ABSTRACT
Here we report an autopsy-verified case of frontotemporal lobar degeneration (FTLD)-transactivation responsive region (TAR) DNA binding protein (TDP) type A with upper motor neuron-predominant motor neuron disease mimicking MM2-thalamic-type sporadic Creutzfeldt-Jakob disease (sCJD). A 69-year-old woman presented with an 11-month history of progressive dementia, irritability, insomnia, and gait disturbance without a family history of dementia or prion disease. Neurological examination revealed severe dementia, frontal signs, and exaggerated bilateral tendon reflexes. Periodic sharp-wave complexes were not observed on the electroencephalogram. Brain diffusion MRI did not reveal abnormal changes. An easy Z score (eZIS) analysis for 99mTc-ECD-single photon emission computed tomography (99mTc-ECD-SPECT) revealed a bilateral decrease in thalamic regional cerebral blood flow (rCBF). PRNP gene analysis demonstrated methionine homozygosity at codon 129 without mutation. Cerebrospinal fluid (CSF) analysis showed normal levels of both 14-3-3 and total tau proteins. Conversely, prion protein was slowly amplified in the CSF by a real-time quaking-induced conversion assay. Her symptoms deteriorated to a state of akinetic mutism, and she died of sudden cardiac arrest, one year after symptom onset.
Despite the SPECT results supporting a clinical diagnosis of MM2-thalamic-type sCJD, a postmortem assessment revealed that this was a case of FTLD-TDP type A, and excluded prion disease. Thus, this case indicates that whereas a bilateral decreasing thalamic rCBF detected by 99mTc-ECD-SPECT can be useful for diagnosing MM2-thalamic-type sCJD, it is not sufficiently specific. Postmortem diagnosis remains the gold standard for the diagnosis of this condition.
Introduction
Early clinical symptoms of sporadic Creutzfeldt-Jakob disease (sCJD) may overlap with those of other neurodegenerative disease like Alzheimer disease and frontotemporal lobar degeneration (FTLD)/ frontotemporal dementia (FTD).Citation1 In most of cases, it is relative straightforward to distinguish between FTD and sCJD by the clinical history, findings on MRI, cerebrospinal fluid (CSF) analyses, and PRNP gene analysis.
However, the diagnosis of MM2-thalamic-type sCJD, also known as sporadic fatal insomnia, is particularly challenging except in postmortem studies due to the lack of distinguishing laboratory or MRI characteristics.Citation2-4 Reportedly, the only supported diagnostic hallmarks among living patients are a bilateral decrease in thalamic cerebral metabolism detected by 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) Citation3 or in regional cerebral blood flow (rCBF) detected by single photon emission computed tomography (SPECT).Citation5,6 Here, we report on a case of autopsy-verified FTLD-transactivation responsive region (TAR) DNA binding protein (TDP) type A with upper motor neuron-predominant motor neuron disease mimicking MM2-thalamic-type sCJD on diagnostic imaging.
Methods and results
Patient Characteristics
A 69-year-old Japanese woman presented with an 11-month history of progressive dementia, irritability, sleep disturbance and gait disturbance, without a family history of prion disease or dementia. Especially, sleep disturbance was noted as follow: hypersomnia started 8 months, and insomnia started 10 months after disease onset, respectively. Neurological examination revealed severe dementia, frontal signs, and exaggerated bilateral tendon reflexes with Babinski sings. Muscle weakness, atrophy and fasciculation were not observed. Anti-serum thyroglobulin antibody levels were elevated. Clinically, a diagnosis of Hashimoto encephalitis/steroid responsive encephalitis was excluded because of steroid unresponsiveness.
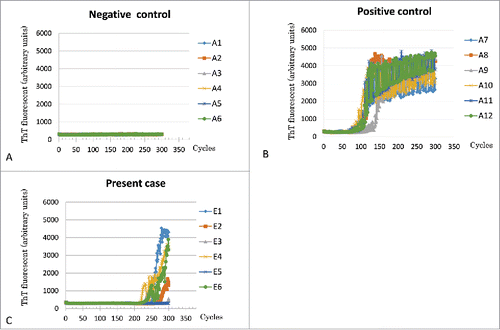
Periodic sharp-wave complexes were not observed on an electroencephalogram. Brain diffusion-weighted MRI (DW MRI) () did not reveal abnormal changes. An easy Z score (eZIS) analysis for 99mTc-ECD-SPECT images (Fujifilm RI Pharma, Tokyo, Japan) revealed decreased rCBF in the bilateral thalami, and punctate areas of decreased rCBF in the frontotemporal cortices () 12 months after symptom onset. Compared with scans performed 5 months after symptom onset, T1-weighted MRI at 12 months showed progression of temporal and mild frontal lobe atrophy (). PRNP gene analysis of peripheral blood DNA revealed methionine homozygosity at codon 129 without mutation. In the CSF analysis, 14-3-3 and total tau protein levels were <500 μg/ml, and 226 pg/ml, respectively. Conversely, prion protein (PrP) was slowly amplified in the CSF by a real-time quaking-induced conversion (RT-QUIC) assayCitation7 (). Her symptoms gradually deteriorated, and she died as a result of sudden cardiac arrest, almost one year after symptom onset. The clinical diagnosis was MM2-thalami-type sCJD.
Figure 1. Diffusion weighted MRI and eZIS analysis of 99mTc-ECD SPECT images. Diffusion-weighted MRI of the brain 10 months after the onset of symptoms (A), and easy Z score (eZIS) analysis of 99mTc-ECD-single photon emission computed tomography (99mTc-ECD-SPECT) images 12 months after onset (B). The eZIS analysis of 99mTc-ECD-SPECT images revealed decreased regional cerebral blood flows (rCBF) in the thalami (arrow). Lanes (a, c, and e) of panel (B) show fusion images of MRI and 99mTc-ECD-SPECT Z score images. A higher Z-score indicates lower rCBF (Z>2.0 means significantly decreased rCBF compared with normal controls. The Z-score scale of 2 to 6 is indicated by the green to red (lower rCBF) color gradient. Lanes (b, d, and f) show plain SPECT images. The red color in the plain SPECT images indicates higher rCBF; the blue color indicates lower rCBF.
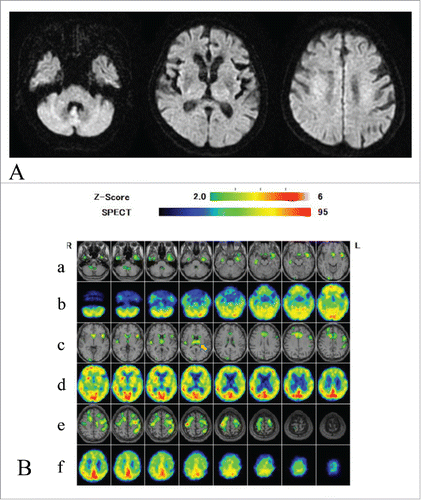
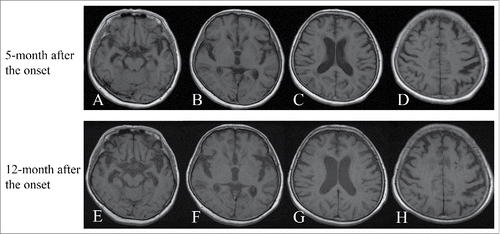
Figure 2. T1-weighted MRI 5 months and 12 months after onset. Panel A-(H)show serial T1-weighted MR images. Serial T1-weighted MR images at 5 months (upper lane, panels A-D) and 12 months (lower lane, panels E-H) after onset showed gradually progressing frontotemporal cortical atrophy, predominantly in the temporal lobes.
Neuropathological Analysis
A postmortem study was performed 62 hours after death with informed consent from her family. The brain and spinal cord were fixed in 20% neutral-buffered formalin for 2 weeks, and tissue blocks were immersed in 95% formic acid for 1 hour to inactivate prion infectivity. For routine neuropathological examinations, sections were subjected to hematoxylin-eosin, Kluver-Barrera’s and Gallyas-Braak staining. Immunohistochemical analyses were performed with the monoclonal antibody (3F4) against PrP (1:100; Dako, Glostrup, Denmark) after hydrolytic autoclaving for antigen retrieval.Citation8 Immunostaining for PrP was conducted as previously described using an EnVision Plus Kit (Dako).Citation9,10 Immunostaining using antibodies against anti-phosphorylated TDP-43 (pTDP-43 ser 409/410; 1:2,500; Cosmo Bio, Tokyo, Japan) were also performed, as appropriate. Primary antibody binding was detected by the labeled streptavidin-biotin method (LSAB kit; Dako). Peroxidase-conjugated streptavidin was visualized with 3, 3’-diaminobenzidine (DAB) (Wako Pure Chemical Industries, Osaka, Japan). Immunostained sections were lightly counterstained with Mayer’s hematoxyline.
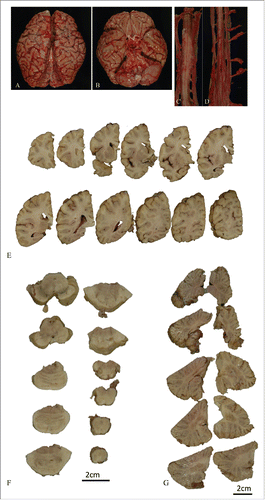
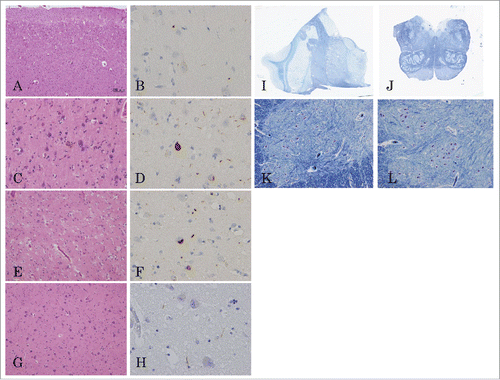
The patient’s brain weighed 1,255g. Macroscopic analysis showed atrophy in the frontal and temporal lobes, predominantly in the left temporal lobe () and slightly atrophic in the anterior roots of the spinal cord (). In serial coronal sections of the left hemisphere, temporal and mild frontal cortical atrophy were evident (). Atrophy of the subiculum and parahippocampal gyrus was also observed. However, the basal ganglia and thalamus, inferior olivary nuclei, and cerebellum were all well preserved ().
Figure 4 (see next page). Macroscopic findings from the brain and spinal cord. The whole brain (A, B), and cervical (C), and lumbar spinal cord (D) before fixation. Serial coronal sections of the left hemisphere (E), axial sections of brainstem (F) and cerebellum (G). Macroscopic analysis showed temporal and mild frontal lobe atrophy, predominantly in the left temporal lobe (A, B). The spinal anterior root was atrophic (C, D). In the coronal serial sections of left hemisphere, temporal and mild frontal cortical atrophy were evident (E). The inferior olivary nuclei and the cerebellum were not atrophic (F, G).
Microscopic analysis showed cerebral spongiform changes and gliosis in cortical surface layers of the frontotemporal lobes () and insula. The precentral gyrus showed reduced numbers of Betz giant cells; the remaining cells were deep-stained and atrophied, and macrophages were observed (). Neuronal loss and gliosis were also observed in the subiculum. Mild neuronal loss and gliosis were also observed in the amygdala (). Large numbers of macrophages were present in the posterior limb of the internal capsule. However, the number of neuron was comparatively preserved in the basal ganglia and thalamus, and brainstem, including the substantia nigra, locus ceruleus, inferior olivary nuclei, and dorsal vagus nuclei (). In spinal cord sections, neurons in the anterior horn were well preserved (); however, the remaining neurons were deeply stained, and lipofuscin-rich neurons were increased. Spheroids and mild gliosis were also observed. Bunina bodies were not observed in the spinal anterior horn. pTDP-43-positive structures characterized by numerous short dystrophic neurites and crescentic or oval neuronal cytoplasmic inclusions were found in the frontotemporal cortices, the amygdala, and the dentate gyrus of the hippocampus (). PrP immunostaining did not reveal obvious PrP deposits in the central nervous system (data not shown). The pathological diagnosis was FTLD-TDP type A.
Figure 5. Microscopic findings from the brain and spinal cord. Tissue sections stained with hematoxyline-eosin (A, C, E, G), pTDP-43 (B, D, F, H), and Kluver-Barrera’s stains (I, J, K, L). Frontal lobe (A, B); precentral gyrus (C, D); amygdala (E, F); thalamus (G, H); coronal section of the thalamus (I); axial section of the medulla (J); C7 anterior horn (K); L4 anterior horn (L). Microscopic analysis showed cerebral spongiform degeneration and gliosis in cortical surface layers of the frontotemporal lobes (A, B). The precentral gyrus exhibited reduced numbers of Betz giant cells, atrophying surviving cells, and the presence of macrophages (C, D). The amygdala showed only mild neuronal loss and gliosis (E, F) and the neurons in the thalamus, brainstem, and anterior horn of the spinal cord were well preserved (G-L). pTDP-43 positive structures characterized by short dystrophic neurites and crescentic or oval neuronal cytoplasmic inclusions were found in the frontotemporal cortices, amygdala, and the dentate gyrus of the hippocampus (B, D, F, H).
Western Blot Analysis of the Protease Resistant PrP
The cryopreserved right frontal lobe and thalamic sections were homogenized, and western blot analysis of protease resistant PrP was performed using 3F4, as previous described.Citation9 The analysis showed no PrP positive band ().
Figure 6. Western blot analyses of frozen brain extracts using 3F4 antibodies. Lane a: a sample from the present case (the frontal lobe); Lane b: a sample from the present case (the thalamus); Lane c: a control sample from a case of MM2-thalamic-type sporadic Creutzfeldt-Jakob disease (sCJD) case (the thalamus); Lane d: a control sample of MM1-type sCJD case (the frontal lobe).

Discussion
In this case, findings on SPECT supported a clinical diagnosis of MM2-thalamic-type-sCJD. In addition, PrP protein was slowly amplified in the CSF by RT-QUIC. However, a postmortem assessment revealed that this was a case of FTLD-TDP type A with upper motor neuron-predominant motor neuron disease following the harmonized classification system for FTLD-TDP pathology.Citation11
Consequently, this case presented false positive result of RT-QUIC. A sensitivity and a specificity of RT-QUIC for diagnosis of CJD were reported to be 85% and 99%, respectively.Citation18 However, 2 potentially false positive cases were also observed; one sample is from a clinically possible Alzheimer disease case, the other is from a patient presenting convulsion with elevated both tau and 14-3-3 protein levels in the CSF, and cortical hyperintensities in MRI.Citation18 Moreover, false positive cases were also reported in a few patients with encephalopathy after convulsion in Japanese study.Citation19 The mechanisms of presenting result of false positive remain unknown. According to the current postmortem study, subclinical or clinical prion diseases were not existed at least in her brain.
Although high sensitivity and specificity of RT-QUIC for typical sCJD were reported, it remains difficult for MM2-thalamic-type sCJD cases to detect abnormal PrP in the CSFCitation6 because of very small amount of abnormal PrP in the affected brain.Citation20
Additionally, most patients with FTD usually show decreased rCBF in the frontotemporal lobes and normal thalamic rCBF in SPECT analysis,Citation12 except for severe thalamic involvement case.Citation13 However, the thalamus has massive connections with the frontal lobe, the limbic system, and the activating reticular formation of the brainstem.Citation13 The dorsal thalamus is also connected with all brain cortices mutually by the thalamocortical circuits. Therefore, remote effects have sometimes appeared in SPECT or 18F-FDG-PET studies. The two major remote effects include crossed cerebellar diaschisisCitation14, and the cerebral cortices-thalamus remote effect.Citation15-17 The cerebral cortices-thalamus remote effect arises from the extensive thalamocortical interconnections and is mainly reported in patients with thalamic infarction. Remote effects have been described extending both from thalamic lesions to the cortices and from cortical lesions to the thalami. Some Alzheimer disease cases exhibit decreasing thalamic rCBF in SPECT studiesCitation17 and it is difficult to distinguish direct or remote effects in cases when there are no obvious lesions in the thalamus or brain cortices by brain MRI.
Our case showed decreased thalamic rCBF in an eZIS analysis in 99mTc-ECD-SPECT, thus mimicking MM2-thalamic-type sCJD.Citation5,6 However, pathological analyses showed very mild pathological neuronal changes in the thalami. Therefore, the bilateral decrease in thalamic rCBF on 99mTc-ECD-SPECT in our case was concluded to represent cortical-thalamus remote effects from frontotemporal lesions to the thalamus.
In general, MM2-thalamic-type sCJD shows no abnormal signal changes in DW MRI for both the brain cortices and the thalami. Pure MM2-thalamic-type sCJD cases, except for MM2-cortical combined cases, do not show any abnormal cortical atrophy in brain MRI in the early phase. However, FTD usually shows progressed frontotemporal lobe atrophy without thalamic signal changes in MRI. Serial MRI studies are also important for clinical diagnosis of FTD. Our data indicate that a bilateral decrease in thalamic rCBF in SPECT study should be interpreted carefully, and remote effects from the cortices should be considered. Combined MRI and SPECT analyses may help distinguish between FTD and MM2-thalamic-type sCJD.
A bilateral decrease in thalamic rCBF detected by SPECT may be diagnostically useful.Citation5,6 However, it is not sufficiently specific to diagnose MM2-thalamic-type sCJD, and postmortem diagnosis remains the gold standard for the diagnosis of this condition.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
FUNDING
This work was supported by grants-in-aid from the Research Committee of Prion Disease and Slow Virus infection (for Y.I., T.K., and K.S), and from the Research Committee of Prion Disease Surveillance (for T.K., K.S., and T.I.), the Ministry of Health, Labour and Welfare of Japan.
REFERENCES
- Riemenschneider M, Wagenpfeil S, Vanderstichele H, Otto M, Wiltfang J, Kretzschmar H, Vanmechelen E, Förstl H, Kurz A. Phospho-tau/total tau ratio in cerebrospinal fluid discriminated Creutzfeldt-Jakob disease from other dementia. Mol Psychiatry 2003; 8:343-7; PMID:12660807; http://dx.doi.org/10.1038/sj.mp.4001220
- Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windl O, Zerr I, Budka H, Kopp N, Piccardo P, et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 1999; 46:224-33; PMID:10443888; http://dx.doi.org/10.1002/1531-8249(199908)46:2%3c224::AID-ANA12%3e3.0.CO;2-W
- Mastrianni JA, Nixon R, Layzer R, Telling GC, Hand D, DeArmond SJ, Prusiner SB. Prion protein conformation in a patient with sporadic fatal insomnia. N Engl J Med 1999; 340:1630-8; PMID:10341275; http://dx.doi.org/10.1056/NEJM199905273402104
- Gambetti P, Kong Q, Zou W, Parchi P, Chen SG. Sporadic and familial CJD: classification and characterization. Br Med Bull 2003; 66:213-9; PMID:14522861; http://dx.doi.org/10.1093/bmb/66.1.213
- Hamaguchi T, Kitamoto T, Sato T, Mizusawa H, Nakamura Y, Noguchi M, Furukawa Y, Ishida C, Kuji I, Mitani K, et al. Clinical diagnosis of MM2-type sporadic Creutzfeldt-Jakob disease. Neurology 2005; 64:643-8; PMID:15728285; http://dx.doi.org/10.1212/01.WNL.0000151847.57956.FA
- Hayashi Y, Iwasaki Y, Yoshikura N, Asano T, Hatano T, Tatsumi S, Satoh K, Kimura A, Kitamoto T, Yoshida M, et al. Decreased regional cerebral blood flow in the bilateral thalami and medulla oblongata determined by an easy Z-score (eZIS) analysis of 99mTc-ECD-SPECT images in a case of MM2-thalamic-type sporadic Creutzfeldt-Jakob disease. J Neurol Sci 2015; 358:447-52; PMID:26421831; http://dx.doi.org/10.1016/j.jns.2015.09.356
- Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, Ishibashi D, Matsubara T, Nakagaki T, Yamanaka H, Shirabe S, et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med 2011; 17:175-8; PMID:21278748; http://dx.doi.org/10.1038/nm.2294
- Kitamoto T, Shin RW, Doh-ura K, Tomokane N, Miyazono M, Muramoto T, Tateishi J. Abnormal isoform of prion proteins accumulates in the synaptic structures of the central nervous system in patients with Creutzfeldt-Jakob disease. Am J Pathol 1992; 140:1285-94; PMID:1351366
- Iwasaki Y, Hashizume Y, Yoshida M, Kitamoto T, Sobue G. Neuropathological characteristics of brainstem lesions in sporadic Creutzfeldt-Jakob disease. Acta Neuropathol 2005; 109:557-66; PMID:15933870; http://dx.doi.org/10.1007/s00401-005-0981-0
- Iwasaki Y, Mimuro M, Yoshida M, Hashizume Y, Kitamoto T, Sobue G. Clinicopathologic characteristics of five autopsied cases of dura mater-associated Creutzfeldt-Jakob disease. Neuropathology 2008; 28:51-61; PMID:18181835; http://dx.doi.org/10.1111/j.1440-1789.2007.00847.x
- Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 2011; 122:111-3; PMID:21644037; http://dx.doi.org/10.1007/s00401-011-0845-8
- Waragai M, Yamada T, Matsuda H. Evaluation of brain perfusion SPECT using an easy Z-score imaging system (eZIS) as an adjunct to early-diagnosis of neurodegenerative disease. J Neurol Sci 2007; 260:57-64; PMID:17493637; http://dx.doi.org/10.1016/j.jns.2007.03.027
- Radanovic M, Rosemberg S, Adas R, Miranda SC, Caramelli P, Caixeta L, Nitrini R. Frontotemporal dementia with severe thalamic involvement: a clinical and neuropathological study. Arq Neuropsyciatr 2003; 61:930-5; http://dx.doi.org/10.1590/S0004-282X2003000600008
- Pantano P, Lenzi GL, Guidetti B, Di Piero V, Gerundini P, Savi AR, Fazio F, Fieschi C. Crossed cerebellar diaschisis in patients with cerebral ischemia assessed by SPECT and 123I-HIPDM. Eur Neurol 1987; 27:142-8; PMID:3497808; http://dx.doi.org/10.1159/000116147
- Lee MS, Choi IS, Chung TS. Thalamic syndrome and cortical hypoperfusion on technetium-99m HM-PAO brain SPECT. Yonsei Med J 1989; 30:151-7; PMID:2800565; http://dx.doi.org/10.3349/ymj.1989.30.2.151
- Meguro K, Akanuma K, Ouchi Y, Meguro M, Nakamura K, Yamaguchi S. Vascular dementia with left thalamic infarction: neuropsychological and behavioral implications suggested by involvement of the thalamic nucleus and the remote effect on cerebral cortex. The Osaki-Tajiri project. Psychiatry Res 2013; 213:56-62; PMID:23693088; http://dx.doi.org/10.1016/j.pscychresns.2012.12.004
- Kobayashi S, Tateno M, Utsumi K, Takahashi A, Saitoh M, Morii H, Fujii K, Teraoka M. Quantitative analysis of brain perfusion SPECT in Alzheimer disease using a fully automated regional cerebral blood flow quantification software, 3DSRT. J Neurol Sci 2008; 264:27-33; PMID:17764699; http://dx.doi.org/10.1016/j.jns.2007.07.015
- Cramm M, Schmitz M, Karch A, Mitrova E, Kuhn F, Schroeder B, Raeber A, Varges D, Kim YS, Satoh K, et al. Stability and reproducibility underscore utility of RT-QuIC for diagnosis of Creutzfeldt-Jakob disease. Mol Neurobiol 2016; 53:1896-904; PMID:25823511; http://dx.doi.org/10.1007/s12035-015-9133-2
- Satoh K. CSF analysis of patients with prion disease by biomarkers and real-time qucking induced conversion (RT-QUIC) method. Rinsho Shinkeigaku 2013; 53:1252-4; http://dx.doi.org/10.5692/clinicalneurol.53.1252
- Takatsuki H, Satoh K, Sano K, Fuse T, Nakagaki T, Mori T, Ishibashi D, Mihara B, Takao M, Iwasaki Y, et al. Rapid and quantitative assay of amyloid-seeding activity in human brains affected with prion diseases. PLoS One 2015; 10:e0126930; PMID:26070208; http://dx.doi.org/10.1371/journal.pone.0126930