ABSTRACT
Background: Sporadic fatal insomnia (sFI) is a rapid progressive neurodegenerative disease characterised by gradual to perpetual insomnia, followed by dysautonomia, coma and death.Citation1 The cause of sFI was recently mapped to a mutation in a protein, the prion, found in the human brain. It is the unfolding of the prion that leads to the generation of toxic oligomers that destroy brain tissue and function. Recent studies have confirmed that a methionine mutation at codon 129 of the human Prion is characteristic of sFI. Current treatment slows down the progression of the disease, but no cure has been found, yet. Methods: We used Molecular Docking and Molecular Dynamics simulation methods, to study the toxic Fatal-Insomnia-prion conformations at local unfolding. The idea was to determine these sites and to stabilise these regions against unfolding and miss-folding, using a small ligand, based on a phenothiazine "moiety". Conclusion: As a result we here discuss current fatal insomnia therapy and present seven novel possible compounds for in vitro and in vivo screening.
FATAL INSOMNIA
Human Prion proteins have long been associated with incurable diseases in the group of Neurodegenerative disorders. These include spongiform encephalopathies such as Parkinson's disease, Alzheimer's disease, Fatal Familial Insomnia, Kuru, Gerstmann-Sträussler-Scheinker (GSS) syndrome and Creutzfeldt-Jakob Disease (CJD). Although these diseases concern prevalently humans, there are other species suffering from transmissible spongiform encephalopathies (TSE), and these include North American Deer and Elks that experience Chronic Wasting Disease (CWD), minks and monkeys, undergoing Mink Transmissible Encephalopathy (MTE), and sheep, where the disease, commonly known as “scrapie”, affects the hypothalamus. Coincidentally, it was through scrapie that Hunter's and Prusiner's studies led to the discovery of the prion protein. Other cases of Feline Transmissible Encephalopathy (FTE) among cats have also been observed and, lastly, Bovine Spongiform Encephalopathy (BSE), whose outbreak became global in the late 90s, was found to be transmitted among cattle and from cattle to consumer humans (CJD). Michael Alpers in 1964Citation2 discovered that human cannibal ritual practices where brains and corpses were eaten by members of a tribe in Papua New Guinea would lead to a disease named Kuru. The incineration of the brains and corpses, along with the “clinical prohibition” of cannibalism, caused a complete eradication of Kuru. Similarly, the transmission of BSE from cattle to humans (CJD) was associated with the consumption of prion infected meat, although not all cases of CJD are caused by the ingestion of infected meat.Citation3
One particular TSE, Fatal familial insomnia (FFI), whose sporadic form has also been unveiled (sFI), is now the subject of epidemiological surveillance due to its rarity. This neurodegenerative disease, is responsible for the death of less than 60 families in the world. From the onset of the first symptoms, it only takes two years ± four months for the disease to progress to the final stage. The first known case was Silvano's Venetian family, investigated in 1984 by Dr Ignazio Roiter and whose tragic impact inspired the book “The family that couldn't sleep” by D. T. Max.Citation4
This disease has led to our own studies of the human prion protein. These prions, which are named after their “proteinaceous” and “infectious” “character”, are a family of short-length disordered proteins whose mutations have been associated with several neurodegenerative diseases and cancers. In the particular case of Sporadic Fatal Insomnia (SFI) the cytotoxicity is exhibited by the mutant “FI-Prion” (or FI-PrP129). Because of the rapid progression of SFI, the search of small molecules targeting FI-Prions is beneficial.
The discovery of new ligands that may target the prion can be facilitated by understanding the structure-activity relationship of these ligands to their receptors. This can be achieved by using different techniques. We particularly based our work on well-known proven computational analytical methods, especially Molecular Docking and Molecular Dynamics. From these preliminary studies we reached several conclusions on how to approach drug-discovery and drug therapy.
BIOLOGY OF FATAL INSOMNIA AND THE ROLE OF PRIONS
When approaching the disease, one main limitation is given by the fact that both sporadic and familial cases of Fatal Insomnia have been described,Citation5 making it difficult to establish exactly the nature of prion disease. However, what is certain in the mechanism of pathogenicity is that somehow these small mutated proteins (FI-Prions) present a methionine residue in position 129 (Met129) in place of a valine residue (Val129) and are not recognised by proteases. This prevents their hydrolysis and correct metabolism, causing them to accumulate at different sites.Citation6
CURRENT THERAPY
Although several therapies have been proposed for Prion Disease,Citation7 including those for FFI, none were particularly effective long term. In the case of SFI, the lack of success has been identified and this has led to further therapy refinement.Citation7-16 Most drugs also showed effectiveness on in vitro models, but not in vivo.Citation7-16 Age onset and ethnicity are non-discriminative, as proven by case reports.Citation10
As mentioned previously, neurodegenerative disorders are characterised by specific mutations on the human prion protein, precipitating in different areas of the brain manifesting clinically as particularly uncomfortable symptoms. Increased incidence of strokes in patients with Fatal Familial Insomnia has been detected among all other specific symptomsCitation7-16 and amenorrhea is common in female patients.
Because symptomatic treatment with vitamins B6, B12, iron and Folic Acid has offered some improvement in the wellbeing of patients affected by SFI, vitamin supplementation remains a helpful nutritional aspect in the management of the illness. These vitamins are found mainly in fish, fruits and vegetables. Dairy products and other aliments also contain them to a lower extent, however dairy products are still important due to their tryptophan content, which is also beneficial. This strategy has led to prolonged symptomatic relief and to sleep restoration effects, up to 5–7 hours nightly sleep for several consecutive days, within months of therapy.Citation9
Melatonin supplements could also be introduced in the early stages of the disease since plasmatic Melatonin is drastically decreased. Melatonin is a natural endogenous molecule, therefore side effects will be less frequent. Modified release tablets are already commercially available, and in countries like the UK they are prescribed for the treatment of short-term insomnia at a dosage of 2 mg once daily in adults over 55 years old. Adjusting the dosage to induce sleep might also be helpful.
According to more recent cases,Citation8-11 some blood tests reveal that ferritin, thyroidal hormone levels in patients suffering from FFI are low, although not all patients are “sampled”, due to excessive weight loss and for the difficulty in managing the symptoms. This is why the prognostic values of early- or late-stage haematological tests are sometimes considered irrelevant in familial cases, but are occasionally available. The blood tests are often accompanied by cerebrospinal fluid (CSF) examinations, all carried out at early stages, indicating no anomalies, however, recent studies produced results demonstrating that the RT-QuIC assay is positive in FFI patients.Citation11 Late-stage blood tests are not always available, due to the rapid progression of the disorder, especially in familial cases.Citation12-14
Drugs that have been used but that have been unsuccessful in the treatment of SFI include the anti-parasitic Quinacrine, which belongs to the group of anti-protozoan and anti-amoebic compounds. The choice of Quinacrine was linked to its reported effectiveness in vitro, but not in vivo. Failure had been associated with induced drug resistance after Quinacrine use, accompanied by increased Pgp transporter activity across the blood brain barrier (BBB). Furthermore, the withdrawal of the anti-protozoan molecule had been justified by its elevated toxicity and consequent adverse drug reactions. All these effects could be factors in the failure of association therapy with the well-known anti-psychotic agent Chlorpromazine (CPZ), where the likelihood of a competition between the two molecules for the prion protein substrate and consequent displacement of the latter is really high.Citation8, 15-17 Quinacrine is still used as a template to reduce toxicity in modern design strategies.
More recent studies have been focused on Doxycycline, a common tetracycline antibiotic for systemic use which has been proven to be effective in disrupting amyloid fibril formation. The function is not that of inhibiting the prion protein, but of early prophylaxis. The efficacy of Doxycycline (DOXY®) is possibly associated with its ability to cross the BBB and it has been frequently reported that its usage brings increased survival in patients.Citation18
Further modern treatments involve the use of murine monoclonal antibodies targeting the human prion protein. These are currently in clinical trial, but preclinical reports have described their success in extending survival on in vivo rat models.Citation19
Concomitantly, the neuroleptic drugs Diazepam, used as anxiolytic, and Midazolam, used for sedation, seem to give short-term relief in people suffering from both SFI and Creutzfeldt-Jackob Disease (CJD), another prion disorder. Midazolam is usually administered at later stages of the disease.Citation20 The effectiveness of both Diazepam and Midazolam may be connected to the fact that the FI-prion displays high affinity for Gabaergic receptors; Midazolam at high doses has also proven to cause side effects similar to the symptoms shown in fatal familial insomnia. Likewise, Amphotericin B, an antifungal systemic drug has proven to be effective both in vitro and in vivo.Citation21-23 Even if the antifungal agent has been recently abandoned, it could serve as a template for more efficient antifungal drug design that targets the thalamus and crosses the blood brain barrier. By contrast, dopamine receptor binders have been favored in the treatment of other TSE.
Lastly, the class of antipsychotic drugs phenothiazines, widely used in the treatment of insomnia, seems to be successful in short-term therapy. Many patients have confirmed that after days of sleeplessness, Promethazine worked for twenty-four hours. This class of compounds is particularly important to consider, because of their ability to bind and stabilise prion proteins.
PHENOTHIAZINES: FROM NEUROLEPTIC AGENTS TO SLEEP-INDUCERS
The reason why phenothiazinesCitation24-27 make perfect candidates as antipsychotic agents is their ability to cure deleterious psychotic features including hallucinations and agitation. In particular, ChlorpromazineCitation25 is able to suppress both unwanted symptoms, thanks to its anxiolytic action and it is also very effective as sleep inducer. The main use of Chlorpromazine in recent years has been that of sleep-inducer in cases of insomnia and in the treatment of anxiety-generated hiccups. The majority of people taking chlorpromazine for the treatment of insomnia experience improvement in sleep patterns.
In the case of patients suffering from SFI, the efficacy of its administration or of the administration of other phenothiazines, i.e. Phenergan, is only temporary (ca ∼ 24 hours).
FURTHER STUDIES ON PHENOTHIAZINES
We therefore justify the consideration of this class of drugs, commonly prescribed for cases of Insomnia or Chronic Insomnia, but whose effects on SFI cases are very short-lasting, as starting patterns for the design of novel drugs. We particularly emphasise the choice of one of the molecules in this class of compounds, Chlorpromazine, as ligand substrate for the prion protein. The eligibility of this drug as template for further drug design is supported by its good hematoencephalic absorption and resistance to variations in the encephalic environment.Citation26-27
SKETCH OF POSSIBLE MOLECULES
Our group conducted several computational studies centred on molecular dynamics simulation and molecular docking methodsCitation28-31 to study the process of protein unfolding, misfolding. These methodologies are well-known and effectively proven. Initially, we performed docking and molecular dynamics studies on chlorpromazine, and the results we obtained were used as groundwork for the modelling of new agents.
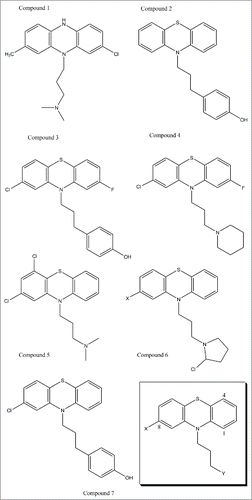
To achieve more sophisticated results, we then analysed both the non-mutated native prion protein and the mutated prion protein forms. We therefore concluded that the class of compounds, mimicking the phenothiazineCitation32 structure that we report sketched below () could be used for further in vitro investigation and for eventual toxicology screens.\raster="rgFigKPRN_A_1368937_F0001_B"
FIGURE 1 (previous page). This figure presents novel drugs designed for in vivo and in vitro studies as possible treatment of Sporadic Fatal Insomnia. The design was achieved with the intent of allowing the methionine in position 129 on the mutated prion protein to be demethylated by proteasic activity. The design was therefore done to increase selectivity, and, effectiveness, by imposing some kind of rigidity on the tail and to refine its directionality. The first compounds were conceived to improve selectivity and toxicity. Some substitutions were executed to enhance affinity, whereas Compound 6 was particularly created to induce N-methylation of the Met129 located on the Fatal Insomnia Prion, however, increased toxicity may be expected, together with potency. Compound 3 and 2 were produced to infiltrate the prion further, perhaps by interacting with tyrosine residues, and to sterically compare with each other. Analogously, Compound 5 could offer further information on both pharmacokinetic and binding behaviours. Compound 4 was designed to underline molecular symmetry and to understand whether fluorine could be interchanged with the chlorine in an aim to decrease nephrotoxicity. Similar observations have been made for Compound 1. Further rigidity may also be affected with the introduction of a double bond in positions 3 and 4 of the tertiary amine. Finally, Compound 7 was docked with its substrate (FI-prion) as a comparative test with the chlorpromazine-substrate (FI-prion) complex and showed promising, although preliminary, results. In the bottom right corner, we can find illustrated the generic pharmacophore for these phenothiazine derivatives.
CONCLUSIONS
This review highlights current and previous drug therapies for the rareCitation33 neurodegenerative disease, Fatal Insomnia. Some of these drug therapies are still being investigated, but have given positive preclinical outcomes to date. The results of our studies are also summarised, and help us justify the reconsideration of abandoned drugs, phenothiazines, and more specifically, possible selective derivatives that we designed and that we here graphically present. Although these designs are still preliminary, we believe that the availability of novel computational and bioinformatics tools which have become more sophisticated in recent years can add to our knowledge. Our findings also suggest that more selective central acting anti-fungal drug molecules could help in the early stages of prion infection.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
ACKNOWLEDGMENTS
We would like to thank the following researchers for their contributions: Professor Philip J. Camp (MD expert), Prof Asa H. Barber, Prof Alex MacKerell (chlorpromazine force fields). We would also like to thank the family of the patients suffering from these disorders.
REFERENCES
- Lugaresi E, Medori R, Montagna P, Baruzzi A, Cortelli P, Lugaresi A, Tinuper P, Zucconi M, Gambetti P. Fatal familial insomnia and dysautonomia with selective degeneration of thalamic nuclei. New England J of Med. 1986;315:997–1003. doi:10.1056/NEJM198610163151605.
- Alpers MP. The epidemiology of kuru: monitoring the epidemic from its peak to its end. Philos Trans R Soc Lond B Biol Sci. 2008;363(1510):3707–17. doi:10.1098/rstb.2008.0071. PMID:18849286.
- McCutcheon S, Alejo Blanco AR, Houston EF, de Wolf C, Tan BC, Smith A, Groschup MH, Hunter N, Hornsey VS, MacGregor IR, et al. All clinically-relevant blood components transmit prion disease following a single blood transfusion: a sheep model of vCJD. PLoS One. 2011;6(8):e23169. doi:10.1371/journal.pone.0023169. PMID:21858015.
- Max DT. The family that couldn't sleep. 1st ed New York: Portobello; 2007
- Montagna P. Fatal familial insomnia: a model disease in sleep physiopathology. Sleep Med Rev 2005;9(5):339–53. doi:10.1016/j.smrv.2005.02.001. PMID:16109494.
- Parchi P, Castellani R, Cortelli P, Montagna P, Chen SG, Petersen RB, Manetto V, Vnencak-Jones CL, McLean MJ, Sheller JR, et al. Regional distribution of protease-resistant prion protein in fatal familial insomnia. Ann Neurol. 1995;38(1):21–29. doi:10.1002/ana.410380107. PMID:7611720.
- Panegyres PK, Armari E. Therapies for human prion diseases. Am J Neurodegener Dis. 2013;2(3):176–86. PMID:24093082.
- Lugaresi E, Provini F. Agrypnia excitata: clinical features and pathophysiological implications. Sleep Med Rev. 2001;5(4):313–22. doi:10.1053/smrv.2001.0166. PMID:12530995.
- Schenkein J, Montagna P. Self-management of fatal familial insomnia. Part 2: case report. MedGenMed. 2006;8(3):66. PMID:17406189.
- Tabernero C. Fatal Familial Insomnia: clinical, neuropathological, and genetic description of a Spanish family. J Neurol Neurosurg Psychiatry. 2000;68(6):774–77. doi:10.1136/jnnp.68.6.774. PMID:10811705.
- Llorens F, Thüne K, Schmitz M, Ansoleaga B, Frau-Méndez MA, Cramm M, Tahir W, Gotzmann N, Berjaoui S, Carmona M, et al. Identification of new molecular alterations in fatal familial insomnia. Human Mol Genet. 2016;25 (12):2417–36. PMID:27056979.
- Zhang B. Fatal familial insomnia: a middle-age-onset Chinese family kindred. Sleep Med. 2010;11(5):498–99. doi:10.1016/j.sleep.2009.11.005. PMID:20133192.
- Sawada H, Oeda T, Umemura A, Tomita S, Kohsaka M, Park K, Yamamoto K, Sugiyama H. Baseline C-reactive protein levels and life prognosis in parkison disease. PLoS One. 2015;10(7):e0134118. doi:10.1371/journal.pone.0134118. PMID:26218286.
- Guaraldi P, Calandra-Buonaura G, Terlizzi R, Montagna P, Lugaresi E, Tinuper P, Cortelli P, Provini F. Oneiric stupor: the peculiar behaviour of agrypnia excitata. Sleep Med. 2011;12:S64–67. doi:10.1016/j.sleep.2011.10.014. PMID:22136903.
- Benito-León J. Combined quinacrine and chlorpromazine therapy in fatal familial insomnia. J Neuropharmacol. 2004;27(4):201–03. doi:10.1097/01.wnf.0000134853.36429.0e.
- Gayrard V, Picard-Hagen N, Viguié C, Laroute V, Andréoletti O, Toutain PL. A possible pharmacological explanation for quinacrine failure to treat prion diseases: pharmacokinetic investigations in a ovine model of scrapie. Br J Pharmacol. 2005;144(3):386–93. doi:10.1038/sj.bjp.0706072. PMID:15655516.
- Huang Y, Okochi H, May BC, Legname G, Prusiner SB, Benet LZ, Guglielmo BJ, Lin ET. Quinacrine is mainly metabolized to mono-desethyl quinacrine by Cyp3a4/5 and its brain accumulation is limited by P-Glycoprotein. Drug Metab Dispos. 2006;34(7):1136–44. doi:10.1124/dmd.105.008664. PMID:16581945.
- Ghaemmaghami S, Ahn M, Lessard P, Giles K, Legname G, DeArmond SJ, Prusiner SB. Continuous quinacrine treatment results in the formation of drug-resistant prions. PLoS Pathog. 2009;5(11):e1000673. doi:10.1371/journal.ppat.1000673. PMID:19956709.
- Noack A, Noack S, Hoffmann A, Maalouf K, Buettner M, Couraud PO, Romero IA, Weksler B, Alms D, Römermann K, et al. Drug-induced trafficking of p-glycoprotein in human brain capillary endothelial cells as demonstrated by exposure to mitomycin C. PLoS One. 2014;9(2):e88154. doi:10.1371/journal.pone.0088154. PMID:24505408.
- Klyubin I, Nicoll AJ, Khalili-Shirazi A, Farmer M, Canning S, Mably A, Linehan J, Brown A, Wakeling M, Brandner S, et al. Peripheral administration of a humanized anti-PrP antibody blocks alzheimer's disease αβ synaptotoxicity. J Neurosci. 2014;34(18):6140–45. doi:10.1523/JNEUROSCI.3526-13.2014. PMID:24790184.
- Wieser HG, Schwarz U, Blättler T, Bernoulli C, Sitzler M, Stoeck K, Glatzel M. Serial EEG findings in sporadic and iatrogenic creutzfeldt-jakob disease. Clin Neurophysiol. 2004;115(11):2467–78. doi:10.1016/j.clinph.2004.05.032. PMID:15465434.
- Mangé A, Nishida N, Milhavet O, McMahon HE, Casanova D, Lehmann S. Amphotericin B inhibits the generation of the scrapie isoform of the prion protein in infected cultures. J Virol. 2000;74(77):3135–40. doi:10.1128/JVI.74.7.3135-3140.2000. PMID:10708429.
- Pocchiari M, Schmittinger S, Masullo C. Amphotericin B delays the incubation period of scrapie in intracerebrally inoculated hamsters. J Gen Virol. 1987;68(Pt 1):219–23. doi:10.1099/0022-1317-68-1-219. PMID:2433387.
- Rosengren LE, Persson LI. Chlorpromazine treatment of blood-brain barrier dysfunction. A quantitative and fluorescence microscopical study on small cerebral stab wounds in the rat. Acta Neuropathol. 1979;46(1-2):145–50. doi:10.1007/BF00684816. PMID:452855.
- Yoshii K, Kobayashi K, Tsumuji M, Tani M, Shimada N, Chiba K. Identification of human cytochrome P450 isoforms involved in the 7-hydroxylation of chlorpromazine by human liver microsomes. Life Sci. 2000;67(2):175–84. doi:10.1016/S0024-3205(00)00613-5. PMID:10901285.
- Molnár J, Szabo D, Mándi Y, Mucsi I, Fischer J, Varga A, König S, Motohashi N. Multidrug resistance reversal in mouse lymphoma cells by heterocyclic compounds. Anticancer Res. 1998;18(4C):3033–38. PMID:9713505.
- Nakamura K, Yokoi T, Inoue K, Shimada N, Ohashi N, Kume T, Kamataki T. Oxidation of histamine H1 antagonist mequitazine is catalyzed by cytochrome P450 2D6 in human liver microsomes. Pharmacogenetics. 1996;6(5):449–57. doi:10.1097/00008571-199610000-00009. PMID:8946477.
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2010;31(2):455–61. PMID:19499576.
- MacKerell, Jr AD, et al. Atomistic models and force fields. Comput Biochem Biophys., Inc. New York: Taylor & Francis; 7–38, 2001
- Tabaee Damavandi P, et al. Master's Thesis. “Computational studies of falcipain-2 irreversible peptidomimetic inhibitors”; 2010
- Tabaee Damavandi P, et al. Doctoral Thesis. “Novel applications of vibrational dynamics in the study of protein misfolding and neurodegenerative disorders”, Queen Mary University of London; 2016
- Widiger, Alexander H Jr. assignor to The Dow Chemical Company, Midland, Mich., “PREPARATION OF PHENOTHIAZINE” a corporation of Michigan No Drawing. Application February 27, 1942, Serial No. 432,641. ( Patented Oct. 10, 1944)
- Blase, JL, Cracco L, Schonberger LB, Maddox RA, Cohen Y, Cali I, Belay ED. Sporadic fatal insomnia in an adolescent. Pediatrics. 2014;133(3):e766–e770. doi:10.1542/peds.2013-1396. PMID:24488737.
