PrPc capability to modulate the dynamics of cell-cell junctions but also a role of its nuclear pool in the regulation of Wnt target genes.
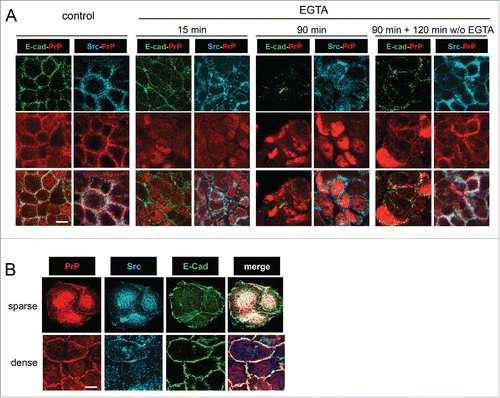
Mechanisms leading to increased levels of PrPc in tumoral cells and the impact of nucleo-junctional PrPc interplay in cancer process remain to be characterized. Comparing several intestinal cell lines, we found no correlation between global PrPc levels and Wnt pathway status.Citation9 Moreover, the impact of Wnt activation on PrPc nuclear accumulation remains to be clarified. The nuclear targeting of PrPc could rely on cell-cell junction remodeling in the course of cell transformation and could involve Src kinase. Indeed, we showed that dismantling of calcium-dependent junctions in Caco-2/TC7 cells was sufficient to impair PrPc targeting at cell-cell contacts and to observe its nuclear localization (). Src kinase, the junctional partner of PrPc,Citation6 accumulated in the cytoplasm after junction disassembly () and colocalized with PrPc in nucleus or perinuclear compartments of proliferative epithelial intestinal cells (). Although the roles of intracellular and nuclear pools of Src kinase in cancer cells are not clear yet, activated Src is known to phosphorylate β-catenin, leading to an impairment of its binding to E-cadherin and to an augmentation of its intracellular levels (for review see ref. Citation38). In the context of SFK-dependent junction remodeling leading to EMT, PrPc would not be targeted to the cell membrane, and we speculate that the resulting increased levels of cytoplasmic PrPc and β-catenin favor the interactions between both proteins, leading to their nuclear translocation with TCF7L2 and to the activation of proliferation- and EMT-associated gene transcription. Thus, in absence of mature junctions and in a context of high cytoplasmic and nuclear levels of PrPc and β-catenin, SFK could be involved in the activation of the final steps of Wnt signaling by PrPc. SFK activity (Src and Yes) has been shown recently to favor YAP nuclear translocation.Citation39 It would be important to determine how, upon cancer-associated dismantling of cell-cell junction, PrPc, which interacts with YAP, could modulate its activation as well. In order to determine how the mechanisms that involve PrPc in cancer process and EMT are integrated, the analysis of the respective proportions and subcellular localizations of PrPc, Src, β-catenin, TCF, γ-catenin, YAP and TAZ in a large-scale study of human tumors would be of great interest.
FIGURE 2. Calcium-dependent dissassembly of cell-cell junctions is sufficient to address PrPc to the nucleus and to delocalize Src from cell-cell contacts. (A) Confluent Caco-2/TC7 cells were treated with 2 mM EGTA for 15 or 90 minutes. For the reversion study, cells were washed after EGTA treatment and incubated for further 120 minutes in control medium. Immunostaining was performed for PrPc, Src and E-cadherin as indicated. As soon as 15 min after the beginning of EGTA treatment, E-cadherin and Src start to be delocalized from cell-cell junctions and to be more diffusely distributed in the cytoplasm, whereas part of PrPc is already detected in the nucleus, this localization being clearly observed at 90 minutes. After 2 hours of reversion, PrPc and Src are re-addressed to cell-cell junctions along with E-cadherin. (B) Src kinase is addressed to the nucleus with PrPc in low density proliferating intestinal cells. Exponentially growing Caco-2/TC7 cells were immunostained for PrPc, Src and E-cadherin as indicated. In small clusters (sparse cells, upper pannel), PrPc and Src accumulate in the nucleus, whereas in large clusters (dense cells, lower pannel), they are addressed at cell-cell contacts where they co-localize with E-cadherin. Bars : 10 µm (note that confluent cells in A are smaller than exponentially growing cells in B, as expected).
In conclusion, the mechanisms of PrPc contribution in cancer biology must be further explored. In a recent extra views report, Santos and colleagues pointed out, as good candidate for therapeutic interventions, the PrPc role as a scaffold protein organizing membrane platforms and the search for specific partners within tumor cells, extracellular matrix, and soluble factors secreted from tumor cells.Citation12 Based on our recent findings, we propose that studies on tumors must integrate the PrPc partners located not only within membrane complexes, including cell-cell junctions, but also within the nucleus. In particular, the interaction of PrPc in nucleus of dividing cells with effectors of Wnt and Hippo pathways, which are both clearly involved in cancer, opens a new field of research on the mechanisms that link PrPc expression and subcellular localization to cancers.
ABBREVIATIONS
| PrPc | = | cellular prion protein |
| ZO-1 | = | zonula occludens-1 |
| NLS | = | nuclear localization signal |
| TCF7/LEF | = | T cell factor/lymphoid enhancing factor |
| EMT | = | epithelial–mesenchymal transition |
| YAP | = | Yes-associated protein |
| SFK | = | Src family kinases |
| TAZ | = | transcriptional co-activator with PDZ-binding motif |
| N-CAM | = | Neural cell adhesion molecule |
| EGTA | = | ethylene glycol tetraacetic acid |
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Funding
This work was supported by institutional funding from INSERM, Université Pierre et Marie Curie and Ecole Pratique des Hautes Etudes and by grant from the Agence Nationale pour la Recherche (ANR-09-JCJC-0052-01).
REFERENCES
- Prusiner SB. Prions. Proc Natl Acad Sci U S A 1998; 95:13363-83; PMID:9811807; http://dx.doi.org/10.1073/pnas.95.23.13363
- Westergard L, Christensen HM, Harris DA. The cellular prion protein (PrP(C)): its physiological function and role in disease. Biochim Biophys Acta 2007; 1772:629-44; PMID:17451912; http://dx.doi.org/10.1016/j.bbadis.2007.02.011
- Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, Brentani RR. Physiology of the prion protein. Physiol Rev 2008; 88:673-728; PMID:18391177; http://dx.doi.org/10.1152/physrev.00007.2007
- Petit CS, Besnier L, Morel E, Rousset M, Thenet S. Roles of the cellular prion protein in the regulation of cell adhesion, cell-cell junctions and barrier function. Tissue Barriers 2013; 1:e24377-1-10; PMID:24665391; http://dx.doi.org/10.4161/tisb.24377
- Mouillet-Richard S, Ermonval M, Chebassier C, Laplanche JL, Lehmann S, Launay JM, Kellermann O. Signal transduction through prion protein. Science 2000; 289:1925-8; PMID:10988071; http://dx.doi.org/10.1126/science.289.5486.1925
- Morel E, Fouquet S, Chateau D, Yvernault L, Frobert Y, Pincon-Raymond M, Chambaz J, Pillot T, Rousset M. The cellular prion protein PrPc is expressed in human enterocytes in cell-cell junctional domains. J Biol Chem 2004; 279:1499-505; PMID:14576159; http://dx.doi.org/10.1074/jbc.M308578200
- Morel E, Fouquet S, Strup-Perrot C, Pichol Thievend C, Petit C, Loew D, Faussat AM, Yvernault L, Pinçon-Raymond M, Chambaz J, et al. The cellular prion protein PrP(c) is involved in the proliferation of epithelial cells and in the distribution of junction-associated proteins. PLoS One 2008; 3:e3000; PMID:18714380; http://dx.doi.org/10.1371/journal.pone.0003000
- Petit CS, Barreau F, Besnier L, Gandille P, Riveau B, Chateau D, Roy M, Berrebi D, Svrcek M, Cardot P, et al. Requirement of cellular prion protein for intestinal barrier function and mislocalization in patients with inflammatory bowel disease. Gastroenterology 2012; 143:122-32 e15; PMID:22446194; http://dx.doi.org/10.1053/j.gastro.2012.03.029
- Besnier LS, Cardot P, Da Rocha B, Simon A, Loew D, Klein C, Riveau B, Lacasa M, Clair C, Rousset M, et al. The cellular prion protein PrPc is a partner of the Wnt pathway in intestinal epithelial cells. Mol Biol Cell 2015; 26:3313-28; PMID:26224313; http://dx.doi.org/10.1091/mbc.E14-11-1534
- McCrea PD, Maher MT, Gottardi CJ. Nuclear signaling from cadherin adhesion complexes. Curr Top Dev Biol 2015; 112:129-96; PMID:25733140; http://dx.doi.org/10.1016/bs.ctdb.2014.11.018
- Mehrpour M, Codogno P. Prion protein: From physiology to cancer biology. Cancer Lett 2010; 290:1-23; PMID:19674833; http://dx.doi.org/10.1016/j.canlet.2009.07.009
- Santos TG, Lopes MH, Martins VR. Targeting prion protein interactions in cancer. Prion 2015; 9:165-73; PMID:26110608; http://dx.doi.org/10.1080/19336896.2015.1027855
- Holthofer B, Windoffer R, Troyanovsky S, Leube RE. Structure and function of desmosomes. Int Rev Cytol 2007; 264:65-163; PMID:17964922; http://dx.doi.org/10.1016/S0074-7696(07)64003-0
- Solis GP, Schrock Y, Hulsbusch N, Wiechers M, Plattner H, Stuermer CA. Reggies/flotillins regulate E-cadherin-mediated cell contact formation by affecting EGFR trafficking. Mol Biol Cell 2012; 23:1812-25; PMID:22438585; http://dx.doi.org/10.1091/mbc.E11-12-1006
- Malaga-Trillo E, Solis GP, Schrock Y, Geiss C, Luncz L, Thomanetz V, Stuermer CA. Regulation of embryonic cell adhesion by the prion protein. PLoS Biol 2009; 7:e55; PMID:19278297; http://dx.doi.org/10.1371/journal.pbio.1000055
- Huc-Brandt S, Hieu N, Imberdis T, Cubedo N, Silhol M, Leighton PL, Domaschke T, Allison WT, Perrier V, Rossel M. Zebrafish prion protein PrP2 controls collective migration process during lateral line sensory system development. PLoS One 2014; 9:e113331; PMID:25436888; http://dx.doi.org/10.1371/journal.pone.0113331
- Viegas P, Chaverot N, Enslen H, Perriere N, Couraud PO, Cazaubon S. Junctional expression of the prion protein PrPC by brain endothelial cells: a role in trans-endothelial migration of human monocytes. J Cell Sci 2006; 119:4634-43; PMID:17062642; http://dx.doi.org/10.1242/jcs.03222
- Watanabe T, Yasutaka Y, Nishioku T, Kusakabe S, Futagami K, Yamauchi A, Kataoka Y. Involvement of the cellular prion protein in the migration of brain microvascular endothelial cells. Neurosci Lett 2011; 496:121-4; PMID:21511010; http://dx.doi.org/10.1016/j.neulet.2011.03.096
- Mehrabian M, Brethour D, Wang H, Xi Z, Rogaeva E, Schmitt-Ulms G. The Prion Protein Controls Polysialylation of Neural Cell Adhesion Molecule 1 during Cellular Morphogenesis. PLoS One 2015; 10:e0133741; PMID:26288071; http://dx.doi.org/10.1371/journal.pone.0133741
- Rybner C, Finel-Szermanski S, Felin M, Sahraoui T, Rousseau C, Fournier JG, Sève AP, Botti J. The cellular prion protein: a new partner of the lectin CBP70 in the nucleus of NB4 human promyelocytic leukemia cells. J Cell Biochem 2002; 84:408-19; PMID:11787070; http://dx.doi.org/10.1002/jcb.10017
- Mange A, Crozet C, Lehmann S, Beranger F. Scrapie-like prion protein is translocated to the nuclei of infected cells independently of proteasome inhibition and interacts with chromatin. J Cell Sci 2004; 117:2411-6; PMID:15126640; http://dx.doi.org/10.1242/jcs.01094
- Hosokawa T, Tsuchiya K, Sato I, Takeyama N, Ueda S, Tagawa Y, Kimura KM, Nakamura I, Wu G, Sakudo A, et al. A monoclonal antibody (1D12) defines novel distribution patterns of prion protein (PrP) as granules in nucleus. Biochem Biophys Res Commun 2008; 366:657-63; PMID:18068119; http://dx.doi.org/10.1016/j.bbrc.2007.11.163
- Strom A, Wang GS, Picketts DJ, Reimer R, Stuke AW, Scott FW. Cellular prion protein localizes to the nucleus of endocrine and neuronal cells and interacts with structural chromatin components. Eur J Cell Biol 2011; 90:414-9; PMID:21277044; http://dx.doi.org/10.1016/j.ejcb.2010.11.015
- Aktary Z, Pasdar M. Plakoglobin: role in tumorigenesis and metastasis. Int J Cell Biol 2012; 2012:189521; PMID:22481945; http://dx.doi.org/10.1155/2012/189521
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012; 149:1192-205; PMID:22682243; http://dx.doi.org/10.1016/j.cell.2012.05.012
- Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 2003; 17:1709-13; PMID:12865297; http://dx.doi.org/10.1101/gad.267103
- Sun J, Wang H, Chen LN, Wang J, Lv Y, Yang XD, Zhang BY, Tian C, Shi Q, Dong XP, et al. Remarkable impairment of Wnt/β-catenin signaling in the brains of the mice infected with scrapie agents. J Neurochem 2016; 136:731-740; http://dx.doi.org/10.1111/jnc.13416
- Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol 2011; 13:877-83; PMID:21808241; http://dx.doi.org/10.1038/ncb2303
- Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer 2015; 15:73-9; PMID:25592648; http://dx.doi.org/10.1038/nrc3876
- Du J, Pan Y, Shi Y, Guo C, Jin X, Sun L, Liu N, Qiao T, Fan D. Overexpression and significance of prion protein in gastric cancer and multidrug-resistant gastric carcinoma cell line SGC7901/ADR. Int J Cancer 2005; 113:213-20; PMID:15386405; http://dx.doi.org/10.1002/ijc.20570
- Li QQ, Sun YP, Ruan CP, Xu XY, Ge JH, He J, Xu ZD, Wang Q, Gao WC. Cellular prion protein promotes glucose uptake through the Fyn-HIF-2alpha-Glut1 pathway to support colorectal cancer cell survival. Cancer Sci 2011; 102:400-6; PMID:21265952; http://dx.doi.org/10.1111/j.1349-7006.2010.01811.x
- de Wit M, Jimenez CR, Carvalho B, Belien JA, Delis-van Diemen PM, Mongera S, Piersma SR, Vikas M, Navani S, Pontén F, et al. Cell surface proteomics identifies glucose transporter type 1 and prion protein as candidate biomarkers for colorectal adenoma-to-carcinoma progression. Gut 2012; 61:855-64; PMID:21890811; http://dx.doi.org/10.1136/gutjnl-2011-300511
- Du L, Rao G, Wang H, Li B, Tian W, Cui J, He L, Laffin B, Tian X, Hao C, et al. CD44-positive cancer stem cells expressing cellular prion protein contribute to metastatic capacity in colorectal cancer. Cancer Res 2013; 73:2682-94; PMID:23418321; http://dx.doi.org/10.1158/0008-5472.CAN-12-3759
- Liang J, Pan Y, Zhang D, Guo C, Shi Y, Wang J, Chen Y, Wang X, Liu J, Guo X, et al. Cellular prion protein promotes proliferation and G1/S transition of human gastric cancer cells SGC7901 and AGS. FASEB J 2007; 21:2247-56; PMID:17409275; http://dx.doi.org/10.1096/fj.06-7799com
- Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol 2011; 27:347-76; PMID:21740232; http://dx.doi.org/10.1146/annurev-cellbio-092910-154036
- Cheng Y, Tao L, Xu J, Li Q, Yu J, Jin Y, Chen Q, Xu Z, Zou Q, Liu X. CD44/cellular prion protein interact in multidrug resistant breast cancer cells and correlate with responses to neoadjuvant chemotherapy in breast cancer patients. Mol Carcinog 2014; 53:686-97; PMID:23681900; http://dx.doi.org/10.1002/mc.22021
- Wielenga VJ, Smits R, Korinek V, Smit L, Kielman M, Fodde R, Clevers H, Pals ST. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol 1999; 154:515-23; PMID:10027409; http://dx.doi.org/10.1016/S0002-9440(10)65297-2
- Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harbor Perspect Biol 2010; 2:a002915; PMID:20182623; http://dx.doi.org/10.1101/cshperspect.a002915
- Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 2015; 519:57-62; PMID:25731159; http://dx.doi.org/10.1038/nature14228
- Sempou E, Biasini E, Pinzon-Olejua A, Harris DA, Malaga-Trillo E. Activation of zebrafish Src family kinases by the prion protein is an amyloid-β-sensitive signal that prevents the endocytosis and degradation of E-cadherin/β-catenin complexes in vivo. Mol Neurodegener 2016; 11:18; PMID:26860872; http://dx.doi.org/10.1186/s13024-016-0076-5
