ABSTRACT
A classical type of bovine spongiform encephalopathy (C-BSE), recognized in 1987, had a large impact on public health due to its zoonotic link to variant Creutzfeldt-Jakob disease by the human consumption of dietary products contaminated with the C-BSE prion. Thus, a number of countries implemented BSE surveillance using rapid post-mortem test kits that were approved for detection of the C-BSE prion in the cattle brain. However, as atypical BSE (L- and H-BSE) cases emerged in subsequent years, the efficacy of the kits for the detection of atypical BSE prions became a matter of concern. In response to this, laboratories in the European Union and Canada evaluated the kits used in their countries. Here, we carried out an evaluation study of NippiBL®, a kit currently used for BSE screening in Japan. By applying the kit to cattle brains of field cases of C-BSE and L-BSE, and an experimental case of H-BSE, we showed its comparable sensitivities to C, L-, and H-BSE prions, and satisfactory performance required by the European Food Safety Authority. In addition to NippiBL®, two kits (TeSeE® and FRELISA®) formerly used in Japan were effective for detection of the L-BSE prion, although the two kits were unable to be tested for the H-BSE prion due to the discontinuation of domestic sales during this study. These results indicate that BSE screening in Japan is as effective as those in other countries, and it is unlikely that cases of atypical BSE have been overlooked.
INTRODUCTION
Transmissible spongiform encephalopathies (TSEs) are fatal neurodegenerative disorders that cause neuronal cell death and spongiosis in the brain of several mammalian species. In human beings, TSEs emerge in such forms as Creutzfeldt-Jakob disease (CJD), Gerstmann-Sträussler-Scheinker syndrome, fatal familial insomnia, and kuru. The causative agent is considered to be solely protein, referred to as ‘prion’, whose major constitutes are disease-associated forms of prion protein (PrPSc, PrP refers to prion protein).Citation1,2 PrPSc is a conformational isoform of glycosylphosphatidylinositol-anchored, non-pathogenic cellular prion protein (PrPC) encoded by the host gene, and it is partially resistant to proteolytic digestion by proteinase K (PK).Citation2,3,4 A key event in prion propagation is the conversion of endogenous PrPC to PrPSc, and PrPSc accumulates in the central nervous system of patients and animals. The cycles of conversion are triggered by pre-existing PrPSc as seeds, where the seeds are initially acquired by unknown processes, due to mutation of the PrPC gene, or by the intake of external PrPSc. Accordingly, TSEs emerge as sporadic diseases of unspecified backgrounds, hereditary diseases, or infectious diseases.Citation4
Bovine spongiform encephalopathy (BSE) is a TSE of cattle. A classical type of BSE (C-BSE) was first reported in 1987 in the United Kingdom,Citation5 and its growing epidemic was recognized later in other countries by infection with the BSE prion through feeding contaminated meat-and-bone meal. Importantly, the epidemic of C-BSE posed an ensuing threat of zoonotic infection of humans after emergence of variant CJD cases in the 1990s, considered to be caused by the human consumption of beef products contaminated with the C-BSE prion.Citation6,7 This prompted a number of countries to implement protective measures including BSE surveillance using rapid post-mortem test kits. These kits are based on the enzyme-linked immunosorbent assay (ELISA), Western blot, or immunochromatography, which detect PrPSc accumulated in the medulla oblongata at the level of the obex in cattle brains after proteolytic digestion and elimination of PrPC, or using antibodies that specifically recognize the conformation(s) of PrPSc. The performance of these kits in detection of the C-BSE prion was evaluated and approved by the European Commission and European Food Safety Authority (EFSA).Citation8-10
Along with C-BSE, two novel atypical forms of BSE named H-type (H-BSE) and L-type BSE (L-BSE) were identified by the mid 2000s.Citation11,12 Cases of L- and H-BSE are less common than those of C-BSE, but they have been reported in several countries of the European Union (EU) as well as Japan, Canada, and United States of America.Citation11-25 Prions of atypical BSE are distinguished biochemically and pathologically from the C-BSE prion. The risk of transmission of atypical BSE prions to human beings is still under investigation. The L-BSE prion has been thought to be more virulent than the C-BSE prion in experimental transmission to non-human primates and transgenic mice expressing a human form of PrPC.Citation26-30 On the other hand, other studies showed inefficient transmission of the L-BSE prion to the transgenic mice, and inefficient in vitro conversion of a human form of PrPC by the L-BSE prionCitation31,32 So far, the H-BSE prion failed to be transmitted to the transgenic mice.Citation29,31 Nevertheless, it is sensible to determine if the rapid tests in current use are also valid for the atypical BSE prions. From this point of view, evaluation studies were carried out on seven tests used in EU countries and three tests used in the Canadian national BSE surveillance program.Citation33,34
In Japan, ELISA test kits such as ‘Platelia®’ and ‘TeSeE® BSE test kit’ (Bio-Rad Laboratories, Inc., Hercules, CA, USA), ‘FRELISA® BSE test kit’ (Fujirebio Inc., Tokyo, Japan), and ‘NippiBL® BSE test kit’ (Nippi Inc., Tokyo, Japan) were mainly used in the BSE screening, and NippiBL® is now the only available kit.Citation8-10,35-37 In harmonization with reports from EU and Canadian laboratories as described above, we carried out an evaluation study of NippiBL®, together with TeSeE® and FRELISA®, to assess their competence to detect atypical BSE prions.
RESULTS
Performance of the Kits to Detect the C-BSE Prion
In a similar way to the preceding evaluation studies by EU and Canadian laboratories, the present study was designed to examine the performance of rapid ELISA tests to detect atypical L- and H-BSE prions in comparison with C-BSE prion.Citation33,34 Hence, we began with reviews of the performance of three kits (NippiBL®, TeSeE®, and FRELISA®) using samples prepared from the brain of a C-BSE-affected cow, although the performances of these kits for detection of the C-BSE prion were already approved elsewhere.Citation8-10,38 We also included the BetaPrion® BSE test kit (Analytik Jena AG - AJ Roboscreen GmbH, Leipzig, Germany) as a reference kit to compare our results with those of EFSA and EU laboratories.Citation9,33
The EFSA has defined two sensitivity criteria: the ‘diagnostic sensitivity’ is the ability to recognize confirmed positive test samples as positive, while the ‘analytical sensitivity’ is a detection limit of positive samples serially diluted by negative brain tissues (i.e., the dilution limit for detection).Citation39 Consistent with the previous evaluation, all kits in the present study determined the brain samples positive for the C-BSE prion as positive.Citation8-10,33,35,38 Thus, the kits fulfilled the criteria of diagnostic sensitivity. In terms of the ‘analytical sensitivity’, shows signal response profiles of the kits using serially diluted positive samples (referred to here as dilution-response profiles). Among the four kits, BetaPrion® had the highest analytical sensitivity under our experimental conditions, achieving a detection limit at a 1:1,024 dilution (210 dilution) of the brain positive for the C-BSE prion ( and ). NippiBL®, whose detection limit was a 1:256 dilution (28 dilution), was the next after BetaPrion®. FRELISA® and TeSeE® (in the conventional assay protocol) followed in descending order (). In analysis by a four-parameter logistic model, the dilution-response profile of each kit was fitted to a regression curve with an adjusted R2 value higher than 0.938 ().
Figure 1. Dilution-response profiles of the kits using the brain homogenate of a C-BSE cow. Raw data were plotted as the mean ± SEM (standard error of the mean) from a set of triplicate wells. The dotted lines indicate thresholds of positivity defined by the manufacturers' protocols. Non-linear curve fitting was applied to the row data using a four-parameter logistic model. (A) TeSeE®, (B) FRELISA®, (C) NippiBL®, and (D) BetaPrion®.
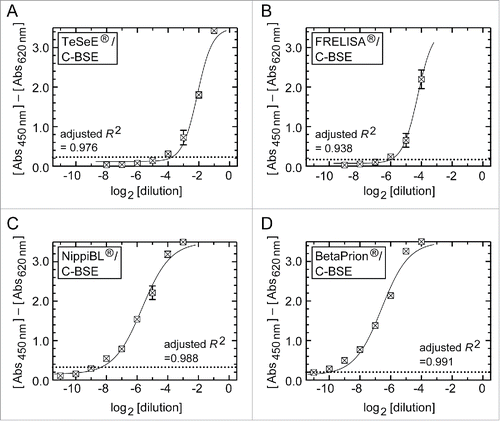
TABLE 1. Detection limits of the ELISA tests for C- and L-BSE samples.
Evaluation of the Kits for Atypical L- and H-BSE Prions
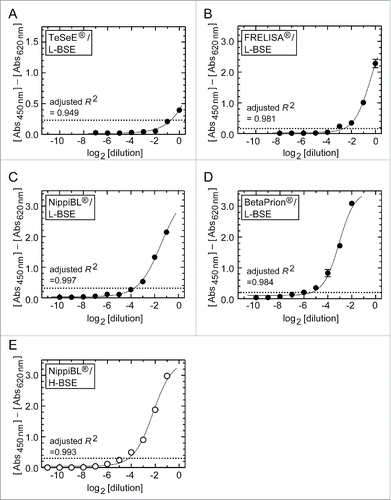
After reviewing the performances of the kits to detect the C-BSE prion, we then applied them to the brain tissues derived from an L-BSE affected cow (). The results are summarized in . All kits distinguished the samples positive for the L-BSE prion and the samples of normal brains, without false-negative or false-positive signals (). Among the kits, BetaPrion® showed the best analytical sensitivity by reaching a detection limit at a 1: 64 dilution (26 dilution) of the brain of the L-BSE cow (). Analytical sensitivities of NippiBL®, FRELISA®, and TeSeE® followed in the same descending order as determined for the C-BSE prion (). Next, we examined the performance of NippiBL® using the brain samples containing the H-BSE prion in a similar way, and obtained its detection limit at a 1: 16 dilution (24 dilution) of the brain (, and ). On four-parameter logistic model analysis, the dilution-response profiles of the kits for the brain samples of atypical L- and H-BSE cows were fitted to regression curves with adjusted R2 values higher than 0.949 ().
Figure 2. Dilution-response profiles of the kits using the brain homogenates of L- and H-BSE cows. Raw data were plotted as the mean ± SEM from a set of triplicate wells. The dotted lines indicate thresholds of positivity defined by the manufacturers' protocols. Non-linear curve fitting was applied to the row data using a four-parameter logistic model. (A – D) TeSeE®, FRELISA®, NippiBL®, and BetaPrion® tested on the samples prepared from the L-BSE cow, respectively. (E) NippiBL® tested on the samples prepared from the H-BSE cow.
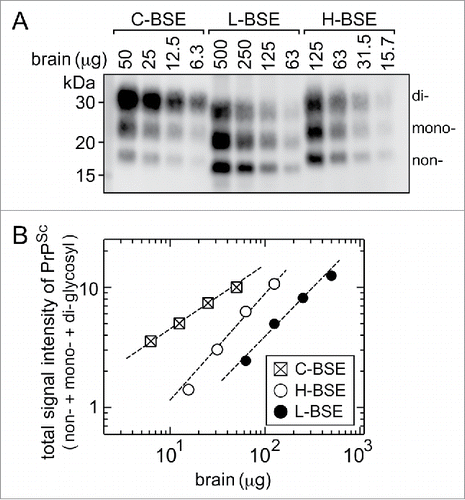
The positive samples in the present study were prepared by serial dilution of 40% (w/v) stock homogenates of the brains of the C-, L-, and H-BSE cows (see Materials and Methods), and the stock homogenates contained different concentrations of PrPSc from each other. To determine the relative concentration of PrPSc in the stock homogenates, the homogenates were digested by PK and subjected to Western blot analysis for quantification of PrPSc (). shows correlations between the amounts of brain tissues and total signal intensities of PrPSc, in which the total signal intensity of PrPSc represents a sum of the intensities of the non-, mono-, and di-glycosylated forms of PrPSc in . The analysis showed that comparable signal intensities of PrPSc were detected in the homogenates corresponding to 12.5 μg of brain tissue of the C-BSE cow, 100 to 200 μg of brain tissue of the L-BSE cow, and 40 to 50 μg of brain tissue of the H-BSE cow (). Accordingly, the relative concentration of PrPSc in the stock homogenates in the brains of C-, L-, and H-BSE cows were calculated to be approximately 24: 1: 21.5. In parallel, indicates that all kits showed a detection limit for the C-BSE sample 23 to 25-times higher than that for the L-BSE sample, and NippiBL® showed a detection limit for the H-BSE sample 21-times higher than that for L-BSE. The parallelism in the relative concentration and detection limits of PrPSc in the C-, L-, and H-BSE samples was an indication of the invariable reactivity of each kit to the three types of BSE prions, though the overall sensitivities were different among the kits.
Figure 3. Western blot analysis after PK digestion to determine the relative amounts of PrPSc in the stock homogenates of the brain. (A) The stock homogenates of the brains of the C-, L-, and H-BSE cows were digested by PK, and aliquots of volumes of the digests corresponding to the indicated weights of tissues were subjected to Western blot analysis. PrPSc was detected using anti-PrP antibody 12F10, with the aid of a chemluminescent detection reagent and a cooled CCD camera imaging system. The letters non-, mono-, and di- denote the non-, N-mono-, and N-di-glycosylated forms of PrPSc. (B) Signal intensities of the non-, mono-, and di-glycosylated forms of PrPSc in each lane in (A) were measured by ImageGuage software, combined as a total signal intensity of PrPSc, and plotted in relative magnitude by taking that of 50 μg of the C-BSE brain tissue as 10.0.
Assessment of the Digestion Condition of NippiBL®
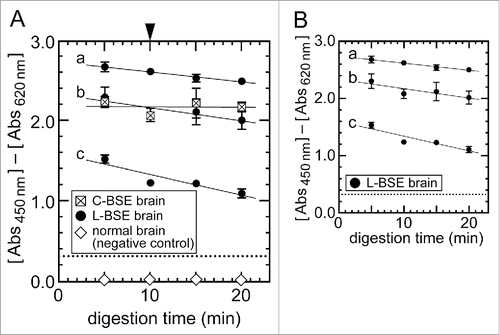
NippiBL® employs a unique protocol of treating the brain tissues with a mixture of protease at 56°C for 10 min for digestion before applying them to the ELISA assay, whereas the other kits in the present study digest the tissues by PK at 37°C.Citation35 Although NippiBL® possessed adequate analytical sensitivity for the detection of atypical BSE prions (, and ), the line of evidence that PrPSc of atypical BSE prions was less resistant to proteolytic digestion under stringent conditions prompted us to examine if the digestion condition of NippiBL® did not cause rapid or irregular decay of the signal intensities of PrPSc when applied to atypical BSE prions.Citation17,40,41 To achieve this, we carried out a time-course study in which the brain samples of the C- and L-BSE cows were processed according to the protocol of NippiBL® but with different duration of digestion for up to 20 min before applying the samples to the ELISA assay. As expected, the signal intensities obtained from the C-BSE brain samples remained at a stable level even when the samples were digested for 20 min (). Under the condition, the L-BSE samples (high, mid, and low concentrations of PrPSc) showed a gradual and time-dependent decrease of the signal intensities during the digestion (). However, the signals were sustained, and did not show a sudden fall or fluctuation that would potentially compromise the accuracy and reproducibility of the analysis. The narrow values of the standard error of the mean for the signal intensities of the triplicate L- and H-BSE samples in the dilution-response profiles () supported this observation.
Figure 4. Effect of the sample digestion condition of NippiBL® on detection of the L-BSE prion. (A) Brain samples of the C-BSE and L-BSE cows were digested according to the manufacturer's protocol but for an extended time. For the L-BSE brain, samples of three different dilutions by normal brains were tested (a: 2−0.6 dilution, b: 2−1 dilution, c: 2−2 dilution). The arrowhead at the top of (A) indicates the digestion time set by the protocol (10 min). Data were plotted as the mean ± SEM from duplicate wells. The dotted lines indicate the thresholds of positivity defined by the protocol. The samples of normal brain (i.e., negative control) gave rise to negative signals throughout the assay. (B) Only data on the brain samples of the L-BSE cow in (A) were plotted for clarity.
DISCUSSION
In addition to the prior approval of three rapid post-mortem BSE test kits used in Japan for detection of the C-BSE prion, we examined the analytical performance of the kits for the detection of atypical BSE prions. Among the three kits, TeSeE® and FRELISA® were used until 2014, and NippiBL® has been used since 2006 and is currently the only available kit.
Our study showed that the kits correctly judged the positive samples as positive, and the negative samples prepared from normal brains as negative (). Although testing a large number of independent samples was beyond the scope of the present study, the results fulfilled the ‘diagnostic sensitivity’ and ‘specificity of the tests’ that the European Commission and the EFSA have defined as the ability to determine specimens of true positive animals to be positive, and true negative animals to be negative.Citation8,39 With respect to the analytical sensitivity, the EFSA regulations require appropriate tests to be within a maximal 2 log10 inferiority range of the most sensitive test.Citation39 In this regard, IDEXX HerdChek® BSE-scrapie (IDEXX Laboratories, Inc., Maine, USA) is viewed currently as the most sensitive test.Citation9,33,34,38 Due to import regulations, we could not include IDEXX HerdChek® in the present study. Instead, based on the results of previous studies showing that TeSeE® (short protocol) and BetaPrion® satisfied the EFSA requirements for the detection of L- and H-BSE prions,Citation33,34 we considered that FRELISA® and NippiBL®, whose sensitivities were between TeSeE® and BetaPrion®, met the EFSA requirements. In fact, when examined using the brain samples of the L-BSE cow, the detection limit of NippiBL® was only 22 factors lower than that of BetaPrion® (, and ). Apart from the analytical sensitivity, it might be intriguing to consider how much tissue is required for a single well of the ELISA plates since the amount differs depending on the kits. In BetaPrion®, for example, a well contains the PK-digested sample corresponding to 28 mg of brain tissue. Thus, the detection limit of BetaPrion® for the authentic C-BSE brain tissue at a 1:1,024 dilution suggests that BetaPrion® can detect PrPSc in as little as 27 μg of the brain of the C-BSE cow used in the present study (). In NippiBL®, a digested sample corresponding to 10 mg of brain tissue is applied to a well; thus, the detection limit of NippiBL® at a 1:256 dilution was equivalent to the ability to detect PrPSc in 40 μg of the brain of the C-BSE cow (). Importantly, despite the kits showing different overall analytical sensitivities, each kit showed no preferential reactivity to a particular type of BSE prions; TeSeE® and FRELISA® had comparable reactivities to C- and L-BSE prions, and NippiBL® had comparable reactivity to C-, L-, and H-BSE prions.
The medulla oblongata at the level of the obex is specified as a general specimen for rapid post-mortem BSE tests.Citation39,42 The distribution and deposition of PrPSc vary in the regions of the brain.Citation41 For example, we found by Western blot analysis that the cerebral specimens of the L-BSE cow we used in the present study contained an approximately 3-fold lower amount of PrPSc than the medulla oblongata at the level of the obex of this cow (data not shown). Similar to the preceding evaluation studies, the test specimens in the present study were not prepared from the medulla oblongata at the level of the obex but from other regions of the brains of the C-, L-, and H-BSE cows (see Materials and Methods).Citation33,34,38 Despite the usage of specimens of the brain region not specified by the protocols, we think the results of the present study strongly support the eligibility and efficacy of the test kits for the purpose of BSE screening.
In the present study, the samples for TeSeE®, FRELISA® and BetaPrion® were prepared by mixing the stock homogenates of the brains of the BSE affected cows in saline and the brain homogenate of normal cows in saline. The mixtures were centrifuged to discard the supernatant, and the pellet fractions of tissues (1-volume) were added with 2-volumes of the 1x concentrated homogenization buffers supplied by the kits (see Materials and Methods). This protocol provided an advantage in accurate dilutions of PrPSc ranging from 20 to 211 in a specified volume of the pellet fractions, but it discarded soluble components of the tissues by centrifugation. Although we have not examined effects of the loss of soluble components on the performances of the kits, we conceived the ultimate goal of the present study was achieved because PrPSc was expected to be retrieved in the pellet fractions almost quantitatively.Citation43 Also, in comparison with the manufacturers' protocols in which the concentrations of the homogenization buffers after addition to the tissues are at 0.80 ± 0.02x, the above protocol brought the concentrations of the buffers after addition to the pellet fractions to 0.67x. However, this did not seem to affect the performance of the test kits, since the buffers are 5% glucose (TeSeE®) or 50 mM Tris buffer supplemented with collagenase and DNase (FRELISA®). After addition of the homogenization buffers, the samples were homogenized and digested by PK according to the manufacturers' protocols, so that the concentrations of PK and the other components such as urea and detergents were at the same as those indicated by the manufacturers. With regard to NippiBL®, the homogenization buffer contains Triton X-100 and urea, and the homogenization of the brain tissues is carried out in the buffer premixed with the proteases supplied with the kit. The manufacturer's protocol instructs that the concentration of the homogenization buffer and the proteases is at 0.90 ± 0.02x after addition to tissues.Citation35 In the present study, the concentrations of the buffer and the proteases after addition to the samples were as follows: 0.83x for the samples at a 2−1 dilution; 0.86x for the samples at a 2−2 dilution, 0.87x for the samples at a 2−3 dilution, 0.89x for the samples at 2−4 and 2−5 dilutions, and 0.90x for the samples at 2−6 to 2−11 dilutions.
An issue specific to NippiBL® is that it treats the brain tissues with a more aggressive digestion before applying them to the ELISA assay.Citation35 We showed that the digestion condition of NippiBL® did not compromise the accuracy of detecting atypical BSE prions (). Conceivably, PrPSc of atypical BSE prions is largely resistant to the digestion condition of NippiBL®, or PrPSc might be degraded to some extent but the degrading peptides retain the epitopes of the antibodies for effective detection. With regard to NippiBL®, we incidentally found that the freezing and thawing of brain tissues in the NippiBL® homogenization buffer significantly impaired the resistance of PrPSc to the proteases of the kit (data not shown), possibly due to the effect of components in the homogenization buffer such as Triton X-100 and urea.Citation35 Of course, the manufacturer's protocol does not instruct users to freeze brain specimens in the homogenization buffer prior to the digestion. This should be avoided.
In conclusion, the present study showed that NippiBL® is suitable for the detection of C-, L-, and H-BSE prions. Also, TeSeE® and FRELISA®, which were discontinued from use in Japan in 2014, were appropriate for the detection of C- and L-BSE prions. These results support the effectiveness of the current BSE surveillance program in Japan, and it is unlikely that cases of atypical BSE have been overlooked due to the test method being used.
MATERIALS AND METHODS
Brain Tissues
Brain tissues negative for the BSE prion (normal brain) were a pool of medulla oblongata tissues proximal to the level of the obex collected from four cows. These specimens were obtained from local abattoirs in Japan, and were determined to be negative for BSE by Western blot and histopathological analyses at the National Institute of Infectious Diseases.
Brain tissues positive for the C-BSE prion were from the thalamus of a field case of a C-BSE cow identified in Ireland (case number: H02-551). The specimen was provided by Prof. W. Hall (University College of Dublin, Ireland) after permission for import given by the Ministry of Agriculture, Forestry and Fisheries, Japan.
Brain tissues positive for the L-BSE prion were from the cerebrum of a field case of an L-BSE cow identified in Japan. The cow (case number: JP24) was positive for BSE by routine screening using the Platelia BSE kit at a local meat-inspection laboratory, and determined as an L-BSE case by confirmatory analysis at the National Institute of Infectious Diseases.Citation16 The DNA sequence of the PrP coding region of the cow had a synonymous codon of asparagine192 (AAT) compared with that of Bos taurus PrP in a public database (accession number: AJ298878, AAC for asparagine192).Citation16
Because no field case of H-BSE has been found to date in Japan, we utilized a cow experimentally infected with the H-BSE prion by intracranial administration (experimental code number: 9458).Citation44 Tissues positive for the H-BSE prion were from the brain stem. The DNA sequence of the PrP coding region of this cow was identical to that of Bos taurus PrP in the database (accession number: AJ298878).Citation44
Sample Preparation
Stock homogenates of 40% (w/v) brains of C-BSE, L-BSE, and H-BSE cows were prepared in saline (Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan). Homogenization was carried out by vigorous shaking of the brains with ceramic YTZ® balls (2.7-mm diameter beads, Nikkato Co., Osaka, Japan) at 2,500 rpm for 5 min in a Multi-beads shocker® tissue disruptor (Yasui Kikai Co., Osaka, Japan). Stock homogenate of the normal brain was prepared in a similar way at a final concentration of 20% (w/v). For NippiBL®, aliquot weights of tissues of normal brains were mashed according to the manufacturer's protocol. Due to the scarcity of brain specimens of atypical BSE cows, the stock homogenates were serially diluted as described below.
TeSeE®, FRELISA®, and BetaPrion®: The 40% stock brain homogenates of C-BSE and L-BSE cows were added to an equal volume of saline, then serially diluted to a 2 base logarithm up to 2−11 with 20% (w/v) normal brain homogenate to obtain 900 μL of 20% (w/v) brain homogenate (i.e., each sample contained 180 mg of brain tissues, in which the net amounts of the BSE-positive tissues were serially diluted). The samples were centrifuged at 19,000 xg at 4°C for 30 min, 600 μL of the supernatant was discarded, and the pellet fractions (equivalent to 180 mg of the brain per tube) were stored at −75°C until use. To obtain pellets of negative control tissues, aliquots of 900 μL of the 20% stock homogenate of the normal brain were centrifuged in the same way. Before the test, the pellet fractions were added to 600 μL of the homogenization buffer supplied by the kits to reconstitute 900 μL of 20% brain homogenate. The samples were then homogenized as instructed in the manufacturers' protocols by using the ceramic beads supplied with the kits of TeSeE® and BetaPrion®, or by using ceramic YTZ® balls (1.5-mm diameter beads, Nikkato Co.) for FRELISA®. The homogenates were dispensed in triplicate tubes by the volumes indicated in the manufacturers' protocols (i.e., dispense 250 μL per tube for TeSeE®, 250 μL for FRELISA®, and 200 μL for BetaPrion®) for digestion with PK, and the assay was performed according to the manufacturers' protocols.
NippiBL®: The kit employs a unique protocol for sample preparation. The dissected brain tissues are mashed through mesh-bottomed cups (BioMasher), the mashed tissues are then directly added to 9-volumes of homogenization buffer supplemented with the proteases of the kit, and disrupted by ceramic beads to prepare 10% homogenate which is ready for digestion at 56°C. 35 To adapt to the protocol, test samples were prepared in the following way to contain 70 mg of the total brain tissues but serially diluted amount of the BSE-positive tissues: a sample at a 2−1 dilution was prepared by the addition of mashed normal brain (35 mg) with 88 μL of the 40% homogenates of BSE-positive brains (equivalent to 35 mg tissue), a sample at a 2−2 dilution was prepared by the addition of mashed normal brain (52 mg) with 44 μL of the 40% homogenates of BSE-positive brains (equivalent to 18 mg tissue), and samples at 2−3 to 2−11 dilutions were prepared by the addition of 70 ± 5.6 mg (mean ± SD) of mashed normal brain with appropriate volumes of the 40% homogenates of BSE-positive brains. Pieces of 70 mg of the normal brains were mashed and used as negative controls. The samples were added to 9-volumes of the homogenizing buffer containing the proteases to adjust the tissue concentration to 10%. Then, as instructed by the manufacturer's protocol, the samples were homogenized by using the ceramic beads that were supplied with the kit, and subjected immediately to digestion.Citation35 After digestion, the samples were dispensed to the wells of the kit in triplicate, and the assay was performed according to the manufacturer's protocol.
Execution of the Test
In the present study, we examined one rapid test kit for either of the brain samples of C-, L-, or H-BSE cow per day, and did not carry out simultaneous examination of different kits or different brain samples on the same day. To minimize variability, only two operators participated in the assay. A Model 680 microplate reader (Bio-Rad Laboratories, Inc.) and an ARVO X4 microplate reader (PerkinElmer Inc., Waltham, MA, USA) were used to measure the absorbance indicated by the manufacturers' protocols (450 nm for reading; 620 nm for reference). All procedures were carried out according to the biosafety guidelines of the National Institute of Infectious Diseases, and the National Institute of Animal Health.
Data Analysis
True-positive and pseudo-positive thresholds were defined by the manufacturers' protocols. If more than two wells in the triplicate wells were positive or pseudo-positive, the overall result was judged as positive. The detection limit was defined as the maximum dilution factor where the overall result was positive. Fitting analysis by a four-parameter logistic model was carried out using Prism 6 software (GraphPad Software, Inc., La Jolla, CA, USA) with the constraint of the maximum absorbance being less than 3.5. Adjusted R-squared (adjusted R2) values were calculated by Prism 6 software using the following equation: Adjusted R2 = 1 - [SSresiduals/(n-K)]/[SStotal/(n-1)], where SSresiduals is the sum of squares of the difference of each point from the fitted-curve, SStotal is the square of the difference of the points from the mean of all absorbance, n is the number of data points, and K is the number of parameters fitted by the regression analysis.
Western Blot Analysis
The stock homogenates of the C-, L-, and H-BSE brains were diluted to 20% (w/v) with saline, and then 50 μL of the 20% (w/v) homogenates (equivalent to 10 mg of tissues) were added with an equal volume of a buffer consisting of 4% zwittergent® 3–14 (Merck Millipore, Darmstadt, Germany), 1% lauroylsarcosine sodium salt (Sigma-Aldrich, St. Louis, MO, USA), 100 mM NaCl, 50 mM Tris-HCl (pH 7.5). The samples were added to 0.625 μL of 80 mg/mL collagenase (Wako Pure Chemical Industries, Osaka, Japan), and incubated at 37°C for 30 min. After brief sonication, the samples were added to 1 μL of PK (at a final concentration of 50 μg/mL; Roche Diagnostics, Basel, Switzerland), and incubated at 37°C for 30 min. The digestion was stopped by the addition of 4-(2-aminoethyl)benzenesulfonyl fluoride at a final concentration of 2 mM (Roche Diagnostics). Following the addition of 50 μL of a mixture of 2-butanol and methanol (5/1, v/v), the samples were centrifuged at 18,000 xg for 10 min at 23°C. The pellet was dissolved in lithium dodecyl sulfate sample buffer (Thermo Fisher Scientific Inc., Novex™, Carlsbad, CA, USA) supplemented with 80 mM dithiothreitol, heated at 100°C for 5 min, and aliquots of the samples were subjected to gel electrophoresis using a NuPAGE® Novex™ 12% Bis-Tris gel (Thermo Fisher Scientific Inc., Invitrogen™) and NuPAGE® MOPS-sodium dodecyl sulfate running buffer (Thermo Fisher Scientific Inc., Novex™). After electrophoresis, proteins were transferred to an Immobilon-P PVDF membrane (Merck Millipore) at 220 mA for 60 min using Tris-glycine buffer (Bio-Rad Laboratories, Inc.) supplemented with 20% methanol. The membrane was incubated at 4°C overnight with anti-prion protein 12F10 antibody (epitope: G153SDYEDRYYRENMHRYPNQ171 of bovine PrP; Cayman Chemical, Ann Arbor, MI, USA) at 0.16 μg/mL in Can Get Signal®-1 immunoreaction enhancer solution (Toyobo Co., Ltd., Osaka, Japan).Citation45 After washing the membrane with 0.05% Tween 20 in phosphate-buffered saline, the membrane was incubated at room temperature for 2 h with horseradish peroxidase-conjugated AffiniPure F(ab')2 anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc., PA, USA) at 0.1 μg/mL in Can Get Signal®-2 solution (Toyobo Co., Ltd.). Detection was carried out using SuperSignal™ West Dura Extended Duration Substrate (Thermo Fisher Scientific Inc., Thermo Scientific™) and a FluorChem IS-8044 imaging system (ProteinSimple, San Jose, CA, USA). Captured images were stored as TIFF files, and signal intensities were quantified by ImageGauge software (Fuji Photo Film, Tokyo, Japan).
Examination of the Effects of the Digestion Condition of NippiBL®
To examine the effects of the digestion condition of NippiBL® on stability of signal intensities of atypical BSE prions, a sample of the C-BSE cattle brain diluted to 2−5 by mashed negative brains, and three samples of the L-BSE cattle brain diluted to 2−0.6 (i.e., 1.5-fold), 2−1, and 2−2 by mashed negative brains were prepared using the method described above. These dilutions were chosen with the expectation of signal intensities (i.e., absorbance at 450 nm) between 1.0 and 3.0, based on the data of the dilution-response profiles of NippiBL® shown in . The samples were processed according to the protocol of NippiBL®, but by setting the digestion time at 5, 10, 15, and 20 min. Normal brain tissue was processed in the same way. After digestion, the samples were dispensed to the duplicate wells of the kit, and developed for detection according to the manufacturer's protocol.
ABBREVIATIONS
| BSE | = | bovine spongiform encephalopathy |
| CJD | = | Creutzfeldt-Jakob disease |
| EFSA | = | European Food Safety Authority |
| ELISA | = | enzyme-linked immunosorbent assay |
| PK | = | proteinase K |
| PrPC | = | cellular prion protein |
| PrPSc | = | disease-associated forms of prion protein |
| TSE | = | transmissible spongiform encephalopathy |
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
ACKNOWLEDGMENTS
The authors thank Dr. Tetsutaro Sata (former affiliation; National Institute of Infectious Diseases, Tokyo, Japan) and Prof. William. Hall (University College of Dublin, Dublin, Ireland) for providing us with specimens of the C-BSE cow, Drs. Hiroyuki Okada, Morikazu Imamura, Yuichi Matsuura, Kentaro Masujin, and Kohtaro Miyazawa (National Institute of Animal Health, Ibaraki, Japan) for specimens of the H-BSE cow. The specimens of the L-BSE cow were given to us by courtesy of the abattoir and Meat Inspection Center of Sasebo city (Nagasaki, Japan).16 We thank Dr. Kentaro Hanada (National Institute of Infectious Diseases, Tokyo, Japan) for advice regarding this study.
FUNDING
This study was supported by the Ministry of Health, Labor and Welfare, Japan under Grant H26-Shokuhin-Ippan-004. The funder did not participate in the study design, data collection and analysis, or preparation of the manuscript.
REFERENCES
- Griffith JS. Nature of the scrapie agent: self-replication and scrapie. Nature 1967; 215:1043-4; PMID:25790189; http://dx.doi.org/10.1038/2151043a0
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science 1982; 216:136-44; PMID:6801762; http://dx.doi.org/10.1126/science.6801762
- Chesebro B, Race R, Wehrly K, Nishio J, Bloom M, Lechner D, Bergstrom S, Robbins K, Mayer L, Keith JM, et al. Identification of scrapie prion protein-specific mRNA in scrapie-infected and uninfected brain. Nature 1985; 315:331-3; PMID:3923361; http://dx.doi.org/10.1038/315331a0
- Prusiner SB. Prion biology and disease. 2nd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2004.
- Wells GA, Scott AC, Johnson CT, Gunning RF, Hancock RD, Jeffrey M, Dawson M, Bradley R. A novel progressive spongiform encephalopathy in cattle. Vet Rec 1987; 121:419-20; PMID:3424605; http://dx.doi.org/10.1136/vr.121.18.419
- Collinge J, Sidle KCL, Meads J, Ironside J, Hill AF. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature 1996; 383:685-90; PMID:8878476; http://dx.doi.org/10.1038/383685a0
- Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, et al. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 1997; 389:498-501; PMID:9333239; http://dx.doi.org/10.1038/39057
- Moynagh J, Schimmel H. Tests for BSE evaluated. Nature 1999; 400:105; PMID:10408430; http://dx.doi.org/10.1038/21981
- European Food Safety Authority. EFSA scientific report on the evaluation of seven new rapid post mortem BSE tests. EFSA J 2004; 2:RN-18, 13. http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2004.18r/epdf
- European Food Safety Authority. Scientific report of the European Food Safety Authority on the evaluation of two rapid post mortem BSE tests. EFSA J 2005; 3: RN-48, 10. http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2005.48r/epdf
- Biacabe A-G, Laplanche J-L, Ryder S, Baron T. Distinct molecular phenotypes in bovine prion diseases. EMBO Rep 2004; 5:110-15; PMID:14710195; http://dx.doi.org/10.1038/sj.embor.7400054
- Casalone C, Zanusso G, Acutis P, Ferrari S, Capucci L, Tagliavini F, Monaco S, Caramelli M. Identification of a second bovine amyloidotic spongiform encephalopathy: Molecular similarities with sporadic Creutzfeldt-Jakob disease. Proc Natl Acad Sci USA 2004; 101:3065-70; PMID:14970340; http://dx.doi.org/10.1073/pnas.0305777101
- Yamakawa Y, Hagiwara K, Nohtomi K, Nakamura Y, Nishijima M, Higuchi Y, Sato Y, Sata T. Expert Committee for BSE Diagnosis, Ministry of Health, Labour and Welfare of Japan. Atypical proteinase K-resistant prion protein (PrPres) observed in an apparently healthy 23-month-old Holstein steer. Jpn J Infect Dis 2003; 56:221-2; PMID:14695437
- De Bosschere H, Roels S, Vanopdenbosch E. Atypical case of bovine spongiform encephalopathy in an east-flemish cow in Belgium. J Appl Res Vet Med 2004; 2:52-4. [accessed 2016 Dec 14]. http://www.jarvm.com/articles/Vol2Iss1/DEBOSSCHERE.htm
- Buschmann A, Gretzschel A, Biacabe A-G, Schiebel K, Corona C, Hoffmann C, Eiden M, Baron T, Casalone C, Groschup MH. Atypical BSE in Germany - Proof of transmissibility and biochemical characterization. Vet Microbiol 2006; 117:103-16; PMID:16916588; http://dx.doi.org/10.1016/j.vetmic.2006.06.016
- Hagiwara K, Yamakawa Y, Sato Y, Nakamura Y, Tobiume M, Shinagawa M, Sata T. Accumulation of mono-glycosylated form-rich, plaque-forming PrPSc in the second atypical bovine encephalopathy case in Japan. Jpn J Infect Dis 2007; 60:305-8; PMID:17881874
- Jacobs JG, Langeveld JPM, Biacabe A-G, Acutis P-L, Polak MP, Gavier-Widen D, Buschmann A, Caramelli M, Casalone C, Mazza M, et al. Molecular discrimination of atypical bovine spongiform encephalopathy strains from a geographical region spanning a wide area in Europe. J Clin Microbiol 2007; 45:1821-9; PMID:17442800; http://dx.doi.org/10.1128/JCM.00160-07
- Richt JA, Kunkle RA, Alt D, Nicholson EM, Hamir AN, Czub S, Kluge J, Davis AJ, Hall SM. Identification and characterization of two bovine spongiform encephalopathy cases diagnosed in the United States. J Vet Diagn Invest 2007; 19:142-54; PMID:17402608; http://dx.doi.org/10.1177/104063870701900202
- Polak MP, Zmudzinski JF, Jacobs JG, Langeveld JP. Atypical status of bovine spongiform encephalopathy in Poland: a molecular typing study. Arch Virol 2008; 153:69-79; PMID:17896076; http://dx.doi.org/10.1007/s00705-007-1062-6
- Gavier-Widén D, Nöremark M, Langeveld JP, Stack M, Biacabe A-G, Vulin J, Chaplin M, Richt JA, Jacobs J, Acín C, et al. Bovine spongiform encephalopathy in Sweden: an H-type variant. J Vet Diagn Invest 2008; 20:2-10; PMID:18182501; http://dx.doi.org/10.1177/104063870802000102
- Biacabe A-G, Morignat E, Vulin J, Calavas D, Baron TGM. Atypical bovine spongiform encephalopathies, France, 2001–2007. Emerg Infect Dis 2008; 14:298-300; PMID:18258124; http://dx.doi.org/10.3201/eid1402.071141
- Dudas S, Yang J, Graham C, Czub M, McAllister TA, Coulthart MB, Czub S. Molecular, biochemical and genetic characteristics of BSE in Canada. PloS One 2010; 5:e10638; PMID:20498835; http://dx.doi.org/10.1371/journal.pone.0010638
- Stack MJ, Chaplin MJ, Davis LA, Everitt S, Simmons MM, Windl O, Hope J, Burke P. Four BSE cases with an L-BSE molecular profile in cattle from Great Britain. Vet Rec 2013; 172:70; PMID:23249774; http://dx.doi.org/10.1136/vr.101158
- [No authors listed] Case of BSE reported in Norway. Vet Rec 2015; 176:163; PMID:25678512; http://dx.doi.org/10.1136/vr.h734
- Orge L, Machado CG, Ramalho L, Carvalho R, Silva J, Almeida P, Tavares P, Ochoa C, Lima C, et al. Identification of H-type BSE in Portugal. Prion 2015; 9:22-8; PMID:25629308; http://dx.doi.org/10.1080/19336896.2014.997615
- Comoy EE, Casalone C, Lescoutra-Etchegaray N, Zanusso G, Freire S, Marcé D, Auvré F, Ruchoux M-M, Ferrari S, Monaco S, et al. Atypical BSE (BASE) transmitted from asymptomatic aging cattle to a primate. PloS One 2008; 3:e3017; PMID:18714385; http://dx.doi.org/10.1371/journal.pone.0003017
- Ono F, Tase N, Kurosawa A, Hiyaoka A, Ohyama A, Tezuka Y, Wada N, Sato Y, Tobiume M, Hagiwara K, et al. Atypical L-type bovine spongiform encephalopathy (L-BSE) transmission to cynomolgus macaques, a non-human primate. Jpn J Infect Dis 2011; 64:81-4; PMID:21266763
- Mestre-Francés N, Nicot S, Rouland S, Biacabe A-G, Quadrio I, Perret-Liaudet A, Baron T, Verdier JM. Oral transmission of L-type bovine spongiform encephalopathy in primate model. Emerg Infect Dis 2012; 18:142-5; PMID:22261009; http://dx.doi.org/10.3201/eid1801.111092
- Béringue V, Herzog L, Reine F, Le Dur A, Casalone C, Vilotte J-L, Laude H. Transmission of atypical bovine prions to mice transgenic for human prion potein. Emerg Infect Dis 2008; 14:1898-901; PMID:19046515; http://dx.doi.org/10.3201/eid1412.080941
- Kong Q, Zheng M, Casalone C, Qing L, Huang S, Chakraborty B, Wang P, Chen F, Cali I, Corona C, et al. Evaluation of the human transmission risk of an atypical bovine spongiform encephalopathy prion strain. J Virol 2008; 82:3697-701; PMID:18234793; http://dx.doi.org/10.1128/JVI.02561-07
- Wilson R, Plinston C, Hunter N, Casalone C, Corona C, Tagliavini F, Suardi S, Ruggerone M, Moda F, Graziano S, et al. Chronic wasting disease and atypical forms of bovine spongiform encephalopathy and scrapie are not transmissible to mice expressing wild-type levels of human prion protein. J Gen Virol 2012; 93:1624-9; PMID:22495232; http://dx.doi.org/10.1099/vir.0.042507-0
- Barria MA, Balachandran A, Morita M, Kitamoto T, Barron R, Manson J, Knight R, Ironside JW, Head MW. Molecular barriers to zoonotic transmission of prions. Emerg Infect Dis 2014; 20:88-97; PMID:24377702; http://dx.doi.org/10.3201/eid2001.130858
- Meloni D, Davidse A, Langeveld JPM, Varello K, Casalone C, Corona C, Balkema-Buschmann A, Groschup MH, Ingravalle F, Bozzetta E. EU-approved rapid tests for bovine spongiform encephalopathy detect atypical forms: a study for their sensitivities. PloS One 2012; 7:e43133; PMID:22984410; http://dx.doi.org/10.1371/journal.pone.0043133
- Gray JG, Dudas S, Graham C, Czub S. Performance analysis of rapid diagnostic tests on atypical bovine spongiform encephalopathy. J Vet Diagn Invest 2012; 24:976-80; PMID:22855378; http://dx.doi.org/10.1177/1040638712455325
- Yamamoto T, Ushiki Y, Hara S, Hall WW, Tsukagoshi-Nagai H, Yokoyama T, Tagawa Y, Sata T, Yamakawa Y, Kinoshita N, et al. An advantageous method utilizing new homogenizing device BioMasher and a sensitive ELISA to detect bovine spongiform encephalopathy accurately in brain tissue. J Virol Methods 2008; 149:316-25; PMID:18346796; http://dx.doi.org/10.1016/j.jviromet.2008.01.018
- Sugiura K, Onodera T, Bradley R. Epidemiological features of the bovine spongiform encephalopathy epidemic in Japan. Rev Sci Tech 2009; 28:945-56; PMID:20462152
- The Ministry of Agriculture, Forestry and Fisheries, Government of Japan. Application for Negligible BSE Risk Status; September, 2012 [accessed 2016 Dec 14]; http://www.maff.go.jp/j/syouan/douei/english/pdf/en_japan_negligible_bse_dossier.pdf
- Gray JG, Dudas S, Czub S. A study on the analytical sensitivity of 6 BSE tests used by the Canadian BSE reference laboratory. PloS One 2011; 6:e17633; PMID:21412419; http://dx.doi.org/10.1371/journal.pone.0017633
- European Food Safety Authority. Protocol for the evaluation of new rapid BSE post mortem tests. Scientific Opinion of the Panel on Biological Hazards on a request from the European Commission on a protocol for the evaluation of new rapid BSE post mortem tests. EFSA J 2007; 508:1-20. http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2007.508/epdf
- Masujin K, Shu Y, Yamakawa Y, Hagiwara K, Sata T, Matsuura Y, Iwamaru Y, Imamura M, Okada H, et al. Biological and biochemical characterization of L-type-like bovine spongiform encephalopathy (BSE) detected in Japanese black beef cattle. Prion 2008; 2:123-8; PMID:19158500; http://dx.doi.org/10.4161/pri.2.3.7437
- Priemer G, Balkema-Buschmann A, Hills B, Groschup MH. Biochemical characteristics and PrPSc distribution pattern in the brains of cattle experimentally challenged with H-type and L-type atypical BSE. PloS One 2013; 8:e67599; PMID:23805320; http://dx.doi.org/10.1371/journal.pone.0067599
- European Food Safety Authority. 2014. Protocol for further laboratory investigations into the distribution of infectivity of Atypical BSE. EFSA J 2014; 12:3798, 55 pp. http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2014.3798/epdf
- Miyazawa K, Okada H, Masujin K, Iwamaru Y, Yokoyama T. Infectivity-associated PrPSc and disease duration-associated PrPSc of mouse BSE prions. Prion 2015; 9:394-403; PMID:26555211; http://dx.doi.org/10.1080/19336896.2015.1111507
- Okada H, Iwamaru Y, Imamura M, Masujin K, Matsuura Y, Shimizu Y, Kasai K, Mohri S, Yokoyama T, Czub S. Experimental H-type bovine spongiform encephalopathy characterized by plaques and glial- and stellate-type prion protein deposits. Vet Res 2011; 42:79; PMID:21699704; http://dx.doi.org/10.1186/1297-9716-42-79
- Krasemann S, Groschup MH, Harmeyer S, Hunsmann G, Bodemer W. Generation of monoclonal antibodies against human prion proteins in PrP0/0 mice. Mol Med 1996; 2:725-34; PMID:8972487
