ABSTRACT
To assess scrapie infectivity associated with caprine-origin tissues, bioassay can be performed using kids, lambs or transgenic mice expressing caprine or ovine prion (PRNP) alleles, but the incubation periods are fairly long. Although several classical ovine scrapie prion permissive cell lines with the ability to detect brain-derived scrapie prion have been available, no classical caprine scrapie permissive cell line is currently available. Therefore, the aims of this study were to generate a rabbit kidney epithelial cell line (RK13) stably expressing caprine wild-type PRNP (cpRK13) and then to assess permissiveness of cpRK13 cells to classical caprine scrapie prion propagation. The cpRK13 and plasmid control RK13 (pcRK13) cells were incubated with brain-derived classical caprine scrapie inocula prepared from goats or ovinized transgenic mice (Tg338, express ovine VRQ allele) infected with caprine scrapie. Significant PrPSc accumulation, which is indicative of scrapie prion propagation, was detected by TSE ELISA and immunohistochemistry in cpRK13 cells inoculated with classical caprine scrapie inocula. Western blot analysis revealed the typical proteinase K-resistant 3 PrPres isoforms in the caprine scrapie prion inoculated cpRK13 cell lysate. Importantly, PrPSc accumulation was not detected in similarly inoculated pcRK13 cells, whether by TSE ELISA, immunohistochemistry, or western blot. These findings suggest that caprine scrapie prions can be propagated in cpRK13 cells, thus this cell line may be a useful tool for the assessment of classical caprine prions in the brain tissues of goats.
INTRODUCTION
Classical scrapie is a transmissible spongiform encephalopathy (TSE), or prion disease, of domestic goats and sheep, the hallmark of which is a slowly progressive fatal neurodegeneration in the central nervous system that is accompanied by accumulation of abnormal conformational isoforms (PrPSc) of the host-encoded normal cellular prion protein (PrPC).Citation1 The relative susceptibility of sheep to infection with classical scrapie depends in part on polymorphisms affecting the amino acid composition of PrPC, especially those occurring at codon 136 (valine [V] or alanine [A]), 154 (arginine [R] or histidine [H]) and 171 (glutamine [Q], R, H, or lysine [K]). Thus, sheep homozygous for the V136R156Q171 (or VRQ) allele show the most susceptibility compared to A136R156Q171 (or ARQ; wild type) allele whereas sheep homozygous for the A136R156R171 (or ARR) allele are the least susceptible.Citation2-4
Like in sheep, prion gene (PRNP) in goats is highly polymorphic.Citation5 In goats, PRNP haplotypes “1” and “2” have been identified as “wild type”.Citation6 Caprine haplotype 2 is identical to the ovine wild type PRNP haplotype, ARQ; caprine haplotype 1 is identical except for a serine [S] to proline [P] substitution occurring at codon 240.Citation6 Goats homozygous for haplotypes 1 and 2 are highly susceptible to scrapie infection.Citation5,7 Classical caprine scrapie prion inoculation studies using kids as recipients revealed that polymorphisms of PRNP at codon 146 (serine [S], haplotype 7), 154 (histidine [H], haplotype 8), 211 (glutamine [Q], haplotype 9) or 222 (lysine [K], haplotype 10) can provide resistance or delayed incubation period.Citation7,8 A recent goat population screening study in the UK and our experimental caprine scrapie inoculation study in goats found that a PRNP polymorphism at codon 127 [S, haplotype 3] is associated with a prolonged incubation period.Citation9,10 Although a polymorphism at codon 142 (methionine [M], haplotype 4) was associated with increased resistance to scrapie infections,Citation10 experimental caprine scrapie inoculation in goats with I/M142 provided only a moderate increase in incubation period.Citation7 Traditionally, mouse bioassays have been utilized to assess prion infectivity, infectious titers and strain typing, but depending on the nature and the origin of the prions, bioassays can take months to years to produce clinical disease. As an alternative, a recent study suggests an “ovinized” model cell culture system achieved equivalent sensitivity as an “ovinized” transgenic mouse bioassay in detecting brain-derived classical ovine scrapie prions but within a month post-inoculation.Citation11 That study used the well-characterized “Rov9” cell line, a rabbit kidney epithelial cell (RK13) transfected to express ovine VRQ allele.Citation12 A cell line permissive to classical caprine-derived scrapie propagation has not been reported and therefore, development of a transfectant cell line expressing caprine PrPC might be an option to overcome this limitation. The susceptibility of RK13-based transfectants to corresponding rodent- and cervid-derived prion propagation has been reported.Citation13-15
In this study, we demonstrate for the first time that RK13 cells stably expressing caprine PrPC (cpRK13) was permissive to certain classical caprine scrapie prion isolates prepared from the brain tissues of scrapie-infected goats and ovinized transgenic mice.
RESULTS AND DISCUSSION
cpRK13 Cells Express Caprine PrPC on the Cell Surface
The RK13 cell line was used in this study to generate caprine PrPC expression due to very low expression of endogenous rabbit PrPC12 and its ability to propagate prions upon expression of exogenous PrPC from multiple species including sheep.Citation12,13,16,17 PCR amplified caprine PRNP haplotype 2 was cloned into a mammalian expression plasmid (pIRESpuro3-cp) (Fig. S1A). It is important to understand that during the posttranslational modification process, both the N-terminal secretory signal sequence (24 amino acids) and the C-terminal glycophosphatidylinositol (GPI) anchor signal sequence (23 amino acids) are removed from PrPC and thus the mature GPI-anchored cell surface expresses PrPC contains only 209 amino acids (residues 25 to 233). Therefore, the mature PrPC expressed on cell surfaces from both PRNP haplotype 1 and haplotype 2 are identical. Following transfection of RK13 cells with pIRESpuro3-cp, stable single cell-derived transfectant clones expressing caprine PrPC were selected using flow cytometry and expanded. N-terminal specific PrP mAb 5B2 which recognizes RYP residues conserved between goat and rabbit PrPC was used to identify both endogenous rabbit PrPC and exogenous goat PrPC expression on RK13 cells.Citation18,19 Cell surface expression of caprine PrPC on cpRK13 cells was confirmed using flow cytometry (). As previously reported,Citation12 plasmid control RK13 cells (pcRK13) did not express detectable level of endogenous rabbit PrPC as assessed by flow cytometry (). The molecular isoforms of PrPC expressed by cpRK13 cells were examined by western blot analysis as well. For comparison, the immunoblot included a brain homogenate prepared from a scrapie-uninfected PRNP haplotype 1,2 goat brain homogenate (animal ID: g4111). Similar banding patterns and distribution of di-, mono- and un-glycosylated PrPC isoforms were readily detected in both cpRK13 cell lysate and goat brain homogenate (). Similar to a previous report,Citation12 PrPC expression was not detected in pcRK13 cell lysates, further confirming the lack of endogenous rabbit PrPC expression in RK13 cells ().
FIGURE 1. Expression of caprine PrPC in cpRK13 cells. (A) Flow cytomtery assay with cpRK13 and pcRK13 cells. cpRK13 (caprine PRNP haplotype 2) and pcRK13 (plasmid control) cells were first incubated with PrP mAb 5B2 (IgG1) or isotype control mAb (IgG1) followed by incubation with FITC-conjugated anti-IgG1 Ab. Cell surface PrPC expression was evaluated by flow cytometry. In total, 10,000 events were counted for each cell type. (B) PrPC western blot assay with cpRK13 and pcRK13 cell lysates. Western blot assays were performed with scrapie-uninfected goat brain homogenate (animal ID: g4111, ∼150 μg wet tissue weight), cpRK13 (3 μl), and pcRK13 (3 μl) cell lysates with PrP mAb F99/97.6.1 (3.5 µg ml−1) without incubation with proteinase K (−). (C) Comparison of deglycosylated PrPC between goat brain homogenates and cpRK13 cell lysate. Scrapie-uninfected goat brain homogenate (animal ID: g4111, ∼100 - 150 μg wet tissue weight) and scrapie-uninoculated cpRK13 cell lysate (3 – 7 μl) were deglycosylated using PNGase F (+) and western blot assays were performed with PrP mAb F99/97.6.1 (3.5 µg ml−1). Scrapie-infected goat brain homogenate (animal ID: g4428, ˜75 μg wet tissue weight) was used as a control and incubated with proteinase K (PK, 100 µg ml−1 (+)), both PK and PNGase F (+) or kept unincubated with both enzymes (−).The positions of the molecular mass markers (in kDa) are shown on the left.
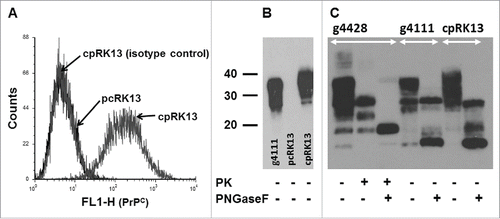
Although the PrPC banding patterns of scrapie-uninfected goat brain homogenates and cpRK13 cell lysates were similar (), the molecular masses of PrPC bands appeared to be slightly higher in cpRK13 cell lysate. This difference might be attributed to the possible glycosylation differences between goats and rabbits. Therefore, to determine any molecular mass differences in PrPC, both samples were deglycosylated using PNGase F and analyzed by western blot assays. At least 4 different deglycosylated PrPC bands with approximate molecular mass of 25 and 27 kDa (full length PrPC), 18 kDa and 16 kDa were observed with scrapie-uninfected goat brain homogenate (). With cpRK13 cell lysate, at least 3 different deglycosylated PrPC bands with approximate molecular mass of 25 kDa (full length PrPC), 18 kDa and 16 kDa were clearly visible on the western blot ().
It is known that PrPC can undergo endoproteolytic activity known as α- and β-cleavage, resulting in C1 (∼16 kDa) and C2 (∼18 kDa) fragments, respectively. Therefore, ∼16 kDa and ∼18 kDa bands in both scrapie-uninfected goat brain and cpRK13 samples are most likely C1 and C2 fragments, respectively (). Similar expression and endoproteolytic cleavage of human and mouse PrPC have been previously reported for RK13 cells.Citation24 In addition to scrapie-uninfected goat brain homogenate, it was of our interest to assess the deglycosylated PrP patterns in scrapie-infected goat brain samples. PrP banding patterns of scrapie-infected goat brain homogenate (animal ID: g4428) was very similar to scrapie-uninfected goat brain homogenate (animal ID: g4111) and both C1 and C2 fragments were clearly visible on the immunoblot (). Proteinase K digestion yielded a characteristic banding patterns of di-, mono- and un-glycosylated PrPres (). After incubation with both proteinase K and PNGase F, a deglycosylated PrPres band (∼18 kDa) and a very weak ∼16 kDa band (possibly C1 fragment) were observed ().
cpRK13 Cells Support Propagation of Classical Caprine Scrapie Prions
In 2001, Vilette and his coworkers demonstrated the permissibility of RK13 cells expressing the ovine PRNP VRQ allele (Rov9) to VRQ/VRQ sheep-derived and Tg338 (transgenic mouse line expressing ovine VRQ allele) mouse-passaged scrapie propagation.Citation12 Several other groups also reported the permissiveness of RK13-cells expressing mouse, bank vole, deer and elk PrPC to propagate prions from the corresponding species.Citation13-15 Scrapie prion propagation was not observed in various established cell lines (non-transfectants) that naturally express endogenous PrPC, including the 2 cell lines derived from fetal tissues of goats.Citation20 Therefore, we decided to use the RK13 cell line to express caprine PrPC and then assess its ability to propagate brain-derived classical caprine scrapie prion isolates. The cpRK13 cell line described in this study is the first reported stable caprine PrPC expressing RK13 cells.
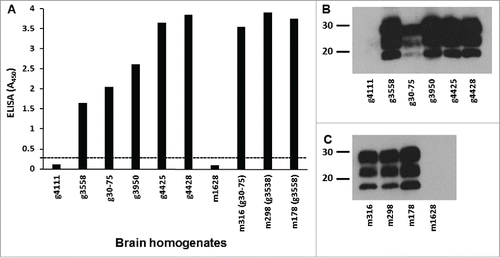
To illustrate amino acid sequence differences among inocula prepared from goats with different PRNP haplotypes, alignment of multiple sequences of PRNP haplotypes is shown in Fig. S1B. To assess whether cpRK13 cells could support classical caprine scrapie prion propagation, a previously described highly sensitive, non-subcultivation-based assay technique developed with ovine RK13 cells (Rov9) was used.Citation11 Prior to the preparation of brain homogenates, PrPSc in clinically scrapie affected goats and Tg338 mice used in this study was confirmed by IHC, a commercially available TSE ELISA kit (IDEXX), and western blot assays and scrapie infectivity was confirmed in bioassay studies using goats and Tg338 mice.Citation9,21,22 For the present study, brain homogenates prepared from all 5 scrapie-infected goats and 3 Tg338 mice were again assessed for PrPSc by TSE ELISA and protein gel blot. As expected, significant amounts of PrPSc in both the caprine and the murine scrapie brain homogenates were detected by TSE ELISA, but not in the scrapie-uninfected goat or mouse brain homogenates (). Furthermore, characteristic proteinase K-resistant di-, mono- and un-glycosylated PrPres isoforms were clearly visible in scrapie brain samples when analyzed by western blot (). As expected, PrPSc was not detected in scrapie-uninfected goat () or mouse brain homogenates ().
FIGURE 2. PrPSc analysis of donor animals' brain homogenates. (A) TSE ELISA analysis of caprine scrapie-infected goat and Tg338 mouse brain homogenates for PrPSc. Approximately 300 μg of 10% w/v brain homogenates of caprine scrapie-infected goat (animal IDs: g3558, g30-75, g3950, g4425, and g4428) and Tg338 mouse (animal IDs: m316, m298, and m178) brain homogenates (in duplicate) was assessed for relative levels of PrPSc using a TSE ELISA kit (IDEXX). Scrapie-uninfected goat (animal ID: g4111) and Tg338 mouse (animal ID: m1628) brain homogenates were also included in the ELISA. Average TSE ELISA absorbance values are shown in the y-axis and animal IDs are shown in the x-axis. Cut-off value for the ELISA (—) was determined as described by the manufacturer. Western blot analysis of caprine scrapie-infected goat (B) and Tg338 mouse (C) brain homogenates for PrPres. Caprine scrapie-infected and scrapie-uninfected goat and Tg338 mouse brain homogenates (˜75 μg wet tissue weight for each sample) were incubated with proteinase K (100 µg ml−1 at 37°C for 60 min) and PrPres was detected using PrP mAbs F99/97.6.1 (3.5 µg ml−1). The positions of the molecular mass markers (in kDa) are shown on the left.
De novo propagation of classical caprine scrapie prions in cpRK13 cells was similarly evaluated for the accumulation of PrPSc; however a TSE ELISA was used for primary screening because it allowed testing of a large number of samples simultaneously. A significant accumulation of PrPSc was detected in cpRK13 cells inoculated with scrapie inocula prepared from 3 goats with PRNP haplotypes 1,1 or 1,2 (animal IDs: g3950, g4425, and g4428) by TSE ELISA, suggesting this cell line was permissive to brain-derived classical caprine scrapie prion propagation (, P<0.01). Despite similar PrPSc levels and PrPres intensities among scrapie inocula, cpRK13 cells inoculated with brain homogenate from g4428 showed the highest TSE ELISA absorbance indicating efficient prion propagation. However, PrPSc propagation was not detected in cpRK13 cells similarly inoculated with scrapie inocula prepared from 2 goats with PRNP haplotypes 3 or 4 (animal IDs: g30-75, G/S127 and g3558, I/M142) (). TSE ELISA absorbance values of pcRK13 cells inoculated with all 5 caprine scrapie inocula were well below the assay cut-off value (), thus clearly suggesting the lack of residual scrapie inocula in the cell lysates under these experimental conditions. One of the limitations of this study was that we used only the recently described non-subcultivation based methodCitation11 to assess the caprine scrapie prion propagation in cpRK13 cells and incubation was limited to maximum of 5 weeks. Perhaps more extended cultivation or a second round of inoculation using the homogenates prepared from first passage could have improved the prion propagation, particularly for PRNP haplotype 3 or 4 scrapie isolates inoculated cpRK13 cells.
FIGURE 3. Detection of de novo PrPSc propagation in cpRK13 cells. (A) TSE ELISA analysis of cpRK13 cells inoculated with caprine scrapie-infected goat brain homogenates. Caprine PrPC expressing cpRK13 (black bars) and plasmid control RK13 cells (pcRK13, open bars) were inoculated with scrapie-infected (animal IDs: g3558, g30-75, g3950, g4425, and g4428,) or scrapie-uninfected (animal ID: g4111) goat brain homogenates and cell lysates were prepared 5 weeks post-inoculation. One hundred μl of cpRK13 or pcRK13 cell lysate was loaded into each well (in triplicate) and relative levels of PrPSc accumulations were evaluated using a TSE ELISA kit (IDEXX). Average TSE ELISA absorbance values with corresponding standard deviations are shown in the y-axis and animal IDs are shown in the x-axis. Cut-off value for the ELISA (—) was determined as described by the manufacturer. (*, P<0.01). (B) Detection of PrPres in cpRK13 cells inoculated with caprine scrapie-infected brain isolates. Western blot assays were performed with cpRK13 cell lysates prepared from scrapie-infected (animal IDs: g3558, g30-75, g3950, g4425, and g4428) or scrapie-uninfected goat brain homogenates (animal ID: g4111). Twenty µl of inoculated cpRK13 or pcRK13 cell lysates were incubated with proteinase K (100 µg ml−1 at 37°C for 60 min) and PrPres was detected using a mixture of PrP mAbs F99/97.6.1. (3.5 µg ml−1) and P4 (0.2 µg ml−1). The positions of the molecular mass markers (in kDa) are shown on the left. (C) and (D). In situ detection of PrPSc accumulation in cpRK13 cells by IHC. Caprine scrapie prion propagation in cpRK13 cells was also assessed by IHC with HistoGel method. PrPSc immunolabeling was clearly visible in cpRK13 cell (D) but not in pcRK13 cells (C) following inoculation with scrapie goat brain homogenates (animal ID: g4428). PrPSc (dark red) in the cells were identified using PrP mAb SAF84.
It is well-known that the certain polymorphisms at caprine PRNP can affect susceptibility to scrapie.Citation7,8 Although PrPSc levels in inocula prepared from 2 goats with PRNP haplotypes 2,3 (animal ID: g30-75) and 1,4 (animal ID: g3558) appeared to be slightly lower than the haplotypes 1,1 or 1,2 scrapie inocula, both PRNP haplotypes 2,3 and 1,4 scrapie inocula produced clinical scrapie in Tg338 mice.Citation21 It was an interesting observation that passage 1 (P1) Tg338 mice receiving haplotype 2,3 inoculum succumb to clinical scrapie earlier than Tg338 mice receiving haplotype 1,2 (animal ID: g3538) scrapie inoculum.Citation21 The same haplotype 39 and 4 (unpublished observation) inocula and a haplotype 1 caprine scrapie inoculum produced clinical scrapie in recipient goats with very similar incubation periods.Citation9
Other research groups have also revealed the lack of propagation of a range of ovine scrapie isolates in ovine RK13 cells (Rov9).Citation23 Likewise human, bank vole, or mouse PrPC expressing RK13 transfectants also failed to propagate certain prion isolates.Citation13,24 The ability of RK13 cells to propagate prions upon expression of exogenous PrPC from multiple speciesCitation12,13,16,17 clearly confirmed that RK13 cells have the required cellular machinery for efficient prion propagation. Although PRNP haplotype 3 and 4 caprine scrapie inocula were able to produce clinical scrapie in recipient goatsCitation9 and Tg338 miceCitation21 which was very similar to PRNP haplotype 1 and 2 caprine scrapie inoculated animals, the lack of scrapie prion propagation in cpRK13cells when inoculated with the same PRNP haplotypes 3 and 4 scrapie inocula highlight the possible involvement of other unidentified factors to establish successful infection.
The molecular isoforms of PrPres in scrapie inocula and PrPSc accumulation in cpRK13 cells were also analyzed by western blot assays. The molecular mass of di- and mono- and un-glycosylated PrPres isoforms of cpRK13 lysates prepared from TSE ELISA positive samples were very similar to each other but appeared to be slightly different from the original goat brain-derived scrapie inocula (). Similar to TSE ELISA findings, PrPres bands were not observed in cpRK13 cells inoculated with 2 scrapie brain homogenates prepared from goats with PRNP haplotypes 3 or 4. As expected, none of the cell lysates prepared from caprine scrapie prion inoculated pcRK13 cells showed any PrPres bands (). In addition to TSE ELISA and western blot assays, we have also performed IHC with scrapie prion isolates inoculated cpRK13 and pcRK13 cell pellets. In situ PrPSc immunolabeling was clearly visible in scrapie-infected cpRK13 cells (), but not with the similarly inoculated pcRK13 cells (). Taken together, these findings clearly indicate that PrPSc accumulation in cpRK13 cells was due to de novo PrPSc synthesis and did not originate from the residual caprine scrapie inocula.
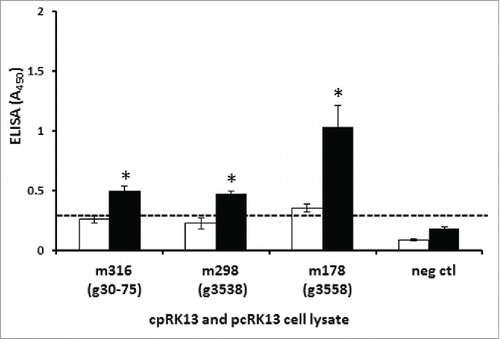
cpRK13 Cells Support Propagation of Tg338 Mouse-Passaged PRNP Haplotypes 3 and 4 Classical Caprine Scrapie Prion Isolates
Cultured neuronal cell lines are generally shown to be permissive to mouse-adapted prion strains.Citation25 Similarly, Rov9 cells were shown to be highly permissive to mouse-passaged classical ovine scrapie prion isolates.Citation11,12 A previous study by our groupCitation21 used bioassay in Tg338 mice to characterize classical scrapie from a goat with PRNP haplotype 1,2 (animal ID: g3538), haplotype 2,3 (animal IDs: g30-75) or haplotype 1,4 (animal ID: g3558). Although the incubation periods in P1 Tg338 mice were prolong and variable for 3 caprine scrapie inocula, incubation periods in P2 mice were short and very similar.Citation21 Furthermore, brain lesion profilesCitation21 and PrPSc accumulation levels in P2 mice were very similar (). To assess whether passage in Tg338 mice might improve prion propagation in cpRK13 cells, a cell culture inoculation assay was performed with the brain homogenates prepared from clinically affected P2 Tg338 mice infected with the above goat scrapie inocula as previously described.Citation11
PrPSc ELISA values of cpRK13 cells inoculated with all 3 scrapie inocula prepared from Tg338 mice were significantly higher than the similarly inoculated pcRK13 cells (, P<0.01). This observation suggests that the observed improvement of scrapie prion propagation in cpRK13 cells (previously non-permissive to PRNP haplotype 3 and 4 goat brain-derived scrapie isolates, (animal IDs: g30-75 and g3558, ()) was most likely due to passage in Tg338 mice. Although cpRK13 cells were able to propagate all 3 mouse-passaged scrapie isolates, several studies have reported the lack of sensitivity of RK13-based transfectants to propagate certain prion isolates. Murine Fukuoka-1 and Chandler strains were able to propagate efficiently in mouse-RK13 cells, but the ME7 murine strain was unable to propagate in the same cells.Citation13 In another study, although mouse-RK13 cells were permissive to mouse-adapted M1000 strain (derived from Fukuoka-1) and mouse-adapted MU-02 strain (derived from sCJD T2MM), similarly inoculated human-RK13 cells were unable to propagate these 2 mouse-adapted prion isolates.Citation24 Furthermore, although bank voles were susceptible to sheep scrapie Ss3 isolate infections, RK13 cells expressing bank vole PrPC were unable to propagate bank vole-adapted scrapie Ss3 isolate.Citation13 Thus, while passage in mice can improve the subsequent propagation of some prion isolates in cell cultures, the presence of additional barriers originating from prion isolates or other cell factors may influence the successful propagation of other prion isolates.
FIGURE 4. Permissibility of cpRK13 cells to brain-derived, Tg338 mouse-passaged heterozygous PRNP classical caprine scrapie prion isolates. Caprine PrPC expressing cpRK13, and plasmid control (pcRK13) cells were inoculated with brain homogenates prepared from scrapie-infected second passaged Tg338 mice inoculated with a caprine PRNP haplotype 1,1 (animal ID: g3538, m298), haplotype 2,3 (animal IDs: g30-75, m316), haplotype 1,4 (animal ID: g3558, m178) or uninoculated mouse (animal ID: m1628, neg ctl). One hundred μl of cpRK13 (black bars), or pcRK13 (open bars) cell lysate was loaded into each well (in triplicate) and relative levels of PrPSc accumulations were evaluated using a TSE ELISA kit (IDEXX). Average TSE ELISA absorbance values with corresponding standard deviations are shown in the y-axis and animal IDs are shown in the x-axis. Cut-off value for the ELISA (—) was determined as described by the manufacturer (*, P<0.01).
In summary, we have generated cpRK13 cell line stably expressing caprine PrPC. We also demonstrated the permissiveness of cpRK13 cells to propagate certain classical caprine scrapie prion isolates derived from goats and Tg338 mice brain tissues. Since experimental goat inoculation studies can take years to produce scrapie, cpRK13 cells may become a useful tool to initially assess the scrapie prions in wild type PRNP goat brain samples.
MATERIALS AND METHODS
Preparation of Inocula for Cell Culture Infection Studies
All experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee (IACUC) at Washington State University. Advanced stages of scrapie infections in animals were diagnosed by clinical signs and confirmed by detection of PrPSc in brain tissues collected at necropsy using immunoassays (). Inocula were prepared from hindbrains of clinically affected wild type PRNP goats (animal IDs: g3950 [haplotype 1,2] , g4425 [haplotype 1,1], and g4428 [haplotype 1,1]) and goats with heterozygous PRNP at codon 127 (animal ID: `g30-75; glycine and serine, G/S127, haplotype 2,3) or 142 (animal ID: g3558; isoleucine and methionine, I/M142, haplotype 1,4) naturally infected with classical caprine scrapie prion isolates and from the hindbrains of a scrapie-uninfected goat (animal ID: g4111, haplotype 1,2). Mouse-passaged inocula were prepared using whole brains of clinically affected ovinized transgenic mice (Tg338, expressing ovine VRQ allele) experimentally inoculated with goats with PRNP haplotype 1,2 (animal IDs: m298, g3538), haplotype 2,3 (animal IDs: m316, g30-75), haplotype 1,4 (animal IDs: m178, g3558), or an uninoculated Tg338 mouse (animal ID: m1628). All brain-derived inocula were homogenized at 10% w/v in a sterile 5% glucose solution or normal saline using a table top tissue homogenizer. Samples were briefly centrifuged, aliquoted and stored at −20°C until use.
Construction of Caprine PrPC Expression Mammalian Vector
Genomic DNA was extracted from peripheral blood mononuclear cells isolated from a PRNP haplotype 1,2 goat. A forward primer with a NheI site (underlined; 5′–GCT AGC ATG GTG AAA AGC CAC ATA GGC) and a reverse primer with an EcoRI site (underlined; 5′–GAA TTC CTA TCC TAC TAT GAG AAA AAT GAG) were designed based on the available caprine PRNP sequence (GenBank accession no. HM038413). The complete open reading frame of PRNP haplotype 2 was amplified by PCR in a 50 μl volume containing 1× PfuUltra™ reaction buffer, 0.25 mM each dNTPs, 0.2 μM each forward and reverse primer, 2 μl genomic DNA, 1 U of PfuUltra™ II Fusion HS DNA polymerase (Agilent) and distilled water (to 50 μl) as described by the manufacturer. A single PCR product (783 bp) was purified from agarose gel and cloned into TOPO cloning vector and transformed into Escherichia coli TOP10 chemically competent cells (Invitrogen). Plasmid DNA was extracted (Qiagen) and sequenced, and clones with the correct PRNP inserts were identified. To release PRNP inserts, plasmid DNA was digested with restriction enzymes NheI and EcoRI. PRNP inserts were gel purified and ligated into a linearized (NheI and EcoRI digested) mammalian expression vector, pIRESpuro3 (Clontech), using T4 DNA ligase (Invitrogen) to produce caprine PRNP haplotype 2 expression construct (pIRESpuro3-cp).
Generation of Stable RK13 Transfectants Expressing Caprine PrPC
The rabbit kidney epithelial cell line (RK13; ATCC® CCL-37™) was maintained in minimum essential medium (Opti-MEM I, Invitrogen) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Atlanta Biologicals), L-glutamine and penicillin-streptomycin (Invitrogen) and incubated at 37°C in a humidified atmosphere of 5% CO2. RK13 cells were transfected with pIRESpuro3-cp or plasmid control (pIRESpuro3 vector without a PRNP insert) using Xfect transfection reagent as described by the manufacturer (Clontech). Twenty-four hours post-transfection, cells were transferred to 100 mm culture dishes containing complete culture medium. The next day, puromycin was added at a concentration of 0.2 µg ml−1 (Invivogen). Culture medium was changed every 4 d and puromycin concentration was gradually increased up to 4 µg ml−1. Puromycin-resistant transfectants were detached from the dishes using protease-free 10 mM ethylenediaminetetraacetic acid (EDTA) solution and then washed with culture medium. Approximately 1 × 106 transfectants were resuspended in complete culture medium and incubated with N-terminal specific PrP mAb 5B2 which recognize RYP residues conserved between goat and rabbit PrPC18, (4 µg ml−1, Santa Cruz Biotechnology), or an isotype control mAb for 30 min on ice, washed and then incubated with FITC-conjugated anti-mouse IgG1 Ab (1:250 dilution, Invitrogen) on ice for 30 min. After washing, labeled cells were resuspended in complete culture medium and surface expression of caprine PrPC was analyzed by flow cytometry (FACSort, Becton-Dickinson). To generate single cell transfectant clones, puromycin-resistant transfectants were similarly labeled and sorted with a fluorescence-activated cell sorter (FACSVantage SE). All the stable transfectant clones expressing caprine PrPC (cpRK13) and plasmid control clones (pcRK13) were maintained in the selective culture medium containing puromycin (2 µg ml−1).
Scrapie Prion Inoculation to Transfectant Cell Lines
To assess the permissiveness of cpRK13 transfectants to propagate classical caprine scrapie prion isolates, a cell culture-based detection assay of prion infectivity was performed as described previously.Citation11,12 Briefly, confluent cpRK13 and pcRK13 cells in 6-well plates were infected with 1 ml of 0.1% brain homogenates (10−3) in triplicate with Opti-MEM I medium (without any additives). Six hours later, 1 ml of complete growth medium containing puromycin (4 µg ml−1) was added to each well and cultures incubated for 4 d. After removing the mixture of inoculum and culture medium, cells were thoroughly rinsed with sterile Hank's balanced salt solution (HBSS) to remove residual inocula before continuing incubation in 2 ml of complete culture medium with puromycin. Culture medium was replaced every 5 – 7 day intervals. Four to five weeks post inoculation, cells were rinsed 3 times with sterile HBSS, detached, transferred to centrifuge tubes and centrifuged. Lysates were prepared from the resulting cell pellets using Triton/DOC lysis buffer (0.5% Triton X-100, 0.5% sodium deoxycholate, 50 mM Tris-HCl, pH 7.5).Citation12 As it has been reported earlier,Citation11 RK13 cells can be kept as a confluent monolayer for several weeks without the loss of viability (data not shown).
Detection of PrPSc by ELISA and PrPres by Immunoblotting
Accumulations of PrPSc in inoculated cpRK13 cell lysates and caprine scrapie brain homogenates were evaluated using a commercially available TSE ELISA kit (CWD Antigen test kit EIA, IDEXX Laboratories). One hundred µl of cell lysate (in triplicate), 300 μg wet weight equivalent goat or Tg338 mouse 10% w/v brain homogenates (in duplicate) were loaded per well, incubated, processed and the corrected optical density (absorbance) was recorded as described by the manufacturer. Negative control samples provided with the TSE ELISA kit were used to determine the assay cut-off values. cpRK13 cells were considered permissive to scrapie propagation if the optical density (absorbance value) was greater than 0.18 plus the negative control sample value. In addition, 10% w/v brain homogenates (∼1.5 μl) and cell lysate of inoculated cpRK13 and pcRK13 (20 μl) were assessed for PrPres (proteinase-K; 100 µg ml−1, incubation at 37°C for 60 min) by western blot using PrP mAbs F99/97.6.1 (3.5 µg ml−1, VMRD) and P4 (0.2 µg ml−1, R-BioPharm) and chemiluminescence (Amersham ECL™) as described earlier.Citation26 To compare molecular masses of unglycosylated PrPC, 10% w/v goat brain homogenates (1.5 – 2 μl) and cpRK13 cell lysates (∼3 – 7 μl) were incubated with peptide-N-glycosidase F (PNGase F, an endoglycosidase) before western blot assays as described by the manufacturer (New England Biolabs).
In Situ Detection of PrPSc Accumulation
Brain homogenate-inoculated cpRK13 and pcRK13 cells were detached using a protease-free 10 mM EDTA solution, washed with HBSS, fixed with 2% neutral-buffered formalin for 10 min at room temperature and again thoroughly washed with HBSS. Fixed cell pellets in the centrifuge tubes were resuspended in 10 µl of HBSS and then 90 µl liquefied Richard-Allan Scientific HistoGel™ (Thermo Scientific) was added, gently mixed and allowed to solidify at room temperature. HistoGel cell pellets were transferred to tissue cassettes, fixed in 10% neutral-buffered formalin and processed for standard immunohistochemistry using an automated immunolabeler (Discovery XT, Ventana Medical Systems) as described previously.Citation27 PrPSc accumulation in inoculated cells was detected using PrP mAb SAF84 (Cayman Chemical) or a mixture of PrP mAbs F89/160.1.5 and F99/97.6.1, and a Fast Red chromogen development kit (Discovery RedMap kit, Ventana).Citation28-30
Data Analysis
PrPSc ELISA absorbance values are shown as means and standard deviations. Data were statistically analyzed by the Student's t-test. The term significant indicates a P value of less than 0.05.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
Authors declare that no potential conflicts of interest exist.
Supplemental_Material.zip
Download Zip (6.5 MB)ACKNOWLEDGMENTS
We thank Donald P. Knowles for critical reading of the manuscript. The authors would also like to thank Linda Hamburg, Lori Fuller, Laetisha O'Rourke, Deborah Wolheter, Jan Luft and Emma Karel for technical assistance. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
Funding
This work was supported by funds from the USDA Agricultural Research Service (CRIS 2090-32000-030-00D).
REFERENCES
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science 1982; 216:136-44; PMID:6801762; http://dx.doi.org/10.1126/science.6801762
- Hunter N, Moore L, Hosie BD, Dingwall WS, Greig A. Association between natural scrapie and PrP genotype in a flock of Suffolk sheep in Scotland. Vet Rec 1997; 140:59-63; PMID:9023905; http://dx.doi.org/10.1136/vr.140.3.59
- Goldmann W, Hunter N, Smith G, Foster J, Hope J. PrP genotype and agent effects in scrapie: change in allelic interaction with different isolates of agent in sheep, a natural host of scrapie. J Gen Virol 1994; 75 ( Pt 5):989-95; PMID:7909834; http://dx.doi.org/10.1099/0022-1317-75-5-989
- Clouscard C, Beaudry P, Elsen JM, Milan D, Dussaucy M, Bounneau C, Schelcher F, Chatelain J, Launay JM, Laplanche JL. Different allelic effects of the codons 136 and 171 of the prion protein gene in sheep with natural scrapie. J Gen Virol 1995; 76:2097-101; PMID:7636494; http://dx.doi.org/10.1099/0022-1317-76-8-2097
- Vaccari G, Panagiotidis CH, Acin C, Peletto S, Barillet F, Acutis P, Bossers A, Langeveld J, van Keulen L, Sklaviadis T, et al. State-of-the-art review of goat TSE in the European Union, with special emphasis on PRNP genetics and epidemiology. Vet Res 2009; 40:48; PMID:19505422; http://dx.doi.org/10.1051/vetres/2009031
- White S, Herrmann-Hoesing L, O'Rourke KI, Waldron D, Rowe J, Alverson J. Prion gene (PRNP) haplotype variation in United States goat breeds. Genetics Sel Evol 2008; 40:553-61
- Lacroux C, Perrin-Chauvineau C, Corbiere F, Aron N, Aguilar-Calvo P, Torres JM, Costes P, Brémaud I, Lugan S, Schelcher F, et al. Genetic resistance to scrapie infection in experimentally challenged goats. J Virol 2014; 88:2406-13; PMID:24284317; http://dx.doi.org/10.1128/JVI.02872-13
- White SN, Reynolds JO, Waldron DF, Schneider DA, O'Rourke KI. Extended scrapie incubation time in goats singly heterozygous for PRNP S146 or K222. Gene 2012; 501:49-51; PMID:22516690; http://dx.doi.org/10.1016/j.gene.2012.03.068
- Dassanayake RP, White SN, Madsen-Bouterse SA, Schneider DA, O'Rourke KI. Role of the PRNP S127 allele in experimental infection of goats with classical caprine scrapie. Anim Genet 2015; 46:341; PMID:25917307; http://dx.doi.org/10.1111/age.12291
- Goldmann W, Ryan K, Stewart P, Parnham D, Xicohtencatl R, Fernandez N, Saunders G, Windl O, González L, Bossers A, et al. Caprine prion gene polymorphisms are associated with decreased incidence of classical scrapie in goat herds in the United Kingdom. Vet Res 2011; 42:110; PMID:22040234; http://dx.doi.org/10.1186/1297-9716-42-110
- Arellano-Anaya ZE, Savistchenko J, Mathey J, Huor A, Lacroux C, Andreoletti O, Vilette D. A simple, versatile and sensitive cell-based assay for prions from various species. PloS one 2011; 6:e20563; PMID:21655184; http://dx.doi.org/10.1371/journal.pone.0020563
- Vilette D, Andreoletti O, Archer F, Madelaine MF, Vilotte JL, Lehmann S, Laude H. Ex vivo propagation of infectious sheep scrapie agent in heterologous epithelial cells expressing ovine prion protein. Proc Natl Acad Sci U S A 2001; 98:4055-9; PMID:11259656; http://dx.doi.org/10.1073/pnas.061337998
- Courageot MP, Daude N, Nonno R, Paquet S, Di Bari MA, Le Dur A, Chapuis J, Hill AF, Agrimi U, Laude H, et al. A cell line infectible by prion strains from different species. J Gen Virol 2008; 89:341-7; PMID:18089759; http://dx.doi.org/10.1099/vir.0.83344-0
- Kim HJ, Tark DS, Lee YH, Kim MJ, Lee WY, Cho IS, Sohn HJ, Yokoyama T. Establishment of a cell line persistently infected with chronic wasting disease prions. J Vet Med Sci 2012; 74:1377-80; PMID:22673102; http://dx.doi.org/10.1292/jvms.12-0061
- Bian J, Napier D, Khaychuck V, Angers R, Graham C, Telling G. Cell-based quantification of chronic wasting disease prions. J Virol 2010; 84:8322-6; PMID:20519392; http://dx.doi.org/10.1128/JVI.00633-10
- Kang HE, Weng CC, Saijo E, Saylor V, Bian J, Kim S, Ramos L, Angers R, Langenfeld K, Khaychuk V, et al. Characterization of conformation-dependent prion protein epitopes. J Biol Chem 2012; 287:37219-32; PMID:22948149; http://dx.doi.org/10.1074/jbc.M112.395921
- Mays CE, Yeom J, Kang HE, Bian J, Khaychuk V, Kim Y, Bartz JC, Telling GC, Ryou C. In vitro amplification of misfolded prion protein using lysate of cultured cells. PloS one 2011; 6:e18047; PMID:21464935; http://dx.doi.org/10.1371/journal.pone.0018047
- Li R, Liu T, Wong BS, Pan T, Morillas M, Swietnicki W, O'Rourke K, Gambetti P, Surewicz WK, Sy MS. Identification of an epitope in the C terminus of normal prion protein whose expression is modulated by binding events in the N terminus. J Mol Biol 2000; 301:567-73; PMID:10966770; http://dx.doi.org/10.1006/jmbi.2000.3986
- Dassanayake RP, Schneider DA, Herrmann-Hoesing LM, Truscott TC, Davis WC, O'Rourke KI. Cell-surface expression of PrPC and the presence of scrapie prions in the blood of goats. J Gen Virol 2012; 93:1127-31; PMID:22278824; http://dx.doi.org/10.1099/vir.0.039032-0
- Oelschlegel AM, Geissen M, Lenk M, Riebe R, Angermann M, Schaetzl H, Groschup MH. A bovine cell line that can be infected by natural sheep scrapie prions. PloS one 2015; 10:e0117154; PMID:25565633; http://dx.doi.org/10.1371/journal.pone.0117154
- O'Rourke KI, Schneider DA, Spraker TR, Dassanayake RP, Highland MA, Zhuang D, Truscott TC. Transmissibility of caprine scrapie in ovine transgenic mice. BMC Vet Res 2012; 8:42; PMID:22472560; http://dx.doi.org/10.1186/1746-6148-8-42
- Madsen-Bouterse SA, Zhuang D, O'Rourke KI, Schneider DA. Differential immunoreactivity of goat derived scrapie following in vitro misfolding versus mouse bioassay. Biochem Biophys Res Commun 2012; 423:770-4; PMID:22713450; http://dx.doi.org/10.1016/j.bbrc.2012.06.034
- Neale MH, Mountjoy SJ, Edwards JC, Vilette D, Laude H, Windl O, Saunders GC. Infection of cell lines with experimental and natural ovine scrapie agents. J Virol 2010; 84:2444-52; PMID:20032176; http://dx.doi.org/10.1128/JVI.01855-09
- Lawson VA, Vella LJ, Stewart JD, Sharples RA, Klemm H, Machalek DM, Masters CL, Cappai R, Collins SJ, Hill AF. Mouse-adapted sporadic human Creutzfeldt-Jakob disease prions propagate in cell culture. Int J Biochem Cell Biol 2008; 40:2793-801; PMID:18590830; http://dx.doi.org/10.1016/j.biocel.2008.05.024
- Vilette D. Cell models of prion infection. Vet Res 2008; 39:10; PMID:18073097; http://dx.doi.org/10.1051/vetres:2007049
- O'Rourke KI, Zhuang D, Truscott TC, Yan H, Schneider DA. Sparse PrP(Sc) accumulation in the placentas of goats with naturally acquired scrapie. BMC Vet Res 2011; 7:7; PMID:21284878; http://dx.doi.org/10.1186/1746-6148-7-7
- Dassanayake RP, Truscott TC, Ozyigit MO, Zhuang D, Schneider DA, O'Rourke KI. Accumulation profiles of PrPSc in hemal nodes of naturally and experimentally scrapie-infected sheep. BMC Vet Res 2013; 9:82; PMID:23601183; http://dx.doi.org/10.1186/1746-6148-9-82
- O'Rourke KI, Baszler TV, Miller JM, Spraker TR, Sadler-Riggleman I, Knowles DP. Monoclonal antibody F89/160.1.5 defines a conserved epitope on the ruminant prion protein. J Clin Microbiol 1998; 36:1750-5; PMID:9620413
- O'Rourke KI, Baszler TV, Parish SM, Knowles DP. Preclinical detection of PrPSc in nictitating membrane lymphoid tissue of sheep. Vet Rec 1998; 142:489-91; PMID:9612916; http://dx.doi.org/10.1136/vr.142.18.489
- O'Rourke KI, Baszler TV, Besser TE, Miller JM, Cutlip RC, Wells GA, Ryder SJ, Parish SM, Hamir AN, Cockett NE, et al. Preclinical diagnosis of scrapie by immunohistochemistry of third eyelid lymphoid tissue. J Clin Microbiol 2000; 38:3254-9; PMID:10970367
