ABSTRACT
The ongoing epidemic of chronic wasting disease (CWD) within cervid populations indicates the need for novel approaches for disease management. A vaccine that either reduces susceptibility to infection or reduces shedding of prions by infected animals, or a combination of both, could be of benefit for disease control. The development of such a vaccine is challenged by the unique nature of prion diseases and the requirement for formulation and delivery in an oral format for application in wildlife settings. To address the unique nature of prions, our group targets epitopes, termed disease specific epitopes (DSEs), whose exposure for antibody binding depends on disease-associated misfolding of PrPC into PrPSc. Here, a DSE corresponding to the rigid loop (RL) region, which was immunogenic following parenteral vaccination, was translated into an oral vaccine. This vaccine consists of a replication-incompetent human adenovirus expressing a truncated rabies glycoprotein G recombinant fusion with the RL epitope (hAd5:tgG-RL). Oral immunization of white-tailed deer with hAd5:tgG-RL induced PrPSc-specific systemic and mucosal antibody responses with an encouraging safety profile in terms of no adverse health effects nor prolonged vector shedding. By building upon proven strategies of formulation for wildlife vaccines, these efforts generate a particular PrPSc-specific oral vaccine for CWD as well as providing a versatile platform, in terms of carrier protein and biological vector, for generation of other oral, peptide-based CWD vaccines.
INTRODUCTION
Chronic wasting disease (CWD) is a prion disease that is currently spreading through North American cervid populations.Citation1 Incidence of CWD has largely been limited to North America, but reported cases in South Korea and Norway indicate the potential for global consequences.Citation2,3 CWD has had a devastating impact on the farmed elk industry and its ongoing spread through wild populations threatens a natural resource that is of considerable economic, ecological and social importance. The circumstantial evidence that human prion diseases can result from exposure to CWD, as well as a recent demonstration of oral transmission of CWD to cynomolgus macaques, add concern of the zoonotic potential of CWD.Citation4,5
At the current time, culling is the primary tool available for management of CWD. While there has been some success in limiting the spread of CWD in wild populations through strategic culling, this is an incomplete and controversial strategy for long-term disease management.Citation6,7 Management of CWD, in particular within wild populations, will likely depend on the coordinated application of several disease management strategies. One important component of this strategy may be the use of vaccines to either reduce PrPSc shedding or prevent infection. In particular, the unique ability of prions to cause long-term environmental contamination, lasting from years to decades, supports vaccination as a strategy to both achieve prophylactic prevention and reduce shedding towards mitigating exposure and infection of animals in contaminated environments.Citation8,9 Emerging evidence of the uptake and presentation of environmentally-shed prions by plants further support the importance of limiting shedding by infected animals.Citation10
Development of a prion vaccine is challenged by the unique nature of the infectious agent; the misfolding of a self-protein (PrPC) into an infectious and pathological conformation (PrPSc).Citation11 While an effective vaccine for CWD remains elusive, there is proof of principle evidence that this is an achievable goal as antibodies to PrPC neutralize prion propagation in vitro and in vivo.Citation12-18 Numerous studies have shown the benefits of immunization to abrogate infectivity, reduce PrPSc loads within relevant tissue, increase survival time, and provide partial protection following infection.Citation19-29 Further, the paradigms of vaccine success are shifting such that reduced shedding, rather than outright prevention of infection, may be a relevant and achievable measure of vaccine efficacy.
While these results provide optimism for the development of a prion vaccine, there are concerns of potentially deleterious consequences associated with the induction of antibodies with reactivity towards a widely-expressed self-protein. While many passive and active immunization trials involving antibodies against PrPC do not cause pathological reactions, other early experiments suggested that antibodies against PrPC trigger neuronal apoptosis or activation of inappropriate signalling cascades.Citation30-32 Further investigation suggested that antibodies to a variety epitopes of the globular domain of PrPC have varying degrees of neurotoxicity.Citation33 Mechanistically, this neurotoxicity seems to mimic the cellular events associated with prion infection and may involve antibody-induced conformational changes in PrPC.Citation34,35
By targeting epitopes that are specifically exposed upon pathological misfolding of PrPC to PrPSc, it is possible to induce antibody responses specific for the pathological conformation, thereby sparing the healthy form of the protein and prioritizing the immune response to the problematic conformation. To date, three DSEs have been translated into injected vaccines that induce PrPSc antibody responses.Citation36,37 Of these, a rigid loop (RL) DSE is considered particularly attractive for a CWD vaccine based on unique structural features of this loop in cervid PrP including its predicted ability to convert from a compact loop into an unstructured region that resembles a free peptide immunogen.Citation38,39
The majority of efforts to develop prion vaccines have been in the context of injected delivery which could have application for farmed animals. For wild animals, an effective vaccine would need to be deployed without direct contact with animals, making oral vaccines the best delivery option. The potential benefits of oral vaccination are perhaps best exemplified by the highly-successful example of rabies that demonstrated it is possible to achieve protective immunity through oral, self-administered vaccines.Citation40,41 Different molecular and biological vectors have been investigated as oral CWD vaccines and these studies have demonstrated that oral vaccine formulations can induce both mucosal and systemic antibody responses in deer and mice.Citation24-27 Perhaps most relevant, Goni et al, using a Salmonella-based oral delivery platform in deer, were able to induce partially protective immune responses to CWD.Citation25 This, however, was achieved with an intensive immunization protocol (8 immunizations, including specific manual application of the vaccine to the tonsils) that would limit the potential for application of this approach in a wildlife setting. Optimistically this study provides proof-of-principle support for the development of an oral CWD vaccine while highlighting the need for delivery platforms that are compatible with a real-world wildlife vaccine from economic, regulatory and practical considerations.
In this work, we describe the construction and immunogenic characterization of an oral prion vaccine based on a replication-incompetent human adenovirus expressing a truncated rabies glycoprotein G recombinant fusion with the RL epitope (hAd5:tgG-RL). Oral delivery of hAd5:tgG-RL to white-tailed deer induced PrPSc-specific systemic and mucosal immune responses after two immunizations. The vaccine showed a satisfactory safety profile as characterized by limited vector shedding and non-reactivity of the induced antibodies to PrPC. Collectively, through strategies of formulation that have proven effective for other wildlife vaccines, these efforts provide a potential PrPSc-specific oral CWD vaccine as well as providing a platform for the generation of other peptide-based oral CWD vaccines.
RESULTS
Characterization of Recombinant Ad5:tgG-RL
Recombinant virus was assayed for both incorporation of the tgG-RL open reading frame as well as expression of the tgG-RL fusion antigen. Total DNA was isolated from either mock-infected HEK293 cells or those infected with either hAd5, hAd5:tgG-RL and subjected to restriction digest analysis. A restriction fragment of the appropriate size (5897 bp) corresponding to the tgG-RL expression cassette and flanking homologous recombination sequence was recovered only from cells infected with hAd5:tgG-RL []. An approximately 30 kB fragment is observed within the viral infected cultures representing the adjacent genomic sequence coding for various structural adenovirus proteins, and is absent from mock-infected isolates [].
FIGURE 1. Vaccine Production. (A) DNA was isolated from HEK293 cells infected with hAd5 and hAd5:tgG-RL and subjected to PacI digestion to identify the presence of tgG-RL coding sequence. (B) Western blot of conditioned media from either mock infected or hAd5:tgG-RL infected HEK293 cells. Proteins were resolved by SDS-PAGE, blotted to nitrocellulose and probed with either anti-tgG or anti-RL sera at 1:1000 or 1:2000, respectively. Protein-antibody complexes were probed with alkaline phosphatase labeled secondary antibody, and visualized following development with BCIP/NBT.
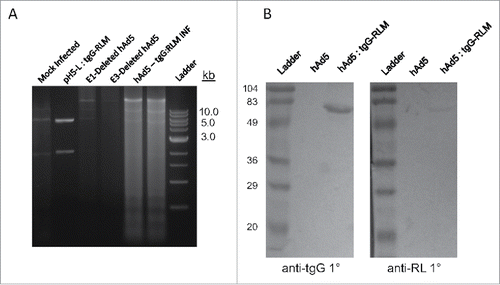
To confirm expression of the heterologous fusion protein, conditioned media from HEK293 cells infected with either hAd5 or hAd5:tgG-RL were analyzed by Western blot. Membranes were probed with polyclonal anti-tgG or anti-RL sera to confirm the composition of expressed antigen. From the media of the hAd5:tgG-RL infected cells both the anti-tgG and anti-RL sera recognized a 65 kDa protein corresponding to the mature, processed form of tgG-RL [].
Systemic and Mucosal Humoral Responses
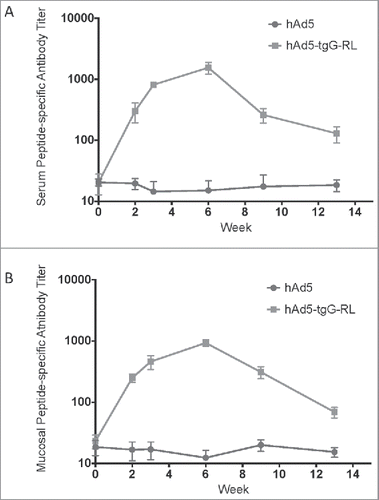
To assess the immunogenicity of hAd5:tgG-RL white-tail deer (n = 5/group) were orally immunized and epitope-specific antibody titers in serum and feces were quantified with a peptide ELISA. Serum titers from 4 of 5 animals receiving the hAd5:tgG-RL oral vaccine displayed seroconversion following primary immunization []. Peptide-specific antibody titers in serum continued to rise following a second immunization 2 weeks later, plateauing at week 6 and the gradually decreased during the remainder of the trial. One of the 5 vaccinated animals failed to seroconvert at any time during the trial. The ability of oral hAd5:tgG-RL vaccine to induce mucosal responses was also evaluated following oral immunization. DSE-specific antibody responses were detected in 4 of the 5 animals receiving hAd5:tgG-RL []. The overall kinetics of the fecal antibody responses mirrored the serum antibody response. Notably, the same animal that failed to develop detectable serum also failed to develop fecal antibody responses. These results confirm that oral delivery of a recombinant viral vector, expressing an appropriate DSE and carrier molecule, is capable of inducing both systemic and mucosal antibody responses in white-tailed deer.
FIGURE 2. Systemic and Mucosal Epitope-specific Antibody Responses. White-tailed deer received an oral administration of 2.0 × 1010 viral particles of either hAd5 (n = 5) or hAd5:tgG-RL (n = 4). The animal who failed to mount an antibody response to the hAd5:tgG-RL vaccine was excluded from this consideration. Animals were orally immunized twice with a two-week interval. Serum antibody titers (A) and fecal antibody titers (B) were quantified with a capture ELISA using RL peptide to coat the wells. Data presented are as the mean ± 1 SD.
Antigen-Specific Lymphocyte Responses
Splenocyte proliferative responses were analyzed to corroborate the induction of serum antibody responses. Splenocytes were isolated 13 weeks after the initial oral immunization, and were co-cultured with purified tgG protein to assess their responsiveness to the tgG carrier protein. Splenocytes from all animals exhibited proliferative responses (SI > 2.0) to Concanavalin A, a polyclonal T cell mitogen (data not shown). Splenocytes from 4 of 5 animals displayed moderate to strong proliferative responses to tgG that were significantly greater (p < 0.05) than that observed for animals receiving hAd5 []. Splenocytes from the animal failing to seroconvert following oral immunization with hAd5:tgG-RL also failed to respond to in vitro tgG stimulation. Proliferative response data corroborate that the peptide-specific antibody responses in animals receiving hAd5:tgG-RL immunizations could be supported by T-helper cells recognizing the tgG carrier protein.
FIGURE 3. Lymphocyte Proliferation Assays. tgG-specific lymphocyte proliferative responses were determined following oral immunization of white-tailed deer with hAd5:tgG-RL vector. Lymphocytes were isolated from spleens 91 days post-immunization. Proliferative capacity of lymphocytes was determined by incorporation of [3H] thymidine follow co-culture with affinity purified tgG protein. Proliferative responses are reported as stimulation index (mean counts per minute with tgG stimulation of triplicate cultures /mean counts per minute of triplicate cultures with medium alone).
![FIGURE 3. Lymphocyte Proliferation Assays. tgG-specific lymphocyte proliferative responses were determined following oral immunization of white-tailed deer with hAd5:tgG-RL vector. Lymphocytes were isolated from spleens 91 days post-immunization. Proliferative capacity of lymphocytes was determined by incorporation of [3H] thymidine follow co-culture with affinity purified tgG protein. Proliferative responses are reported as stimulation index (mean counts per minute with tgG stimulation of triplicate cultures /mean counts per minute of triplicate cultures with medium alone).](/cms/asset/658cf79c-a645-4cf2-9945-121c07bec269/kprn_a_1367083_f0003_b.gif)
Immune Sera Specificity to PrPC
To determine if the antibodies induced by oral immunization retained the same PrPSc specificity as previously characterized for the parenteral vaccine, serum from hAd5:tgG-RL vaccinated animals was assayed for PrPC reactivity by ELISA, using recombinant cervid PrP 90–231 for antibody capture. Serum samples corresponding to peak antibody titers at week 6 post-vaccination was selected based on the assumption that this provided sufficient time for affinity maturation of antibody responses. PrPC reactive monoclonal antibodies, 6D11 and M2188, were used as positive controls, while a polyclonal antibody, affinity-purified from serum samples collected following parenteral vaccination with the RL construct, was included as a negative control. Antibody titers for serum samples collected from animals receiving hAd5 were designated 101 and were considered to reflect background activity when assaying serum samples in ELISA []. Serum samples from animals receiving hAd5:tgG-RL did not display detectable reactivity with cervid PrPC as the group titers were calculated to be less than 101, similar to the control serum samples [].
FIGURE 4. Specificity of Immune Responses. (A) Antibody titers were quantified by capture ELISA using cervid PrPC 90–231 as coating antigen, and are reported as mean values ± 1 SD. Monoclonal antibodies 6D11 and M2188 served as positive controls, while polyOv.RL sera served as a negative control. (B) Immunoprocipitation of PrP from healthy and prion-infected brains. Pooled serum from deer orally immunized with hAd5:tgG-RL was assessed for reactivity with PrPSc and PrPC. Serum antibodies were cross-linked to magnetic beads and incubated with non-infected and infected 10% brain homogenate. A PrPC/Sc reactive monoclonal antibody, AH6B, as well as polyclonal antibodies affinity-purified from serum samples collected following parenteral vaccination with a YML construct were included as controls. The YML antibody has demonstrated reactivity with PrPSc in immunoprecipitation assays.Citation37
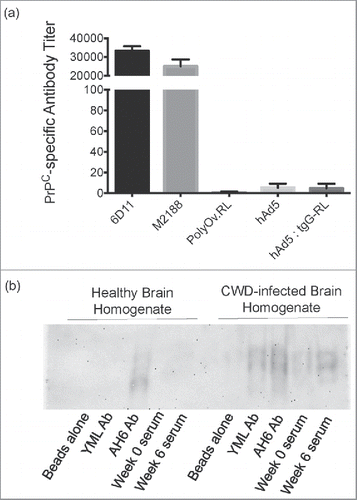
The PrPSc specificity and reactivity of the serum antibodies induced by hAd5:tgG-RL were also analyzed with immunoprecipitation assays, using both healthy and prion-infected brain tissue. A PrPC/Sc reactive monoclonal antibody, AH6B, as well as polyclonal antibodies affinity-purified from serum samples collected following parenteral vaccination with a YML construct were included as controls. The YML antibody has demonstrated reactivity with PrPSc in immunoprecipitation assaysCitation37 Pooled serum samples collected from animals six weeks after hAd5:tgG-RL vaccination demonstrated reactivity with PrPSc brain tissue but failed to react with PrPC in healthy brain material []. Further, pre-immune serum from the hAd5:tgG-RL vaccinated animals failed to react with PrPSc.
Vector Shedding
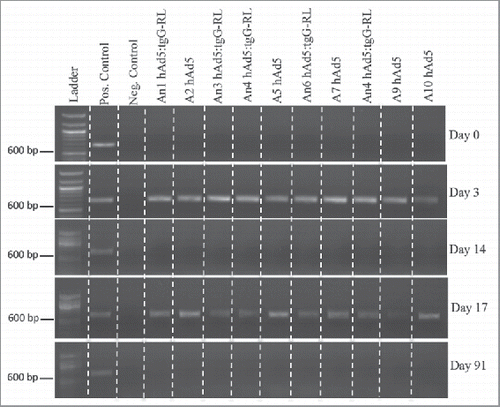
To assess the duration of hAd5 vector shedding, fecal samples were assayed for viral DNA using an adenovirus hexon-specific PCR. Fecal samples collected prior to immunization were negative for adenovirus []. At three days post immunization, fecal samples from all animals vaccinated with either hAd5:tgG-RL or hAd5 were positive for the adenovirus hexon sequence. This vector shedding reflects passive transmission of the oral vector through the animal since the hAd5 vector was not replication competent. At 14 days after primary immunization all animals were PCR negative for Ad5. Similarly, three days after the secondary vaccination, fecal samples from all animals were again positive for adenoviral DNA []. Finally, all animals were confirmed to not be shedding detectable levels of virus when the trial concluded 91 days post-vaccination. Sequencing of the PCR product generated from day 3 fecal samples confirmed the amplified sequence matched human group C adenovirus hexon sequence (AC_000008.1).
FIGURE 5. Vector Shedding. Ten white-tailed deer (n = 5/group) orally received 2.0 × 1010 viral particles of either hAd5 or hAd5:tgG-RL. Animals were orally immunized at D0 and D14. Total DNA was isolated from fecal samples on individual animals, and served as template for diagnostic PCR to identify viral sequence coding for the Ad5 hexon protein. DNA concentration was standardized for each 20 ul PCR, and entire reactions were resolved in 1% agarose gels.
DISCUSSION
Prion vaccine development by our group has prioritized epitopes that induce antibody responses specific to the misfolded PrPSc species. To date, peptide-based vaccines, corresponding to three different DSEs, have been delivered parenterally and their immunogenicity and specificity has been characterized.Citation23,36,37 The priority of the current investigation was to translate the DSE corresponding to the PrP rigid loop region to an oral vaccine vector. In terms of philosophy and molecular construction, these efforts build upon the highly successful example of oral vaccines for rabies.
The haptenic nature of peptides requires the use of an immunogenic carrier protein to generate the T-cell help required for the induction of an antibody response. Our previous CWD vaccines utilized the Leuktoxin (Lkt) protein of Mannheimia haemolytica to support the induction of T-cell dependent antibody responses.Citation36 However, this large prokaryotic protein is poorly suited for expression in eukaryotic systems and viral vectors. In contrast, rabies glycoprotein G (gG) is an extremely potent immunogen capable inducing robust and prolonged immune responses.Citation42 As a carrier, gG is capable of inducing immune responses to heterologous antigens and peptide epitopes.Citation43 In a direct comparison with Lkt, a truncated version of gG (tgG) induced significantly higher titre, and longer duration, DSE-specific antibody responses.Citation44 For these reasons, the tgG-RL fusion antigen was selected for translation into an oral delivery vector.
Human adenovirus serotype 5 (hAd5) was selected as a mucosal vaccine vector for its broad species and tissue tropism, as well as propensity to induce both systemic and mucosal humoral immunity to heterologous antigens.Citation45,46 Many mammals share habitats with cervids, making a replication-incompetent hAd5 a priority to ensure limited environmental contamination by the vaccine. Shedding of the hAd5 vector was limited to three days immediately following vaccination. In contrast, shedding could be detected for weeks following oral delivery of a replication-competent vector.Citation47 That the replication-defective vector was able to infect cells in the gastro-intestinal tract of white-tailed deer and express the transgene was evident in the induction of RL-specific systemic and mucosal antibody responses. Although larger antigen expression, and therefore greater immune responses would likely be possible with the use of a replication-competent virus, safety issues surrounding environmental contamination or secondary immunization of non-target species influenced our choice of a replication-defective virus.
Within these efforts, only four of the five animals receiving the hAd-tgG-RL vaccine demonstrated detectable epitope-specific antibody responses. Based on virus shedding, we can be confident that the non-responding animal (An3) received the vaccine but (based on the splenocyte proliferation assays and antibody titres) did not mount an immunological response to the vaccine. There are a number of potential explanations for this vaccine non-responder. Firstly, it is important to appreciate that the purpose of this investigation was to provide proof-of-principle for the development of an oral vaccine for cervids utilizing vectors that are consistent with a real-world vaccine from financial and regulatory perspectives. This did not include efforts to optimize either the dose or timing of the vaccinations which could certainly impact the magnitude and consistency of the induced responses. As such, it may be possible to achieve a more prominent and uniform response through optimizing the dose and/or timing of vaccination. Secondly, within outbred populations, even for commercialized vaccines, it is not unusual to observe variability in vaccine responsiveness, including non-responders.
Further to that, the antibody titres induced following oral vaccination with the hAd5:tgG-RL vector were approximately two orders of magnitude lower than those achieved when using an adjuvanted parenteral vaccine with the same DSE.Citation36,37 This may be due to the delivery of an unadjuvanted oral vaccine which was not dose optimized as well as the use of a replication-incompetent vector that generated a limited amount of antigen. Further, with the oral vaccine, there wasn't a significant anamnestic response following the secondary immunization. This may be due to the induction of hAd5-specifc immunity, which prevented virus infection or uptake by mucosa-associated lymphoid tissue. The parenteral DSE-specific vaccine induced antibody responses that were also detectable in nasal secretions and cerebrospinal fluid.Citation36 The failure of the oral vaccine to induce detectable humoral responses within either nasal secretions or CSF [data not shown] may be attributable to the induction of a much lower systemic antibody response. There are several options to increase the immunogenicity, and potentially protective capacity, of the hAd5 vector. These include optimizing the dose and timing of vaccination as well as incorporating accessory immunostimulatory proteins in the transgene, chemical modification or encapsulation of the recombinant virus to decrease degradation in gastrointestinal tract, and possibly reducing interference by vector immunity by increasing the interval between multiple immunizations.Citation48-50
While developing a CWD vaccine for use in wild cervids was the primary objective of this study, these results may also be applicable in captive cervids and domestic livestock. A properly formulated and sufficiently immunogenic vaccine could be delivered in the feed of animals, thereby providing a method to eliminate the handling of individual animals during vaccination. While oral immunization is the only option for implementing a cervid-specific wildlife vaccination program, a commensurate benefit to mucosal vaccination is the induction of a broad anti-PrP humoral response. Recent studies indicate the importance of inducing both systemic and mucosal immunity, where high levels of IgG and IgA correlated to increased survival.Citation25-27 While the current efforts prioritized a particular DSE, the platform could easily be adapted for other peptide epitopes, including those associated with antibody responses against PrPC.
MATERIALS AND METHODS
Construction of Oral CWD Vaccine
Total RNA was isolated from rabies-positive fox brain tissue using RNeasy Mini Kit (Qiagen) and a cDNA library was synthesized using Superscript III cDNA Library Construction Kit (Life Technologies). A truncated version of the Rabies Glycoprotein G (gG) was amplified using primers that truncated the 3′ region to eliminate the transmembrane and cytosolic domains, as well as to facilitate the introduction the codon-optimized sequence corresponding to the rigid-loop epitope. The resulting gene product encodes a product in which the RL epitope is presented as a C-terminal fusion of the truncated tgG protein. DNA sequence was synthesized (Genscript) and further amplified to contain a stop codon and flanking restriction sites to facilitate further cloning. Both tgG and RL were restricted and co-cloned into pH5L vector containing signal transcription and regulatory elements to facilitate antigen expression.51 PacI-digested pH5L:tgG-RL and pH5R were co-transfected into HEK293 cells using Calcium-phosphate HEK293 transfection kit (Promega) to facilitate homologous recombination and the production of recombinant hAd5:tgG-RL virus. An E1/partial E3-deleted hAd5 lacking any heterologous protein expression was included as a negative control. Both hAd5 and hAd5:tgG-RL viruses was amplified following several passages in HEK293 cells, purified by CsCl gradient centrifugation and concentration was determined from the formula: viral particles/mL = (optical density at 260 nm) × (dilution factor) × 1012).Citation52
Isolation of Recombinant Adenoviral DNA from Infected Tissue Culture
HEK293 cells in T75 format were infected (1 × 105 vp) with hAd5:tgG-RL, hAd5 control virus, or mock infected. Cells exhibiting full CPE 48 h post infection were harvested, pelleted and incubated in extraction buffer (400 mM NaCl, 0.5% Triton X-100, 50 mM Tris-HCl pH 7.5) for 30 min at 4°C. Following centrifugation, supernatant was incubated with 1% SDS and 0.8 mg/mL Proteinase K at 37°C for 1 h. DNA isolated by Phenol:Chloroform:Isoamyl alcohol extraction and ethanol precipitation. Isolated DNA was restricted with PacI to identify the tgG-RL open reading frame. Restriction digest products were subjected to electrophoresis in 1% agarose gels.
Western Blotting
HEK293 cells were infected with either 1 × 105 vp hAd5:tgG-RL, hAd5 control or mock infected. Conditioned media containing 20 ug total protein was isolated from all cultures 72 h post infection and subject to electrophoresis in 10% SDS-polyacrylamide gels, and transferred to a nitrocellulose membrane (Bio-Rad). Membranes were probed with either anti-tgG or anti-RL polyclonal serum at 1:1000 or 1:2000, respectively. Immune complexes were further probed with alkaline phosphatase-conjugated goat anti-mouse IgG (H+L) (KPL) at 1:4000. Specific proteins were visualized following the addition of SigmaFAST BCIP/NBT substrate (Sigma-Aldrich).
Oral Immunization of White-Tailed Deer
Ten six-week old female white-tailed deer (n = 5/group) orally received 2 × 1010 viral particles of Ad5:tgG-RL or Ad5 virus in 4 mL unsupplemented Eagles Minimum Essential Medium at days 0 and 14. Animals were housed under biosafety level 3 containment for the duration of the trial. Individual serum, nasal swabs, feces, and cerebrospinal fluid were isolated, and stored at −20°C. An independent research team performed all animal work and experiments were done according to the Guide to the Care and Use of Experimental Animals, provided by the Canadian Council on Animal Care. The Saskatchewan Animal Care Committee approved all experimental protocols.
Extraction of Antibody from Feces
Fecal samples were thawed and suspended to 0.1 g/mL in chilled extraction buffer (PBS, 0.05% Tween 20, 1x complete protease inhibitor cocktail (Roche)). Suspensions were vortexed, nutated for 30 min at 4°C, cleared of cellular debris by centrifugation at 16,000 × g for 10 min at 4°C, and stored at −20°C.
Vector Shedding from Vaccinated Animals
DNA was isolated from approximately 200 mg of fecal material of individual animals using QIAamp DNA Stool Mini Kit (Qiagen) to manufacturers specifications. Eluted DNA was supplemented with 0.1 ug/ul BSA and served as template for PCR to identify viral DNA. Template DNA was standardized to 50 ng/20 ul reaction, and PCRs were conducted using Phusion High-Fidelity Polymerase, to manufacturers specifications (NEB). hAd5 primers were constructed based on the published sequence of the human adenovirus serotype 5 hexon (AC_000008.1): hAd5-hexF3: GGACATGGCTTCCACGTACT hAd5-hexR3: GCCTGTTGGGCAATAGATTGT. Genomic human adenovirus serotype 5 DNA purified from spiked white-tailed deer fecal samples served as positive control template, and DNA purifications from non-spiked samples served as negative control.
ELISAs
Serum and mucosal epitope-specific antibody responses were quantified by ELISA through previously described protocols.Citation37 Serum PrPC-specific antibody responses were quantified by ELISA using samples from peak titer at week 6, performed similarly as previously described, using affinity purified truncated cervid PrP 90–231 as coating antigen.Citation43 Control antibodies 6D11, M2188, and ovine polyclonal anti-RL serum were used at 1:40 initial dilution. ELISA titres are expressed as the reciprocal of the highest serum dilution resulting in an OD reading exceeding two standard deviations above the value for the pre-immune serum.
Antibody Purification and Immunoprecipitation of PrPSc
Serum from immunized deer were evaluated for interaction with PrPSc and PrPC. Immunoglobulin isolated using Protein-A column-affinity purification was conjugated to magnetic beads for brain homogenate immunoprecipitation assays as described.Citation37 A PrPC/Sc reactive monoclonal antibody, AH6B, as well as polyclonal antibodies affinity-purified from serum samples collected following parenteral vaccination with a YML construct were included as controls. The YML antibody has demonstrated reactivity with PrPSc in immunoprecipitation assays.Citation37
Spenocyte Stimulation with Carrier
Spleens were harvested from immunized animals at day 91 post oral immunization. Spenocyte isolation and culture were described previously.Citation53 Splenocytes were cultured for 72 h in triplicate with affinity-purified tgG (1.0 and 3.0 μg/mL), concanavalin A (1.0 μg/mL). Cells were cultured in a final volume 200 ul and pulsed with 0.4 uCi / mL [3H] thymidine. Following 72 h incubation, incorporation of [3H]-thymidine was determined using standard scintillation counting methods. Proliferative responses were calculated as a stimulation index (counts per minute with stimulating antigen/counts per minute with media alone) and expressed as the mean of triplicate cultures.
Statistical Analysis
The data represent repeated measures of ELISA antibody titres in animals over time and did not adhere to a normal distribution. To account for the repeated measures study design, data for each animal were first summed over time. Summed data were then ranked to account for their non-normal distribution and a one-way ANOVA analysis performed on the ranked sums. Where appropriate, Tukey's test was used to examine differences among treatment groups. P values less than 0.05 were considered significant.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
None.
ACKNOWLEDGMENTS
Dr. Trent Bollinger and the Canadian Cooperative Wildlife Health Centre Western/Northern for supplying rabies infected tissue. Dr. Alexander Zakhartchouk who kindly provided plasmids pH5L and pH5R for construction of recombinant adenoviruses. Dr. Claudia Madampage for providing purified cervid prion protein. Dr. Patricia Cano for technical expertise in splenocyte proliferation assays. Dr. Philip Griebel is supported by a Tier I CRC with funds provided by the CIHR.
FUNDING
This work was funded by the Saskatchewan Agriculture Development Fund under grant #20140043.
REFERENCES
- Saunders SE, Bartelt-Hunt SL, Bartz JC. Occurrence, transmission, ad zoonotic potential of chronic wasting disease. Emerg Infect Dis. 2012;18:369–76. doi:10.3201/eid1803.110685. PMID:22377159.
- Lee YH, Sohn HJ, Kim MJ, Kim HJ, Lee WY, Yun EI, Tark DS, Cho IS, Balachandran A. Strain characterization of the Korean CWD cases in 2001 and 2004. J Vet Med Sci. 2013;75:95–98. Epub 2012 Aug 28. doi:10.1292/jvms.12-0077. PMID:22972463.
- Benestad SL, Mitchell G, Simmons M, Ytrehus B, Vikoren T. First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Vet Res. 2016;1:88. doi:10.1186/s13567-016-0375-4
- Belay ED, Gambetti P, Schonberger LB, Parchi P, Lyon DR, Capellari S, McQuiston JH, Bradley K, Dowdle G, Crutcher JM, et al. Creutzfeldt-Jakob disease in unusually young patients who consumed venison. Arch Neurol. 2001;58:1673–78. doi:10.1001/archneur.58.10.1673. PMID:11594928.
- Czub S. First evidence of intracranial and peroral transmission of Chronic Wasting Disease (CWD) into Cynomolgus macaques: A work in progress. Prion 2017 Deciphering Neurodegenerative Disorders; 2017 May 2017; Edinburgh, Scotland.
- Mateus-Pinilla N, Weng HY, Ruiz MO, Shelton P, Novakofski J. Evaluation of a wild white-tailed deer population management program for controlling chronic wasting disease in Illinois, 2003–2008. Prev Vet Med. 2013;110:541–48. doi:10.1016/j.prevetmed.2013.03.002. PMID:23558033.
- Uehilnger FD, Johnston AC, Bollinger TK, Waldner CL. Systematic review of management strategies to control chronic wasting disease in wild deer populations in North America. BMC Vet Res. 2016;12:173. doi:10.1186/s12917-016-0804-7. PMID:27549119.
- Almberg ES, Cross PC, Johnson CJ, Heisey DM, Richards BJ. Modeling routes of chronic wasting disease transmission: Environmental prion persistence promotes deer population decline and extinction. PLoS One. 2011;6:e19896. Epub 2011 May 13. doi:10.1371/journal.pone.0019896. PMID:21603638.
- Seidel B, Thomzig A, Buschmann A, Groschup MH, Peters R, Beekes M, Terytze K. Scrapie agent (strain 263) can transmit disease via the oral route after persistence in soil for years. PLoS One. 2007;9:e435. doi:10.1371/journal.pone.0000435
- Pritzkow S, Morales R, Moda F, Khan U, Telling GC, Hoover E, Soto C. Grass plants bind, retain, uptake, and transport infectious prions. Cell Res. 2015;11:1168–75.
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–44. doi:10.1126/science.6801762. PMID:6801762.
- Enari M, Flechsig E, Weissmann C. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc Natl Acad Sci U S A. 2001;98:9295–9. doi:10.1073/pnas.151242598. PMID:11470893.
- Perrier V, Solassol J, Crozet C, Frobert Y, Mourton-Gilles C, Grassi J, Lehmann S. Anti-PrP antibodies block PrPSc replication in prion-infected cell cultures by accelerating PrPC degradation. J Neurochem. 2004;89:454–63. doi:10.1111/j.1471-4159.2004.02356.x. PMID:15056288.
- Peretz D, Williamson RA, Kaneko K, Vergara J, Leclerc E, Schmitt-Ulms G, Mehlhorn IR, Legname G, Wormald MR, Rudd PM, et al. Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature. 2001;412:739–43. doi:10.1038/35089090. PMID:11507642.
- Taschuk R, Van der Merwe J, Marciniuk K, Potter A, Cashman N, Griebel P, Napper S. In vitro neutralization of prions with PrP(Sc)-specific antibodies. Prion. 2015;9:292–303. doi:10.1080/19336896.2015.1071761. PMID:26284508.
- Sigurdsson EM, Brown DR, Daniels M, Kascsak RJ, Kascsak R, Carp R, Meeker HC, Frangione B, Wisniewski T. Immunization delays the onset of prion disease in mice. Am J Pathol. 2002;161:13–7. doi:10.1016/S0002-9440(10)64151-X. PMID:12107084.
- Sigurdsson EM, Sy M-S, Li R, Scholtzova H, Kascsak RJ, Kascsak R, Carp R, Meeker HC, Frangione B, Wisniewski T. Anti-prion antibodies for prophylaxis following prion exposure in mice. Neurosci Lett. 2003;336:185–7. doi:10.1016/S0304-3940(02)01192-8. PMID:12505623.
- Moda F, Vimercati C, Campagnani I, Ruggerone M, Giaccone G, Morbin M, Zentilin L, Giacca M, Zucca I, Legname G, et al. Brain delivery of AAv9 expressing an anti-PrP monovalent antibody delays prion disease in mice. Prion. 2012;6:383–90. doi:10.4161/pri.20197. PMID:22842862.
- Koller MF, Grau T, Christen P. Induction of antibodies against murine full-length prion protein in wild-type mice. J Neuroimmunol. 2002;132:113–6. doi:10.1016/S0165-5728(02)00316-8. PMID:12417440.
- Wisniewski T, Goni F. Immunomodulation for prion and prion-related diseases. Expert Rev Vaccines. 2010;6:75–85.
- Marciniuk K, Taschuk R, Napper S. Evidence for prion-like mechanisms in several neurodegenerative diseases: Potential implications for immunotherapy. Clin Dev Immunol. 2013;2013:473706. doi:10.1155/2013/473706. PMID:24228054.
- Xanthopoulos K, Lagoudaki R, Kontana A, Kyratsous C, Panagiotidis C, Grigoriadis N, Yiangou M, Sklaviadis T. Immunization of recombinant prion protein leads to partial protection in a murine model of TSEs through a novel mechanism. PLoS One. 2013;8:e59143. doi:10.1371/journal.pone.0059143. PMID:23554984.
- Taschuk R, Marciniuk K, Maattanen P, Madampage C, Hedlin P, Potter A, Lee J, Cashman N, Griebel PJ, Napper S. Safety, specificity and immunogenicity of a PrP(Sc)-specific prion vaccine based on the YYR disease specific epitope. Prion. 2014;8:51–59. doi:10.4161/pri.27962. PMID:24509522.
- Bade S, Baier M, Boetel T, Frey A. Intranasal immunization of Balb/c mice against prion protein attenuates orally acquired bovine spongiform encephalopathy. Vaccine. 2006;24:1242–53. doi:10.1016/j.vaccine.2005.12.051. PMID:16455168.
- Goñi F, Mathiason CK, Yim L, Wong K, Hayes-Klug J, Nalls A, Peyser D, Estevez V, Denkers N, Xu J, et al. Mucosal immunization with an attenuated Salmonella vaccine partially protects white-tailed deer from chronic wasting disease. Vaccine. 2015;33:726–33. doi:10.1016/j.vaccine.2014.11.035. PMID:25539804.
- Goñi F, Prelli F, Schreiber F, Scholtzova H, Chung E, Kascsak R, Brown DR, Sigurdsson EM, Chabalgoity JA, Wisniewski T. High titers of mucosal and systemic anti-PrP antibodies abrogate oral prion infection in mucosal-vaccinated mice. Neuroscience. 2008;15:679–86. Epub 2008 Mar 6. doi:10.1016/j.neuroscience.2008.02.051
- Goñi F, Knudsen E, Schreiber F, Scholtzova H, Pankiewicz J, Carp R, Meeker HC, Rubenstein R, Brown DR, Sy MS, et al. Mucosal vaccination delays or prevents prion infection via an oral route. Neurosci. 2005;133:413–21. doi:10.1016/j.neuroscience.2005.02.031
- Schwarz A, Krätke O, Burwinkel M, Riemer C, Schultz J, Henklein P, Bamme T, Baier M. Immunisation with a synthetic prion protein-derived peptide prolongs survival times of mice orally exposed to the scrapie agent. Neurosci Lett. 2003;350:187–9. doi:10.1016/S0304-3940(03)00907-8. PMID:14550926.
- Magri G, Clerici M, Dall'Ara P, Biasin M, Caramelli M, Casalone C, Giannino ML, Longhi R, Piacentini L, Della Bella S, et al. Decrease in pathology and progression of scrapie after immunisation with synthetic prion protein peptides in hamsters. Vaccine. 2005;23:2862–8. doi:10.1016/j.vaccine.2004.11.067. PMID:15780734.
- Solforosi L, Criado JR, McGavern DB, Wirz S, Sánchez-Alavez M, Sugama S, DeGiorgio LA, Volpe BT, Wiseman E, Abalos G, et al. Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science. 2004;303:1514–6. doi:10.1126/science.1094273. PMID:14752167.
- Mouillet-Richard S, Ermonval M, Chebassier C, Laplanche JL, Lehmann S, Launay JM, Kellermann O. Signal transduction through prion protein. Science. 2000;289:1925–8. doi:10.1126/science.289.5486.1925. PMID:10988071.
- Arsenault RJ, Li Y, Potter A, Griebel PJ, Kusalik A, Napper S. Induction of ligand-specific PrP (C) signaling in human neuronal cells. Prion. 2012;6(5):477–88. doi:10.4161/pri.21914. PMID:22918447.
- Sonati T, Reimann RR, Falsig J, Baral PK, O'Connor T, Hornemann S, Yaganoglu S, Li B, Herrmann US, Wieland B, et al. The toxicity of antiprion antibodies is mediated by the flexible tail of the prion protein. Nature. 2013;501:102–09 doi:10.1038/nature12402. PMID:23903654.
- Reimann RR, Sonati T, Hornemann S, Herrmann US, Arand M, Hawke S, Aguzzi A. Differential toxicity of antibodies to the prion protein. PLoS Pathog. 2016;12(1):e1005401. ppat.1005401. doi:10.1371/journal.ppat.1005401. PMID:26821311.
- Herrmann US, Sonati T, Falsig J, Reimann RR, Dametto P, O'Connor T, Li B, Lau A, Hornemann S, Sorce S, et al. Prion infections and Anti-PrP antibodies trigger converging neurotoxic pathways. PLoS Pathog. 2015;11(2):e1004662. doi:10.1371/journal.ppat.1004662. PMID:25710374.
- Hedlin PD, Cashman NR, Li L, Gupta J, Babiuk LA, Potter AA, Griebel P, Napper S. Design and delivery of a cryptic PrP(C) epitope for induction of PrP(Sc)- specific antibody responses. Vaccine. 2010;28:981–8. doi:10.1016/j.vaccine.2009.10.134. PMID:19925901.
- Marciniuk K, Maattanen P, Taschuk R, Airey D, Potter A, Cashman NR, Griebel P, Napper S. Development of a Multivalent, PrPSc-specific prion vaccine through rational optimization of three disease-specific epitopes. Prion. 2014;32:1988–97.
- Gossert AD, Bonjour S, Lysek DA, Fiorito F, Wuthrich K. Prion protein NMR structures of elk and of mouse/elk hybrids. Proc Natl Acad Sci U S A. 2005;102:646–50. doi:10.1073/pnas.0409008102. PMID:15647363.
- Uger MD, Chai V, Ciolfi V, Chen H, Plawinski E, Mutukura T, Sage A, Demers R, Lau L, Lee P, et al. Abstract 4636: AMF-1c-120: An antibody specific for misfolded prion protein expressed on ovarian cancer cells. Exp Mol Ther. 2013;73:4636–36.
- Mahl P, Clinquet F, Guiot AL, Niin E, Fournials E, Saint-Jean N, Aubert M, Rupprecht CE, Gueguen S. Twenty-year experience of the oral rabies vaccine SAG2 in wildlife: A global review. Vet Res. 2014;45:77. doi:10.1186/s13567-014-0077-8. PMID:25106552.
- Sterner RT, Meltzer MI, Shwiff SA, Slate D. Tactics and economics of wildlife oral rabies vaccination, Canada and the United States. Emerg Infect Dis. 2009;15:1176–84. doi:10.3201/eid1508.081061. PMID:19757549.
- Gupta PK, Sharma S, Walunj SS, Chaturvedi VK, Raut AA, Patial S, Rai A, Pandey KD, Saini M. Immunogenic and antigenic properties of recombinant soluble glycoprotein of rabies virus. Vet Microbiol. 2005;108:207–14. doi:10.1016/j.vetmic.2005.04.007. PMID:15916870.
- Smith ME, Koser M, Xiao S, Siler C, McGettigen JP, Calkins C, Pomerantz RJ, Dietzschold B, Schnell MJ. Rabies virus glycoprotein as a carrier for anthrax protective antigen. Virol. 2006;30:344–56. doi:10.1016/j.virol.2006.05.010
- Taschuk R, Griebel P, Napper S. Carrier protein significantly alters the magnitude, duration and type of antibody response to a peptide epitope from a self-protein. J Immol and Vacc. 2015;1(1):101.
- Buge SL, Richardson E, Alipanah S, Markham P, Cheng S, Kalyan N, Miller CJ, Lubeck M, Udem S, Eldridge J, et al. An adenovirus-simian immunodeficiency virus env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J Virol. 1997;71:8531–41. PMID:9343211.
- Alejo DM, Moraes MP, Liao X, Dias CC, Tulman ER, Diaz-San S, Rood D, Grubman MJ, Silbart LK. An adenovirus vectored mucosal adjuvant augments protection of mice immunized intranasally with an adenovirus-vectored foot-and-mouth disease virus subunit vaccine. Vaccine. 2013;26:2302–09. doi:10.1016/j.vaccine.2013.02.060
- Griscelli F, Opolon P, Saulnier P, Mami-Chouib F, Gautier E, Echchakir H, Angevin E, Chevalier T, Bataille V, Squiban P, et al. Recombinant adenovirus shedding after intratumoral gene transfer in lung cancer patients. Gene Ther. 2003;10:386–95. doi:10.1038/sj.gt.3301928. PMID:12601393.
- Facciabene A, Aurisicchio L, Elia L, Palombo F, Mennuni C, Ciliberto G, La Monica N. Vectors encoding carcinoembryonic antigen fused to the B subunit of heat-labile entertoxin elicit antigen-specific immune responses and antitumor effects. Vaccine. 2007;26:47–58. doi:10.1016/j.vaccine.2007.10.060. PMID:18055074.
- Yamanaka H, Ishibashi D, Yamaguchi N, Yoshikawa D, Nakamura R, Okimura N, Arakawa T, Tsuji T, Katamine S, Sakaguchi S. Enhanced mucosal immunogenicity of prion protein following fusion with B subunit of Escherichia coli heat-labile enterotoxin. Vaccine. 2006;5:2815–23. doi:10.1016/j.vaccine.2005.12.054
- Croyle MA, Callahan SM, Auricchio A, Schumer G, Linse KD, Wilson JM, Brunner LJ, Kobinger GP. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J Virol. 2004;78:912–92. doi:10.1128/JVI.78.2.912-921.2004. PMID:14694122.
- Zakhartchouk AN, Viswanathan S, Mahony JB, Gauldie J, Babiuk LA. Severe acute respiratory syndrome coronavirus nucleocapsid protein expressed by an adenovirus vector is phosphorylated and immunogenic in mice. J Gen Virol. 2005;86:211–15. doi:10.1099/vir.0.80530-0. PMID:15604448
- Croyle MA, Cheng X, Sandhu A, Wilson JM. Development of novel formulations that enhance adenoviral-mediated gene expression in the lung in vitro and in vivo. Mol Ther. 2001;4:22–28. doi:10.1006/mthe.2001.0411. PMID:11472102.
- Mutwiri G, Watts T, Lew L, Beskorwayne T, Papp Z, Baca-Estrada ME, Griebel P. Ileal and jejunal Peyer's patches play distinct roles in mucosal immunity of sheep. Immunol. 1999;97:455–61. doi:10.1046/j.1365-2567.1999.00791.x
