ABSTRACT
Prion diseases are fatal neurodegenerative disorders that affect humans and animals. Although various small molecules have been evaluated for application in the treatment of prion diseases, none have been shown to be efficacious. Expanding our knowledge of these molecules is important for understanding of the complex mechanisms of prion diseases. To improve access to the scattered information on small molecules related to prion diseases, we built a database of therapeutic molecules associated with prion diseases (THERPA, therpa.pythonanywhere.com). THERPA includes 119 small molecules and their 283 relationships with prion diseases. THERPA is an interactive visual database and useful for improving search efficiency which can help researchers identify intrinsic small molecules that can be used for developing therapeutics for prion diseases.
Introduction
Prion diseases are rare, fatal, neurodegenerative disorders that affect humans and animals and are characterized by abnormal accumulation of misfolding prion protein (PrPSc), affecting the central nervous system [Citation1–3]. Small molecules that interact with proteins can influence biological processes and can be used to attenuate and treat diseases and bacterial infections [Citation4,Citation5]. Although various small molecules have been also used to inhibit PrPSc accumulation or alleviate prion diseases, appropriate therapies for prion diseases have not yet been developed [Citation6–9], and a meta-analysis of published articles on treatment strategies for prion diseases is needed. However, such studies are difficult because data on this topic are not easily accessed. Accordingly, we developed a manually curated database containing detail information on small molecules that may inhibit PrPSc or cure prion diseases, designated THERPA. THERPA is a public small molecule database that collects information from public sources and Pathway studio software. THERPA includes relevant data such as treatment concentrations, studied species, and roles of small molecules in PrP function and prion diseases progression. Moreover, the website also includes a pie chart and a treemap for understanding characteristics of small molecules, facilitating the analysis of prion diseases-related small molecules. All data, including detail information on various small molecules can be found on the navigation bar at the top in the main page of THERPA website.
Results and discussion
THERPA was designed to allow researchers to easily explore and analyze data of interest. A total of 119 small molecules and their 283 relationships from 129 articles (Supplementary Table 1) are included in THERPA. The database consist of six submenus.
Main table
Detailed information for the 119 small molecules is listed and summarized in the Main table. The data are classified into 14 categories, and all categories can be filtered according to selected terms in each search field. Articles (abstracts or full articles) are listed according to the pubmed identifier (PMID). The chemical structures of each small molecule can be observed by clicking the PubChem column.
Materials for experiments
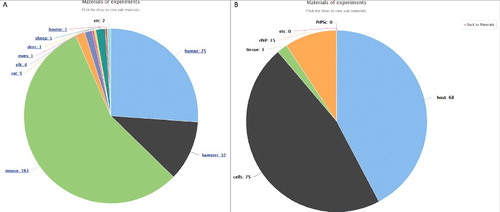
All materials described in the Main table are shown on a pie chart. Detailed information of the materials can be found by clicking the slices on the pie chart ().
Figure 1. Example pie chart of materials used in experiments described in the curated public articles. (A) All materials organized in this study were visualized. (B) Sub-pie chart of materials originated from mice. Click “Back to Materials” on the upper right panel of image to return to the original pie chart.
Treemap
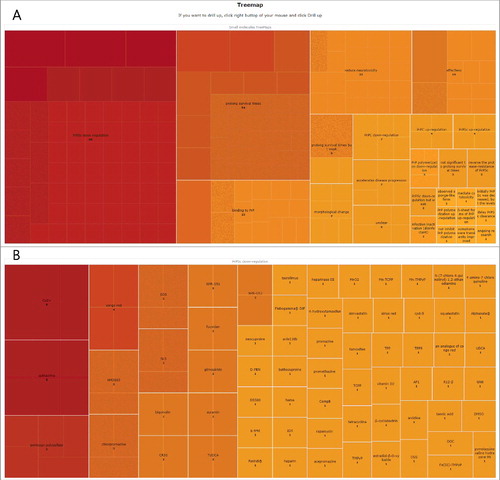
Tree-structured data describing the relationships of the 119 small molecules to PrP and/or prion diseases are provided (). Each branch of the tree is given as a rectangle, tiled with smaller rectangles representing sub-branches. Each branch indicates a relationship of small molecules to PrP and/or prion diseases progression. The first sub-branch provides small molecules included in each branch. The second sub-branch provides the PMID describing the role of each small molecule. This treemap facilitates our understanding of the studied roles of the small molecules in prion diseases.
Figure 2. A tree map. Each branch of the tree is given as a rectangle, tiled with smaller rectangles representing sub-branches. Each branch indicates a relationship of small molecules to PrP and/or prion diseases progression. The first sub-branch provides small molecules included in each branch. The second sub-branch provides the PMID describing the role of each small molecule. (A) Two hundred eighty-three relationships between small molecules and PrP and/or prion diseases are shown. (B) Sub-tree map structure of PrPSc downregulation is shown.
File export
All data or filtered data can be downloaded as CSV or EXCEL files on this page. Copy and print are also available.
Submit data
Users can upload data using the prescribed form on this page.
List of submitted data
All submitted data by users will be listed here automatically.
Details on 119 small molecules, including copper and quinacrine, are provided in THERPA. The most commonly curated molecule was copper; copper has been reported to be involved in downregulation of PrPSc and prolong survival [Citation6, Citation10, Citation11]. However, the role of copper in prion diseases is unclear because some studies have also reported that copper does not alleviate prion diseases [Citation7, Citation12, Citation13]. Quinacrine has been shown to be efficacious in cell models for prion diseases [Citation8, Citation14]. However, quinacrine has failed to provide clinical benefits in humans [Citation9, Citation15, Citation16]. These were found in the Main table.
The materials used in the articles are visualized in the pie chart, under the submenu “Materials for experiments”. Samples from mice were commonly used, and most data were obtained by studying the incubation period or PrPSc regulation. Over 90% of materials were from humans, mice, and hamsters.
The treemap displays hierarchical images of the roles of small molecules. The structure of the treemap facilitates the identification of molecules that have been extensively studied and of small molecules that have been studied with regard to PrP and prion diseases. The most frequently reported role was PrPSc downregulation (34.98%), and copper and quinacrine, which were described above, were commonly reported as small molecules in the study of PrPSc downregulation. 32 small molecules were found to be related to prolonged survival, and pentosan polysulfate was one of the most commonly studied molecules related to prolonged survival times.
THERPA is the first repository for prion diseases data and has a user-friendly interface that allows easy access to data of 119 small molecules and their 283 relationships with PrP and/or prion diseases progression. Curated data from hundreds of published studies are included in THERPA. The detail information in THERPA will facilitate meta-analyses and the development of therapeutic strategies for prion diseases.
Materials and methods
PMIDs were collected using Pathway Studio software and a literature search using the keywords “prion diseases” with “small molecules” found in the title or full text up to December 2017. All information curated in the database was collected from full text articles using collected PMIDs. All small molecules listed in the database were selected as described in . A total of 119 small molecules and their 283 relationships were curated.
The web interface for THERPA was built using Django (https://www.djangoproject.com/) with the SQLite database engine. To create the HTML part of the interface, we used Bootstrap (http://getbootstrap.com/) and JQuery, a Java Script framework (https://jquery.com/). The Asynchronous JavaScript and XML (AJAX) techniques were implemented to efficiently search the curated information.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary_Table_1.xlsx
Download MS Excel (50.3 KB)Additional information
Funding
References
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 09;216(4542):136–44. doi:10.1126/science.6801762
- Kitamoto T, Muramoto T, Mohri S, et al. Abnormal isoform of prion protein accumulates in follicular dendritic cells in mice with Creutzfeldt-Jakob disease. J Virol. 1991 Nov;65(11):6292–5.
- Brandner S, Isenmann S, Raeber A, et al. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996 Jan 25;379(6563):339–43. doi:10.1038/379339a0
- Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001 Jun;65(2):232–60. doi:10.1128/MMBR.65.2.232-260.2001
- Pullman TN, Craige B, Jr., et al. Comparison of chloroquine, quinacrine (atabrine) and quinine in the treatment of acute attacks of sporozoite-induced vivax malaria, Chesson strain. J Clin Invest. 1948 May;27(3 Pt1):46–50. doi:10.1172/JCI101970
- Giese A, Levin J, Bertsch U, et al. Effect of metal ions on de novo aggregation of full-length prion protein. Biochem Biophys Res Commun. 2004 Aug 06;320(4):1240–6. doi:10.1016/j.bbrc.2004.06.075
- Mitteregger G, Korte S, Shakarami M, et al. Role of copper and manganese in prion disease progression. Brain Res. 2009 Oct 06;1292:155–64. doi:10.1016/j.brainres.2009.07.051
- Doh-Ura K, Iwaki T, Caughey B. Lysosomotropic agents and cysteine protease inhibitors inhibit scrapie-associated prion protein accumulation. J Virol. 2000 May;74(10):4894–7. doi:10.1128/JVI.74.10.4894-4897.2000
- Scoazec JY, Krolak-Salmon P, Casez O, et al. Quinacrine-induced cytolytic hepatitis in sporadic Creutzfeldt-Jakob disease. Ann Neurol. 2003 Apr;53(4):546–7. doi:10.1002/ana.10530
- Sigurdsson EM, Brown DR, Alim MA, et al. Copper chelation delays the onset of prion disease. J Biol Chem. 2003 Nov 21;278(47):46199–202. doi:10.1074/jbc.C300303200
- Hijazi N, Shaked Y, Rosenmann H, et al. Copper binding to PrPC may inhibit prion disease propagation. Brain Res. 2003 Dec 12;993(1–2):192–200. doi:10.1016/j.brainres.2003.09.014
- Siggs OM, Cruite JT, Du X, et al. Disruption of copper homeostasis due to a mutation of Atp7a delays the onset of prion disease. Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13733–8. doi:10.1073/pnas.1211499109
- Varela-Nallar L, Gonzalez A, Inestrosa NC. Role of copper in prion diseases: deleterious or beneficial? Curr Pharm Des. 2006;12(20):2587–95.
- Korth C, May BC, Cohen FE, et al. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc Natl Acad Sci U S A. 2001 Aug 14;98(17):9836–41. doi:10.1073/pnas.161274798
- Martinez-Lage JF, Rabano A, Bermejo J, et al. Creutzfeldt-Jakob disease acquired via a dural graft: failure of therapy with quinacrine and chlorpromazine. Surg Neurol. 2005 Dec;64(6):542–5. discussion 545. doi:10.1016/j.surneu.2005.03.035
- Wroe SJ, Pal S, Siddique D, et al. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: A case report. Lancet. 2006 Dec 09;368(9552):2061–7. doi:10.1016/S0140-6736(06)69835-8