ABSTRACT
The surveillance of CJD or human prion diseases (PrDs) has been conducted for 10 y in China. To evaluate the quality of China CJD surveillance system, the collections of the clinical and epidemiological information, the sampling, the clinical examinations and laboratory tests and follow-up survey were separately analyzed based on the data from 2010 to 2015. The obtaining rates of clinical-information table, epidemiological-information table, sample inspection sheet and medical record of the referring patients from reporting units to the center of CJD surveillance maintained or reached at very high levels, being close to 100% in the past 3 y. 93.82%, 85.23%, 96.21% and 94.70% of the reported cases had the data of MRI, EEG, CSF 14-3-3 and PRNP sequencing, respectively. Follow-up surveys were conducted in about 50% cases in 2010 and 2011, 93.39% cases in 2012 and 100% cases in the last 3 y. High obtaining rates of the clinical and epidemiological data, high conducting rates of the relevant clinical examinations and laboratory tests, high performing rates of follow-up survey for every referring case reflect a good implemental capacity in China CJD surveillance system, which supplies solid basis for recognition and diagnosis of human prion diseases and guarantees good quality of China CJD surveillance system.
Human prion diseases (PrD) are a group of rare fatal and transmissible neurodegenerative diseases, covering Creutzfeldt-Jakob disease (CJD), Kuru, Gerstmann–Sträussler–Scheinker syndrome (GSS) and fatal familial insomnia (FFI).Citation1-2 Human PrD has an incidence of 1 or 2 cases per million ones per year with rapidly progressive dementia, cerebellar ataxia, myoclonus and behavioral changes. About 85% of all CJD cases are sporadic (sCJD), 10-15% are inherited or genetic form (gCJD) and less than 1% is acquired, namely iatrogenic CJD (iCJD).Citation3 The clinical, pathological and pathogenic features of various subtypes of human prion diseases may vary largely.
In the last decade of the 20th century, countries in Europe, North America, as well as Australia and Japan started and restarted national surveillance for human PrDs,Citation1,4-12 because of the outbreak of bovine spongiform encephalopathy (BSE) in the middle of 1980s and emergence of variant CJD (vCJD) 10 y late. Later, some other countries and regions in South America and Asia began their national or regional CJD surveillance, gradually forming global network for human PrDs. As lacking of special clinical manifestations and convenient diagnostic tool, it is sometimes difficult to recognize and diagnose human PrD promptly and correctly, which needs combinations of the data of clinical manifestations, epidemiology, clinical examinations and laboratory tests.Citation13-14 Therefore, obtaining the clinical and epidemiological information and performing relative clinical examinations and laboratory tests for the suspected PrD patients are essential in PrD surveillance. Moreover, follow-up survey in CJD surveillance is also important for correction and revision of the diagnosis of the reporting patients.
CJD Surveillance in China from 2010 to 2015
China CJD surveillance consists of 12 provincial CDCs and 15 reference hospitals, covering Beijing, Shanghai, Tianjin, Chongqing, Jilin, Shaanxi, Hubei, Guangdong, Guizhou, Anhui, Henan and Xinjiang.Citation15-18 The surveillance program and the relevant documents were issued by China CDC. The diagnostic criteria of CJD and other human prion diseases using in the surveillance system were based on the relevant documents issued by WHOCitation19-20 with slightly modification. Based the surveillance documents, the “suspected case of human prion disease” is the patient who has the symptoms in accordance with the diagnosis criteria for any subtype of human prion diseases, or the case that is believed by the physicians to be necessary to exclude the possibility of CJD.
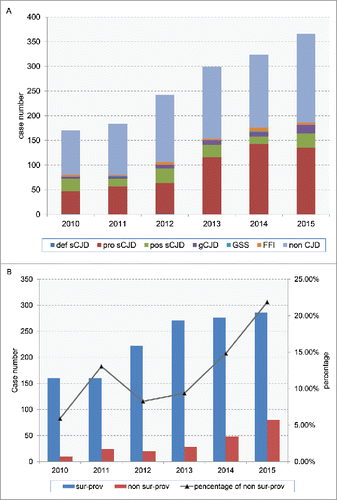
From 2010 to 2015, totally 1585 suspected CJD cases were referred to the China surveillance system of human prion disease. Among them, 700 cases were diagnosed as sCJD (2 definite, 560 probable and 138 possible), 81 cases were diagnosed as genetic human prion disease (5 GSS, 25 FFI and 51 different gCJD) (). Majority (86.75%, 1375 cases) of the reported cases came from the surveillance provinces, while 13.25% (210 cases) were reported from non-surveillance provinces. Either the numbers and rates of reporting cases from non-surveillance provinces or the numbers of non-surveillance provinces that reported suspected cases increased along with the surveillance years ().
FIGURE 1. The referring cases in CJD surveillance system from 2010 to 2015. A. Distributions of various human PrDs and non-CJD cases among the 6 surveillance years. def-sCJD: definite sCJD; pro sCJD: probable sCJD; pos sCJD: possible sCJD. B. The numbers of the referring cases reported via surveillance provinces (sur-prov) and non-surveillance provinces (non sur-prov) among the 6 surveillance years. Percentage of non sur-porv represents the percentages of the referring cases reported by non surveillance provinces in all referring cases.
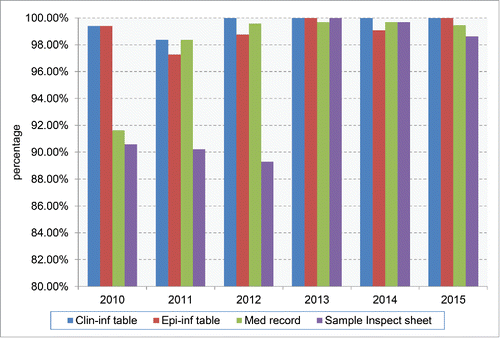
Collection of the Information of the Suspected Patients from the Reporting Units
In practice, the clinical data and specimens of the suspected patients were collected by the clinician from hospitals, while the epidemiological data were collected by the staff of provincial CDCs. The collected data and sample were referred to the national reference laboratory for human prion disease in China CDC for laboratory tests and final diagnosis. Several tables or sheets were designed for the information collection. Clinical Information Table (Clin-inf table), which was filled by the clinician, contained the data of the reporting unit, the general information of the patients, the onset time and symptoms, the major clinical manifestations, the death time (if it had) and the results of clinical examinations (EEG and MRI). Epidemiological Information table (Epi-inf table) contained the data of reporting unit, the permanent residence of the patients, the family disease history, the past medical history (such as neurosurgical operation, organ transplantation, usages of extracted hormones and blood transfusion), special profession (such as medical or veterinary staff, butcher). Specimen inspection sheet covered the information of the specimens, such as the sampling time, kinds and amounts of the samples (including CSF, blood, brain or other tissues if it is possible). In addition, the medical record, sometimes the graphs of EEG and MRI, of the suspected CJD patient was also asked to be provided.
As shown in , the integrity of the documents for each reporting case maintained at high level in the past surveillance years. Both Clin-inf tables that were filled and uploaded by local physicians and Epi-inf tables that were filled and uploaded by local CDC staffs were obtained close to 100%, especially in the past 3 y. Medical records of the suspected cases were also copied and submitted at high level (from 91.62% in 2010 to more than 99% in the past 4 y). The inspection sheets of the collected CSF and/or blood samples of the suspected CJD cases were obtained from 95.84% cases, increasing from about 90% in the first 3 y to more than 99% in the last 3 y.
Performance of Clinical Examinations and Laboratory Tests
According to the diagnostic criteria, the diagnoses of probable and definite PrD cases need further clinical examinations and laboratory tests.Citation20 For the clinical examinations, 93.82% (1497 out of 1585) of the reporting cases relieved MRI scanning at least one time during the hospitalizations. The examining rates of MRI increased from roughly 90% in 2010 and 2011 to more than 94% in the past 4 y (). Average 85.23% (1351 out of 1585) cases had EEG examinations. However, the examining rates of EEG decreased year by year, from 89.14% in 2010 to 81.15% in 2015 ().
FIGURE 3. The performing percentages of the clinical examinations and laboratory tests of the referring cases among the 6 surveillance years. A. MRI and EEG. B. CSF 14-3-3 and PRNP sequencing.
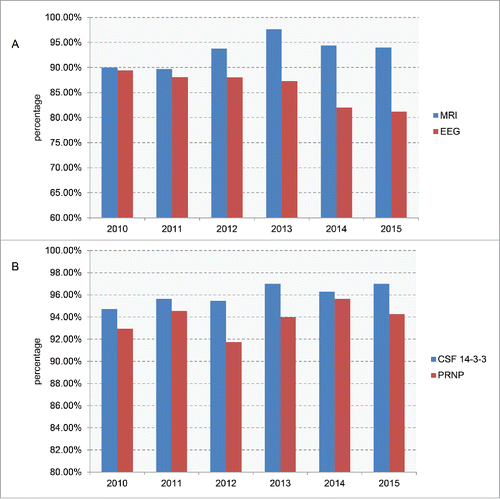
The enrolled laboratory tests in CJD surveillance currently included Western blot for CSF protein 14-3-3, PRNP PCR and sequencing based on blood sample, neuropathology, PrPSc specific Western blot and immunohistochemistry (IHC) based on brain tissues. The standard operation protocol (SOP) for those tests were described previously and issued by China CDC. 96.21% (1525/1585) cases underwent CSF 14-3-3 tests, varying from 94.70% to 96.99% in the 6 surveillance years, while 94.01% (1490/1585) cases had PRNP sequencing data ranging from 91.73% to 95.65% (). Only two cases in the past 6 surveillance years underwent brain postmortem, which let us conduct neuropathological and PrPSc assays.
Follow-Up Survey, Preparation of the Master Table of the Patient and Feed-Back of the Recommendation for Diagnosis and Handling
All data of a suspected case, including the clinical and epidemiological information, the results of clinical examinations and laboratory tests, were summarized and prepared as master table for this patient. During this process, some information was usually updated and amended, according to the review of the medical record and/or follow-up survey. The suggestion for diagnosis and further handling each reporting case was made by the center of CJD surveillance and feedback to the reporting local CDC and hospital.
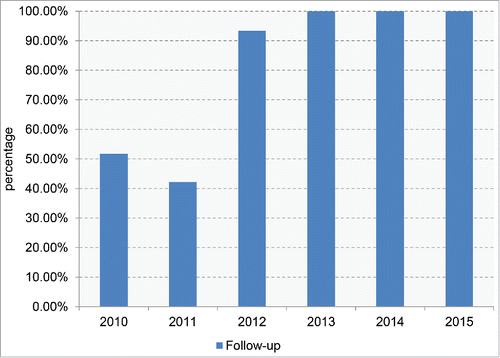
Follow-up survey was conducted either the staffs from China CDC or from local provincial CDCs. The interviewee was either the family member or the physician for confirmation of main items or disease development. The follow-up results were noted in the follow-up sheet. The patients who had at least one time telephone-interview or face to face interview were considered to be positive in follow-up survey. As shown in , the follow-up rates were about 50% in 2010 and 2011, increased to 93% in 2012 and reached to 100% in the last 3 y. The master table of the reporting patient was prepared by the staff in China CDC and revised after the follow-up survey. Review of the master tables of all referring cases in the past 6 surveillance years illustrated that almost all items were completed, except 3 cases in 2010 lacking of the onset times and one lacking of initial symptoms.
FIGURE 4. The conducting rates of follow-up survey for the referring cases among the 6 surveillance years.
After comprehensive analysis of the clinical, epidemiological and laboratory data of each reporting case, the center of CJD surveillance gave a written suggestion of diagnosis to the reporting units (local CDC and/or hospital). The types of the suggested diagnosis included definite, probable, possible PrD (sCJD, gCJD, FFI, GSS), not suggested as CJD (non-CJD) and not supported as CJD for the moment (pending case). Meanwhile, a written recommendation for further follow-up and daily handling of the diagnosed PrD case was given to the physician in change and/or the family members. Any diagnostic correction or revision of the referring cases based on the follow-up survey, especially when the patients discharged from the original reporting hospitals, was promptly feed-back to the reporting units.
Discussion
In this study, we have verified very high obtaining rates of 3 information sheets (Clin-Inf table, Epi-Inf table and Specimen inspection sheet) of the referring cases in the past 6 years, which are required to be uploaded from the reporting units to the center of CJD surveillance in China CDC based on the document of CJD surveillance. The supplying rates of the medical records of the reporting cases are also very high. It reflects a good implemental capacity in our CJD surveillance system, which supplies solid basis for PrDs recognition and diagnosis.
The performance rates of the clinical examinations maintain at high levels in the past 6 surveillance years, especially MRI scanning that has been brought into the diagnostic criteria for probable sCJD since 2012. We have also noticed that the rates of EEG decline along with years, which is possibly associated with the more accessible MRI scanning in clinical. High sampling rates for CSF and blood let more than 94% and 92% referring patients be conducted with CSF 14-3-3 assay and PRNP sequencing. No doubt, performances of those assays increase the reliability and accuracy of the diagnosis of PrDs, especially probable sCJD. Obtaining of the data of PRNP sequences of the overwhelming majority of the referring cases let us efficiently avoid of misdiagnosis of genetic PrDs, especially for those without clear disease associated family histories. In the past few years, RT-QuIC assay of CSF has been used in some European countries, USA and Japan, showing desired sensitivity and specificity for sCJD. Such method has been recently established in our laboratory and the clinical trial is being conducted (Xiao et al, in preparation). It is undoubted that RT-QuIC will benefit greatly to the CJD surveillance system in China where the rates of brain biopsy and autopsy are extremely low.
We believe that continue follow-up survey is absolutely benefit to our CJD surveillance. Actually, numbers of the required items for the information of the reporting cases have been replenished, amended and revised during the follow-up processes. Revision of the diagnoses, particularly for the group of “pending case,” largely depends on follow-up survey. In practice, due to the limited time for feed-back, some numbers of the patients are considered as pending cases for uncertain of clinical signs and/or clinical examinations.
Many other issues also contribute to the quality of our CJD surveillance. Among them, knowledge education and technique training are particularly important. We have conducted CJD surveillance annual meeting each year and CJD laboratory technique training course every 2 y for the staffs from local units in the surveillance provinces since 2006, and expended to the staffs from the provinces officially not belonging to our CJD surveillance network yet in the past 2 y. As a positive response, both the numbers referring cases and reporting provinces show constantly increase in the past years.Citation17-18 As a rare disease, CJD or other subtypes of human PrDs is still not well recognized in China yet. Brain postmortem rate in China maintains extremely low, probably related with the tradition. Hence, comprehensive obtaining of the clinical and epidemiological data, conducting of the relevant clinical examinations and laboratory tests, performing of follow-up survey for every referring case are particularly important for the Chinese CJD surveillance system.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
ACKNOWLEDGMENTS
We appreciate all staffs in Chinese CJD surveillance system for their great work. We also thank all referring patients and their family members for supplying the required information and donating specimens.
FUNDING
This work was supported by Chinese National Natural Science Foundation Grants (81301429, 81572048) and SKLID Development Grant (2012SKLID102, 2015SKLID503).
REFERENCES
- Chen C, Dong XP. Epidemiological characteristics of human prion diseases. Infect Dis Poverty 2016; 5:47; PMID:27251305; http://dx.doi.org/10.1186/s40249-016-0143-8
- Prusiner SB. The prion diseases. Brain Pathol 1998; 8:499-513; PMID:9669700; http://dx.doi.org/10.1111/j.1750-3639.1998.tb00171.x
- Puoti G, Bizzi A, Forloni G, Safar JG, Tagliavini F, Gambetti P. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol 2012; 11:618-28; PMID:22710755; http://dx.doi.org/10.1016/S1474-4422(12)70063-7
- Budka H, Will RG. The end of the BSE saga: do we still need surveillance for human prion diseases? Swiss Med Wkly 2015; 145:w14212
- Centers for Disease Control and Prevention (CDC). Surveillance for Creutzfeldt-Jakob disease–United States. MMWR Morb Mortal Wkly Rep 1996; 45:665-8; PMID:8769655
- Cousens SN, Zeidler M, Esmonde TF, De Silva R, Wilesmith JW, Smith PG, Will RG. Sporadic Creutzfeldt-Jakob disease in the United Kingdom: analysis of epidemiological surveillance data for 1970–96. BMJ 1997; 315:389-95; PMID:9277601; http://dx.doi.org/10.1136/bmj.315.7105.389
- Zerr I, Brandel JP, Masullo C, Wientjens D, de Silva R, Zeidler M, Granieri E, Sampaolo S, van Duijn C, Delasnerie-Lauprêtre N, et al. European surveillance on Creutzfeldt-Jakob disease: a case-control study for medical risk factors. J Clin Epidemiol 2000; 53:747-54; PMID:10941953; http://dx.doi.org/10.1016/S0895-4356(99)00207-3
- Lu CJ, Sun Y, Chen SS. Incidence of Creutzfeldt-Jakob disease in Taiwan: a prospective 10-year surveillance. Eur J Epidemiol 2010; 25:341-7; PMID:20333444; http://dx.doi.org/10.1007/s10654-010-9446-4
- Litzroth A, Cras P, De Vil B, Quoilin S. Overview and evaluation of 15 years of Creutzfeldt-Jakob disease surveillance in Belgium, 1998–2012. BMC Neurol 2015; 15:250; PMID:26630984; http://dx.doi.org/10.1186/s12883-015-0507-x
- Stratton E. Surveillance for Creutzfeldt-Jakob disease in Canada. Can Commun Dis Rep 1999; 25:7-8; PMID:9926624
- Heinemann U, Krasnianski A, Meissner B, Varges D, Kallenberg K, Schulz-Schaeffer WJ, Steinhoff BJ, Grasbon-Frodl EM, Kretzschmar HA, Zerr I. Creutzfeldt-Jakob disease in Germany: a prospective 12-year surveillance. Brain 2007; 130:1350-9; PMID:17472986; http://dx.doi.org/10.1093/brain/awm063
- Lim JS, Kwon HM, Jang JW, Ju YR, Kim S, Park YH, Park SY, Kim S. Characteristics of Korean patients with suspected Creutzfeldt-Jakob disease with 14-3-3 protein in cerebrospinal fluid: Preliminary study of the Korean Creutzfeldt-Jakob disease active surveillance program. Prion 2015; 9:136-43; PMID:25996401; http://dx.doi.org/10.1080/19336896.2015.1022020
- Zerr I, Poser S. Clinical diagnosis and differential diagnosis of CJD and vCJD. With special emphasis on laboratory tests. APMIS 2002; 110:88-98; PMID:12064260; http://dx.doi.org/10.1034/j.1600-0463.2002.100111.x
- Wood H. Prion disease: New approaches to CJD diagnosis. Nat Rev Neurol 2012; 8:241; PMID:22487751; http://dx.doi.org/10.1038/nrneurol.2012.59
- Shi Q, Gao C, Zhou W, Zhang BY, Chen JM, Tian C, Jiang HY, Han J, Xiang NJ, Wang XF, et al. Surveillance for Creutzfeldt-Jakob disease in China from 2006 to 2007. BMC Public Health 2008; 8:360; PMID:18928564; http://dx.doi.org/10.1186/1471-2458-8-360
- Gao C, Shi Q, Tian C, Chen C, Han J, Zhou W, Zhang BY, Jiang HY, Zhang J, Dong XP. The epidemiological, clinical, and laboratory features of sporadic Creutzfeldt-Jakob disease patients in China: surveillance data from 2006 to 2010. PLoS One 2011; 6:e24231; PMID:21904617; http://dx.doi.org/10.1371/journal.pone.0024231
- Shi Q, Zhou W, Chen C, Zhang BY, Xiao K, Zhang XC, Shen XJ, Li Q, Deng LQ, Dong JH, et al. The Features of Genetic Prion Diseases Based on Chinese Surveillance Program. PLoS One 2015; 10:e0139552
- Shi Q, Zhang XC, Zhou W, Xiao K, Chen C, Zhang HY, Sun JY, Chen LN, Zhang XM, Han J, et al. Analysis of the advantage features of Beijing surveillance network for Creutzfeldt-Jakob disease. Prion 2015; 9:304-14; PMID:26251963; http://dx.doi.org/10.1080/19336896.2015.1075115
- Budka H, Aguzzi A, Brown P, Brucher JM, Bugiani O, Gullotta F, Haltia M, Hauw JJ, Ironside JW, Jellinger K, et al. Neuropathological diagnostic criteria for Creutzfeldt-Jakob disease (CJD) and other human spongiform encephalopathies (prion diseases). Brain Pathol 1995; 5:459-66; PMID:8974629; http://dx.doi.org/10.1111/j.1750-3639.1995.tb00625.x
- Perry DC, Geschwind MD. Thorough work-up and new diagnostic criteria needed for CJD. Nat Rev Neurol 2011; 7:479-80; PMID:21892213; http://dx.doi.org/10.1038/nrneurol.2011.118