ABSTRACT
Cellular prion protein (PrPC) is expressed in a wide variety of stem cells in which regulates their self-renewal as well as differentiation potential. In this study we investigated the presence of PrPC in human dental pulp-derived stem cells (hDPSCs) and its role in neuronal differentiation process. We show that hDPSCs expresses early PrPC at low concentration and its expression increases after two weeks of treatment with EGF/bFGF. Then, we analyzed the association of PrPC with gangliosides and EGF receptor (EGF-R) during neuronal differentiation process. PrPC associates constitutively with GM2 in control hDPSCs and with GD3 only after neuronal differentiation. Otherwise, EGF-R associates weakly in control hDPSCs and more markedly after neuronal differentiation.
To analyze the functional role of PrPC in the signal pathway mediated by EGF/EGF-R, a siRNA PrP was applied to ablate PrPC and its function. The treatment with siRNA PrP significantly prevented Akt and ERK1/2 phosphorylation induced by EGF.
Moreover, siRNA PrP treatment significantly prevented neuronal-specific antigens expression induced by EGF/bFGF, indicating that cellular prion protein is essential for EGF/bFGF-induced hDPSCs differentiation.
These results suggest that PrPC interact with EGF-R within lipid rafts, playing a role in the multimolecular signaling complexes involved in hDPSCs neuronal differentiation.
1. Introduction
Stem cells are primitive and unspecialized cells with the ability to develop into different cells types using a process called cell differentiation [Citation1]. The concept of “staminality” is often associated with embryonic stem cells but in recent years there has been a growing interest in adult stem cells that do not require embryo manipulation.
A kind of adult stem cells, mesenchymal stem cells, show self-renewal, multilineage differentiation and in vitro proliferation ability after long-term cultures [Citation2]. This kind of cells have been isolated from several tissues, including bone marrow, umbilical cord blood, human dental pulp and adipose tissue [Citation3–6].
Their proliferative capacity, multipotency, and high differentiation power besides the ability to repair tissues make these cells useful in regenerative medicine [Citation7]. Among the possible sources, dental pulp is particularly interesting for ease retrieval, multipotency and bioethical considerations.
Human dental pulp-derived stem cells (hDPSCs) show plastic adherence and are characterized by a typical fibroblast-like morphology. They express specific markers for mesenchymal stem cells (i.e. CD44, CD90, CD105, STRO-1) and are negative for hematopoietic markers (CD14, CD19), but capable of in vitro differentiation into odontoblasts, osteoblasts, chondrocytes, adipocytes and neurons [Citation8–10]. Several works have shown that hDPSCs represent a highly heterogeneous population with distinct clones and differences in proliferative and differentiating capacity [Citation11,Citation12]. In particular, hDPSCs show the ability to differentiate into neuronal-like cells [Citation13] or dopaminergic neuron-like cells [Citation14]. It makes them as a cellular model candidate for the study and treatment of neurodegenerative diseases, such as Alzheimer, Parkinson and Huntington disease [Citation15–17].
Strong evidence shows relationship between cellular prion protein (PrPC) and stem cells. In fact, PrPC, a cell surface protein, is expressed in a wide variety of stem cells, including embryonic and hematopoietic stem cells and its function has been linked to stem cells biology modulating the proliferation and self-renewal of these cells [Citation18–20].
PrPC is highly conserved in mammalian and is present on all nucleated cells, although it's mainly expressed in the central and peripheral nervous system. PrPC is involved in many cellular processes, such as synaptic plasticity, calcium homeostasis, copper metabolism, apoptosis and cellular resistance to oxidative stress [Citation21–25]. A recent implication concerns the possible role of PrPC in neuronal differentiation processes of stem cells. In fact, during the neurogenic differentiation process, PrPC expression increases [Citation26], since PrPC plays a role in neuritogenesis [Citation20, Citation27]. Moreover, PrPC drives the differentiation of human embryonic stem cells into neurons, oligodendrocytes and astrocytes [Citation28].
The expression of PrPC makes mesenchymal stem cells good candidates to develop in vitro system for the study of prion infectivity and multiplication [Citation15].
Previous works suggest that lipid rafts and their components, as gangliosides, are essential for neuronal differentiation of different types of stem cells [Citation29–31].
Since PrPC is constitutively present in lipid rafts [Citation32, Citation33] and in a wide variety of stem cells [Citation18, Citation19], the purpose of this study was to investigate the possible role of PrPC during neuronal differentiation of human dental pulp-derived stem cells.
2. Results
2.1. Characterization of hDPSCs and neuronal differentiation
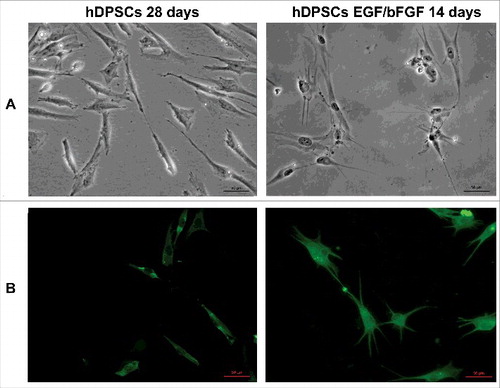
Stem cells were established from human dental pulp tissue isolated from third molars and cultivated as described above and in a precedent work [Citation30]. In fact, the established cells expressed multipotent mesenchymal stromal specific surface antigens, such as CD44, CD90, CD105 and STRO1 [Citation8, Citation30], but not the hematopoietic markers CD14 and CD19 [Citation30]. Moreover, after stimulation with EGF/bFGF, hDPSCs slow their growth and after two weeks it was possible to observe neurites outgrowth () and the presence of specific neuronal markers, as β3-tubulin ().
2.2. Presence of PrPC in established and neuronal differentiated hDPSCs by flow cytometry, western blot and immunofluorescence analysis
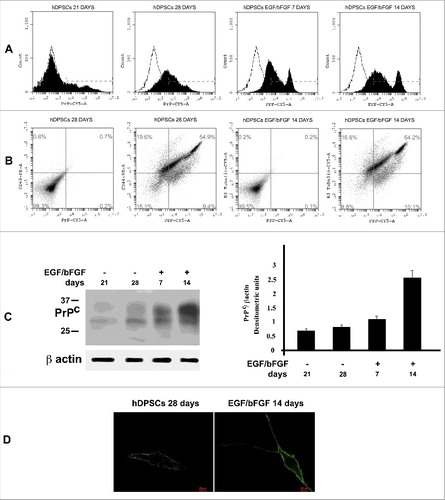
In order to verify the presence of PrPC, we performed flow cytometry analysis of hDPSCs at 21 and 28 days from dental pulp isolation and after neuronal differentiation with EGF/bFGF for additional 1 o 2 weeks (7 and 14 days). As shown in , flow cytometry analysis showed at 21 days a weakly positive staining for PrPC expression. This value increased at 28 days and after differentiation with EGF/bFGF a further increase in the expression (7 and 14 days) of the PrPC was observed. Moreover, to analyze simultaneous expression of antigens of staminality and PrPC in untreated hDPSCs or of neuronal marker and PrPC in EGF/bFGF stimulated cells, we developed double staining with anti-CD44 or anti-β3-tubulin and anti-PrP. Flow cytometric analysis highlighted that in control hDPSCs more than 54% of cells were positive for CD44 and PrPC and, after neuronal differentiation, more than 64% of the cells were positive for both antigens (PrPC and β3-tubulin) (). The expression of PrPC in hDPSCs were confirmed by western blot () and fluorescence analysis ().
Figure 2. PrPC expression in hDSPCs. (A) Flow cytometry analysis of PrPC expression at 21 and 28 days from dental pulp separation and after additional 7 and 14 days with EGF/bFGF. Histograms represent log fluorescence vs cell number, gated on cell population of a side scatter/forward scatter (SS/FS) histogram. Cell number is indicated on the y-axis and fluorescence intensity is represented on the x-axis. Each panel was compared with the corresponding IgG negative isotype control. A representative experiment among 3 is shown. (B) Double staining flow cytometry analysis of PrP/CD44 in control hDPSCs (28 days from pulp separation) and PrP/ β3-tubulin after stimulation with EGF/bFGF for additional 14 days. Histograms represent log fluorescence PE vs log fluorescence CY5, gated on cell population of a side scatter/forward scatter (SS/FS) histogram. CD44-PE fluorescence is indicated on the y-axis and PrP-CY5 fluorescence intensity is represented on the x-axis. Each panel was compared with the corresponding IgG negative isotype control. A representative experiment among 3 is shown. (C) Western blot analysis of PrPC expression at 21 and 28 days from dental pulp separation and after additional 7 and 14 days with EGF/bFGF, using anti-PrP SAF32. Loading control was evaluated using anti-β-actin. Densitometric analysis of bands from the representative western blot is reported in panel on the right as Mean ± SD. (D) Immunofluorescence analysis of hDPSC untreated o treated with EGF/bFGF, using anti-PrP SAF32.
2.3. Association of PrPC with gangliosides and EGF-R in hDPSCs during neuronal differentiation
Since several gangliosides [Citation30, Citation31], as well as EGF receptor [Citation34], have been shown to be components of the signaling complex within microdomains involved in neuronal differentiation of hDPSCs, we investigated the interaction of PrPC with gangliosides (GM2 and GD3) or EGF receptor. PrPC immunoprecipitates from both hDPSCs, untreated or stimulated with EGF/bFGF for 2 weeks, were subjected to dot blot analysis. Immunolabeling with anti-GM2 showed that PrPC and GM2 were associated in control hDPSCs, but not in differentiated cells (). On the contrary, labeling with anti-GD3 showed association only in differentiated cells (). Moreover, immunostaining with anti-EGF-R revealed that PrPC and EGF-R were weakly associated in control hDPSCs and this association was increased in differentiated cells for 2 weeks with EGF/bFGF ().
Figure 3. Analysis of PrPC association with gangliosides and EGF-R by coimmunoprecipitation. hDPSCs, untreated or treated with EGF/bFGF for 14 days, were lysed in lysis buffer, followed by immunoprecipitation with anti-PrP SAF32. A mouse IgG isotypic control was employed. The immunoprecipitates were spotted onto nitrocellulose, and incubated with anti-GM2 (A), anti-GD3 (B) and anti-EGF-R (C), as described in Materials and methods. A representative experiment among 3 is shown. Bar graph in the right panel shows densitometric analysis. Results represent the Mean ± SD from 3 independent experiments, *p < 0.01. The immunoprecipitates were checked using the anti-PrP 6H4 (D).
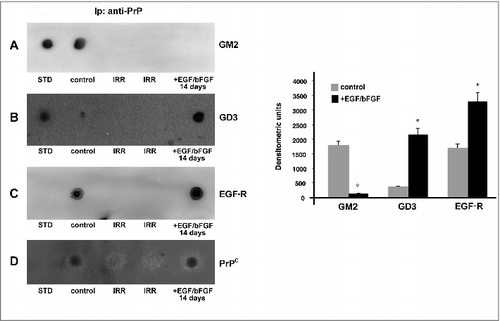
The immunoprecipitate was revealed as PrPC, as detected by dot blot, using the anti-PrP 6H4 mAb (). In control samples the immunoprecipitation with IgG with irrelevant specificity, under the same condition, did not result in detectable levels of gangliosides or EGF-R ().
2.4. PrPC regulates signal pathways induced from EGF/EGF-R during neuronal differentiation of hDPSCs
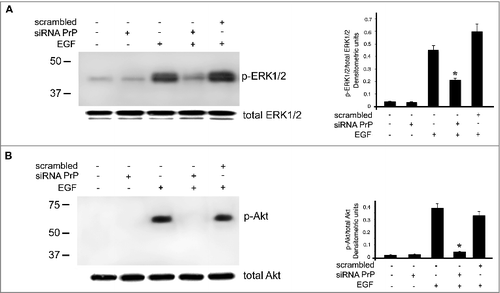
As reported from other authors [Citation34], PrPC was able to regulate the activation of MAP kinase and Protein Kinase B (Akt) by the modulation of EGF-R in N2a cells. In this context, we hypothesize that in hDPSCs PrPC may be able to regulate the activation of Akt and ERK1/2. hDPSCs, either untreated or treated with siRNA PrP for 72 h at 37°C, were stimulated with EGF for 5 min at 37°C. Western blot analysis showed the activation of Akt and ERK1/2 in the sample stimulated with EGF, while the activation was prevented in the sample pretreated with siRNA PrP plus EGF ().
Figure 4. Effects of PrPC silencing on ERK and Akt phosphorylation induced by EGF. hDPSCs, untreated or treated with 20 ng/ml EGF, in the presence or in the absence of pre-treatment with siRNA PrP or scrambled siRNA, were analyzed by Western blot, using anti-pERK1/2, anti-total ERK1/2 (A), anti-pAkt and anti-total Akt (B), Densitometric analysis is shown in the right. Results represent the Mean ± SD from 3 independent experiments, *p <0.01 siRNA PrP treated cells vs EGF treated cells. As control, scrambled siRNA was employed in each experiment.
2.5. Plasma membrane PrPC is required for ERK and Akt activation
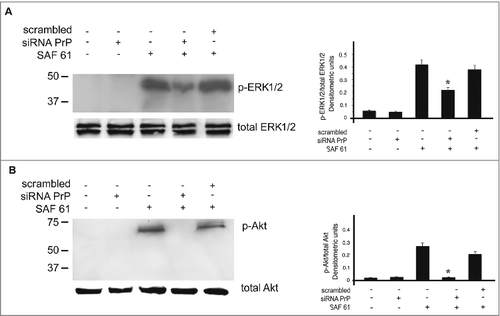
PrPC is a GPI-anchored protein present in lipid rafts [Citation35] and in intracellular compartment, such as endosomes, rough endoplasmic reticulum and Golgi apparatus [Citation36]. To understand which PrPC fraction is responsible for the activation of ERK1/2 and Akt, we used an anti-PrP SAF61, which is able to cluster PrPC into cell membrane [Citation37, Citation38]. hDPSCs, untreated or pre-treated with siRNA PrP for 72 h, were stimulated with SAF61 for 5 min at 37°C. Western blot analysis of p-ERK1/2 and p-Akt indicated that SAF61 induced their phosphorylation, which is significantly reduced after PrPC silencing (). The result show that PrPC plasma membrane is responsible for the activation of ERK and Akt signaling pathway in hDPSCs.
Figure 5. Plasma membrane PrPC is required for signal transduction. hDPSCs cells, treated with siRNA PrP or scrambled for 72 hours, were stimulated with anti PrP SAF61 mAb for 10 min and analyzed by Western blot, using anti-pERK1/2, anti-total ERK1/2 (A), anti-pAkt, and anti-total Akt (B). Densitometric analysis is shown in the right panel. Results represent the Mean±SD from 3 independent experiments, *p <0.01 siRNA PrP treated cells vs SAF61 treated cells.
2.6. Role of PrPC during neuronal differentiation of hDPSCs
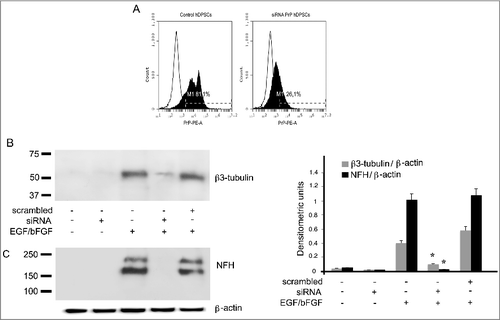
In order to evaluate the role of PrPC in the process of neuronal differentiation of hDPSCs, a small interfering RNA (siRNA) was applied to ablate PrPC and its function. Flow cytometry analyses of siRNA-treated cells revealed that PrPC expression appeared significantly reduced as compared to control cells (); to verify the neuronal differentiation, hDPSCs were tested by western blot analysis with β3-tubulin () and NFH (). The data show that silencing of PrPC by siRNA affected the neuronal differentiation process of hDPSCs, induced by EGF/bFGF after 2 weeks ().
Figure 6. Role of PrPC during neuronal differentiation of hDPSCs induced by EGF/bFGF. (A) Flow cytometry analysis of PrPC expression in hDPSCs, untreated or treated with siRNA PrP for 72 hours. Histograms represent log fluorescence vs cell number, gated on cell population of a side scatter/forward scatter (SS/FS) histogram. Cell number is indicated on the y-axis and fluorescence intensity is represented on the x-axis. Each panel was compared with the corresponding IgG negative isotype control. A representative experiment among 3 is shown. (B-C) Western blot analysis of β3-tubulin, and NFH expression in hDPSCs, untreated or treated with 20 ng/ml EGF and 40 ng/ml bFGF for 14 days, in the presence or in the absence of pre-treatment with siRNA PrP or scrambled siRNA for 72 hours. Densitometric analysis is shown in the right panel. Results represent the Mean±SD from 3 independent experiments. *p <0.01 siRNAPrP treated cells vs EGF/bFGF treated cells.
3. Discussion
In this study we analyzed the role of cellular prion protein in hDPSCs isolated from third molars of healthy subjects and demonstrated a key role for PrPC during neural differentiation. hDPSCs were characterized in our previous work [Citation30], revealing that these cells express well known multipotent mesenchymal stromal-specific surface antigens, such as CD44, CD90, CD105 and STRO-1 [Citation9, Citation39, Citation40]. The scientific literature has shown that these cells were able to differentiate to neurons after EGF/bFGF treatment [Citation41, Citation42].
In a previous work [Citation30] we reported that in hDPSCs lipid rafts were present and gangliosides represented major constituents [Citation31]. GM2 was the most representative ganglioside in hDPSCs, while GD3 was present exclusively during neuronal differentiation of human pulp-derived stem cells [Citation30, Citation31]. In the present work we analyzed the presence of PrPC and its involvement in the neural differentiation process. First, we investigated the presence of PrPC in control and differentiated hDPSCs. Flow cytometry analysis showed a weak staining at 21 days for PrPC expression and this value increased at 28 days. After differentiation with EGF/bFGF, a further increase in the expression (7 and 14 days) of the PrPC was observed.
These data are agreement with several authors who showed that PrPC is expressed in a wide variety of stem cells, including embryonic and hematopoietic stem cells [Citation18, Citation19], where it is involved in the regulation of self-renewal, in their differentiation potential and in fate restriction of embryonic stem cell [Citation43, Citation44].
Since it was already reported [Citation45] that PrPC is associates with gangliosides within lipid rafts, we analyzed whether PrPC was associated with GM2 or GD3 in hDPSCs.
Our results showed that PrPC is able to associate with GM2 specifically in control hDPSCs whereas, after neuronal differentiation of hDPSCs, it associates preferentially with GD3.
On the other hand, EGF receptor has been shown to be a component of the signaling complex within microdomains involved in several functions in N2a [Citation34]. Thus, we investigated the interaction of PrPC with EGF receptor in hDPSCs. Dot blot analysis with anti-EGF-R of PrPC immunoprecipitates revealed that these two molecules are weakly associated within lipid rafts in control hDPSCs and the association increases after neuronal differentiation of hDPSCs.
These data are supported by other authors, who highlighted the association of PrPC with two components of the EGF-R macromolecular complex, such as Grb2 and p-Src. This indicates that PrPC may be part of the cell membrane complexes that regulate EGF/EGF-R signaling [Citation34].
We hypothesized that, after neuronal induction, EGF-R is recruited within lipid rafts, where, interacting with PrPC, triggers the signal transduction.
Indeed, lipid rafts are structures capable to concentrate specific receptors at cell plasma membrane, involved in signal transduction starter. On this basis, we evaluated whether PrPC might be able to regulates signal pathways induced from EGF/EGF-R during neuronal differentiation of hDPSCs. As reported by other authors [Citation34], PrPC is able to regulate the activation of MAP kinase and Protein Kinase B (Akt) by the modulation of EGF-R in N2a cells and SK-N-SH. In this context, we hypothesized that in hDPSCs PrPC may be able to regulate the activation of ERK1/2 and Akt. In fact, siRNA PrP pretreatment for 72 h resulted in reduction of the activation of ERK1/2 and Akt induced by EGF.
In our cellular model, we demonstrated that kinase activation (e.g., ERK1/2 and Akt) was mediated by the GPI-anchored plasma membrane PrPC and the association with EGF-R was increased after neuronal induction.
Since some authors had highlighted the role of PrPC during differentiation process and, in particular, [Citation27] PrPC contribution to the acquisition of neuronal polarization by modulating β1 integrin activity, we investigated the role of PrPC in the process of neuronal differentiation of hDPSCs. A small interfering RNA (siRNA PrP) was employed to ablate PrPC and its function. Silencing of PrPC by siRNA affected the neuronal differentiation process on hDPSCs, induced by EGF/bFGF for 2 weeks.
These data pointed out the key role of PrPC in the process of neuronal differentiation in hDPSCs. Taken together, our results suggest that PrPC interact with EGF-R within lipid rafts, playing a role in the multimolecular signaling complexes involved in hDPSCs neuronal differentiation.
4. Materials and methods
4.1. Isolation of stem cells derived from human dental pulp
hDPSCs, isolated from third molar of healthy adult subjects (13 to 19 years old), were maintained in Dulbecco's Modified Eagle's Medium low glucose (DMEM-L), containing 100 units/ml penicillin, 10 mg/ml streptomycin, plus 0,1% amphotericin (Sigma-Aldrich, Milan, Italy), plus 10% foetal bovin serum (FBS) (Life Technologies, Monza, Italy), at 37°C in humified CO2 atmosphere, as already described extensively in a previous work [Citation30]. All samples were collected with informed consent of the patients according to ethics considerations and after subscribed suitable set of forms and with the approval of the ethics committee.
4.2. Treatments
To induce neuronal differentiation, hDPSCs were cultured in appropriate induction media as previously described [Citation41, Citation42]. Briefly, the cells were cultured up to 28 days from the pulp separation and subsequently stimulated with Neurobasal A medium containing L-Glutamine, supplemented with B27 (Life Technologies, Monza, Italy), basic Fibroblast Growth Factor 40 ng/ml (bFGF) and Epidermal Growth Factor 20 ng/ml (EGF) (PeproThec, DBA, Milan, Italy) for 7 and 14 days. For signaling experiments, undifferentiated hDPSCs were stimulated with EGF for 5 min at 37°C in 5% CO2; alternatively we used a mouse anti-PrP SAF61 mAb (Spi-Bio, Bertin Pharma, France), to cluster PrPC into the cell membrane.
4.3. Western blot analysis
hDPSCs, untreated or treated with siRNA PrP for 72 h and stimulated with EGF alone (5 min) or in association with bFGF (for 7 or 14 days) obtained as described below, were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Briefly, hDPSCs were lysed in lysis buffer containing 0,1% Triton X-100, 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4 and 75 U of aprotinin and allowed to stand for 20 min at 4°C. The cell suspension was mechanically disrupted by Dounce homogenization (10 strokes). The lysate was centrifuged for 5 min at 1300 x g to eliminate nuclei and large cellular debris and, after protein concentration analysis by Bradford Dye Reagent assay (Bio-Rad, Milano, Italia), the lysate was tested with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Subsequently, the proteins were electrophoretically transferred to PVDF membranes (Bio-Rad, Milan, Italia) that were blocked with 5% nonfat dried milk in TBS (Bio-Rad, Milan, Italia), containing 0.05% Tween 20 (Bio-Rad, Milan, Italia), and probed with rabbit anti-p-ERK1/2 pAb, rabbit anti-total ERK1/2 pAb, rabbit anti-p-Akt pAb, rabbit anti-total Akt pAb, mouse anti-β3-tubulin mAb, mouse anti-NFH mAb (Cell Signaling Technology Danvers, MA, USA), mouse anti-PrP SAF32 mAb (Spi-Bio, Bertin Pharma, France) and mouse anti-actin mAb (Sigma-Aldrich, Milan, Italy). Antibodies were visualized with horseradish peroxidase (HRP)-conjugated anti-mouse IgG or anti-rabbit IgG (GE Healthcare Amersham Biosciences, Uppsala, Sweden) and immunoreactivity assessed by chemiluminescence reaction, using the ECL detection system (Amersham, Buckingamshire, UK). Densitometric scanning analysis was accomplished with NIH Image 1.62 software by Mac OS X (Apple Computer International).
4.4. Flow cytometry analysis
Expression of PrPC on hDPSCs was quantified by flow cytometry after cell staining with rabbit anti-PrP mAb EP1802Y (Abcam, Cambridge, USA). As a negative control, we used rabbit IgG-CY5. hDPSCs at several times (21–28 day from dental pulp separation) or treated with EGF/bFGF (20 ng/ml and 40 ng/ml respectively) for additional 7 and 14 day were fixed with 4% paraformaldehyde and permeabilized by 0.1% (v/v) Triton X-100. After washing, cells were incubated with rabbit anti-PrP EP1802Y mAb for 1 h at 4 °C, followed by CY5-conjugated anti-rabbit IgG H&L (Abcam, Cambridge, USA) for additional 30 min. Alternatively we performed a double staining with rabbit anti-PrP EP1802Y mAb and mouse anti-CD44 mAb or mouse anti-β3-tubulin mAb (Cell Signaling Technology, Danvers, MA, USA). All samples were analyzed with a FACScan cytometer (BD accuri C6 Flow cytometer) equipped with a blue laser (488 nm) and a red laser (640 nm). At least 20,000 events were acquired.
4.5. Immunoprecipitation
hDPSCs, untreated or treated with EGF/bFGF for 14 days were immunoprecipitated with mouse anti-PrP SAF32 mAb (Spi-Bio, Bertin Pharma, France). Briefly, hDPSCs, stimulated as above, were lysed in lysis buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 1 mM PMSF, 10 mg of leupeptin/ml). After preclearing, the supernatant was immunoprecipitated with mouse anti-PrP SAF32 mAb (Spi-Bio, Bertin Pharma, France) plus protein A-acrylic beads. A mouse IgG isotypic control (Sigma-Aldrich, Milan, Italy) was used. The PrPC immunoprecipitates were used for dot blot analysis.
4.6. Dot blot analysis
The PrPC immunoprecipitates, obtained as reported above, were transferred onto nitrocellulose membranes (Bio-Rad, Milan, Italia). Dot blot analysis was performed as described by Garofalo et al 2016 (46). Briefly nitrocellulose strips were probed with: rabbit anti-EGF-R pAb (Santa Cruz, CA, USA), mouse anti-GM2 IgM mAb (NANA) and mouse anti-GD3 IgM mAb (Seikagaku Corp., Chou-ku, Tokyo, Japan). They were then incubated with the corresponding species-specific secondary antibodies and immunoreactivity was visualized using the ECL Western detection system. The immunoprecipitates were checked by anti-PrP 6H4 mAb (Prionics, Milan, Italy).
4.7. Knockdown PrPC by siRNA
hDPSCs were seeded (8 × 104 cells/ml) 6-well plates, in DMEM-L containing serum and antibiotics. Twenty-four hours after seeding, cells were transfected with 5 nM siRNA PrP (Flexitube GeneSolution GS5621 for PRNP), using HiPerFect Transfection Reagent (Qiagen, Valencia, CA), according to the manufacturer's instructions. As experimental control, cells were also transfected with 5 nM scrambled siRNA (AllStars Negative Control – Qiagen). After 72 h, cells were incubated with EGF (PeproThec, DBA, Milan, Italy) for 5 min at 37°C or, alternatively, with EGF/bFGF for 7 and 14 days. PrPC expression was verified by flow cytometry analysis by using rabbit anti-PrP EP1802Y mAb (Abcam, Cambridge, USA).
4.8. Immunofluorescence analysis
hDPSCs were seeded (2 × 104 cells/ml) 6-well plates, in DMEM-L containing serum and antibiotics. Twenty-four after seeding, cells were stimulated with EGF/bFGF (20 ng/ml and 40 ng/ml respectively) for 14 days and used for immunofluorescence analysis. Briefly, hDPSCs treated as above were fixed with 4% paraformaldehyde and permeabilized by 0.1% (v/v) Triton X-100. After washing, cells were incubated with mouse anti-β3-tubulin mAb (Cell Signaling Technology, Danvers, MA, USA) or alternatively, with mouse anti-PrP SAF32 mAb (Spi-Bio, Bertin Pharma, France) for 1 h at 4°C, followed by anti-mouse Alexa fluor 488 (ThermoFisher Scientific, Rockford, USA) for additional 30 min. Finally, cells were observed with a Zeiss Axio Vert. A1 fluorescence microscope (Zeiss, Milan, Italy).
4.9. Statistical analysis
Western blot images were subjected to densitometric scanning analysis, performed by Mac OS X (Apple Computer International), using NIH Image 1.62 software. All data reported in this paper were verified in at least 3 different experiments and reported as mean ± standard deviation (SD). Only p values -less than 0.01 were considered as statistically significant.
Disclosure of potential conflict of interest
The authors declare that they have no competing interests.
Author contribution
Conceptualization: ViM SM CS.
Data curation: ViM VaM.
Formal analysis: SM CS.
Funding acquisition: ViM.
Investigation: FS LP.
Methodology: VaM SM LP FS.
Project administration: ViM MS.
Resources: ViM MS.
Software: VaM.
Supervision: ViM MS CS.
Validation: ViM MS.
Visualization: MS VaM.
Writing – original draft: ViM MS SM VaM.
Writing – review & editing: ViM MS SM VaM.
Acknowledgments
This work was supported by Rieti University Hub “Sabina Universitas” to V.M.
References
- Watt FM, Driskell RR. The therapeutic potential of stem cells. Philos Trans R Soc Lond B Biol Sci. 2010;365:155–163.
- Ksiazek K. A comprehensive review on mesenchymal stem cell growth and senescence. Rejuvenation Res. 2009;12:105-116.
- Robey PG, Kuznetsov SA, Riminucci M, et al. Bone marrow stromal cell assays: in vitro and in vivo. Methods Mol Biol. 2014;1130:279-293.
- Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:1-49.
- Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294-1301.
- Zannettino ACW, Paton S, Arthur A, et al. Multi-potential Human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413-421.
- Rohban R, Pieber TR. Mesenchymal Stem and Progenitor Cells in Regeneration: Tissue Specificity and Regenerative Potential. Stem Cells Int. 2017;2017:5173732.
- Atari M, Gil-Recio C, Fabregat M, et al. Dental pulp of the third molar: a new source of pluripotent-like stem cells. J Cell Sci. 2012;125:3343-3356.
- Koyama N, Okubo Y, Nakao K, et al. Evaluation of pluripotency in human dental pulp cells. J Oral Maxillofac Surg. 2009;67:501-506.
- Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531-535.
- Young FI, Telezhkin V, Youde SJ, et al. Clonal heterogeneity in the neuronal and glial differentiation of dental pulp stem/progenitor cells. Stem Cells Int. 2016;2016:1290561.
- Pisciotta A, Carnevale G, Meloni S, et al. Human dental pulp stem cells (hDPSCs): isolation, enrichment and comparative differentiation of two sub-populations. BMC Dev Biol. 2015;15:14.
- Ullah I, Subbarao RB, Kim EJ, et al. In vitro comparative analysis of human dental stem cells from a single donor and its neuronal differentiation potential evaluated by electrophysiology. Life Sci. 2016;154:39-51.
- Chun SY, Soker S, Jang YJ, et al. Differentiation of human dental pulp stem cells into dopaminergic neuron-like cells in vitro. J Korean Med Sci. 2016;31:171-177.
- Mediano DR, Sanz-Rubio D, Ranera R, et al. The potential of mesenchymal stem cell in prion research. Zoonoses and public Health. 2015;62:165-178.
- Park S, Kim E, Koh SE, et al. Dopaminergic differentiation of neural progenitors derived from placental mesenchymal stem cells in the brains of Parkinson's disease model rats and alleviation of asymmetric rotational behaviour. Brain Res. 2012;1466:158-166.
- Nesti C, Pardini C, Barachini S, et al. Human dental pulp stem cells protect mouse dopaminergic neurons against MPP+ or rotenone. Brain Res. 2011;1367:94-102.
- Zhang CC, Steele AD, Lindquist S, et al. Prion protein is expressed on long-term repopulating hematopoietic stem cells and is important for their self-renewal. Proc Natl Acad Sci USA. 2006;103:2184-2189.
- Lee YJ, Baskakov IV. The cellular form of the prion protein is involved in controlling cell cycle dynamics, self-renewal, and the fate of human embryonic stem cell differentiation. J Neurochem. 2013;124:310-322.
- Steele AD, Emsley JG, Ozdinler PH, et al. Prion protein (PrPC) positively regulates neural precursor proliferation during developmental and adult mammalian neurogenesis. Proc Natl Acad Sci USA. 2006;103:3416-3421.
- Wulf MA, Senatore A, Aguzzi A. The biological function of the cellular prion protein: an update. BMC Biol. 2017;15:34.
- Mattei V, Matarrese P, Garofalo T, et al. Recruitment of cellular prion protein to mitochondrial raft-like microdomains contributes to apoptosis execution. Mol Biol Cell. 2011;22:4842-4853.
- Garofalo T, Manganelli V, Grasso M, et al. Role of mitochondrial raft-like microdomains in the regulation of cell apoptosis. Apoptosis. 2015;20:621-634.
- Watt NT, Taylor DR, Gillott A, et al. Reactive oxygen species-mediated beta-cleavage of the prion protein in the cellular response to oxidative stress. J Biol Chem. 2005;280:35914-35921.
- Hu W, Kieseier B, Frohman E, et al. Prion proteins: physiological functions and role in neurological disorders. J Neurol Sci. 2008;264:1-8.
- Lyahyai J, Mediano DR, Ranera B, et al. Isolation and characterization of ovine mesenchymal stem cells derived from peripheral blood. BMC Vet Res. 2012;8:169.
- Loubet D, Dakowski C, Pietri M, et al. Neuritogenesis: the prion protein controls β1 integrin signaling activity. FASEB Journal. 2012;26:678-690.
- Lee YJ, Baskokov IV. The cellular form of the prion protein guides the differentiation of human embryonic stem cell into neuron-, oligodendrocyte- And astrocyte-committed lineages. Prion. 2014;8:266-275.
- Bieberich E. It's a Lipid's World: Bioactive Lipid Metabolism and Signaling in Neural Stem Cell Differentiation. Neurochem Res. 2012;37:1208-1229.
- Mattei V, Santacroce C, Tasciotti V, et al. Role of lipid rafts in neuronal differentiation of dental pulp-derived stem cells. Exp Cell Res. 2015;339:231-240.
- Ryu JS, Ko K, Lee JW, et al. Gangliosides are involved in neural differentiation of human dental pulp-derived stem cells. Biochem Biophys Res Commun. 2009;387:266-271.
- Botto L, Cunati D, Coco S, et al. Role of lipid rafts and GM1 in the segregation and processing of prion protein. PLoS One. 2014;9:e98344.
- Sorice M, Mattei V, Tasciotti V, et al. Trafficking of PrPC to mitochondrial raft-like microdomains during cell apoptosis. Prion. 2012;6:354-358.
- Llorens F, Carulla P, Villa A, et al. PrP(c) regulates epidermal growth factor receptor function and cell shape dynamics in Neuro2a cells. J Neurochem. 2013;127:124-138.
- Mattei V, Martellucci S, Santilli F, et al. Morphine Withdrawal Modifies Prion Protein Expression in Rat Hippocampus. PLoS One. 2017;12:e0169571.
- Harris DA. Trafficking, turnover and membrane topology of PrP. Br Med Bull. 2003;66:71-85.
- Mouillet-Richard S, Ermonval M, Chebassier C, et al. Signal transduction through prion protein. Science. 2000;289:1925-1928.
- Toni M, Spisni E, Griffoni C, et al. Cellular prion protein and caveolin-1 interaction in a neuronal cell line precedes Fyn/Erk 1/2 signal transduction. J Biomed Biotechnol. 2006;2006:69469.
- Huang GT, Sonoyama W, Chen J, et al. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res. 2006;324:225-236.
- Suchanek J, Soukup T, Visek B, et al. Dental pulp stem cells and their characterization. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009;153:31-35.
- Arthur A, Rychkov G, Shi S, et al. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787-1795.
- Vollner F, Ernst W, Driemel O, et al. A two-step strategy for neural differentiation in vitro of human dental follicle cells. Differentiation. 2009;77:433-441.
- Martin-Lannerée S, Hirsch TZ, Hernandez-Rapp J, et al. PrP(C) from stem cells to cancer. Front Cell Dev Biol. 2014;2:55.
- Peralta OA, Huckle WR, Eyestone WH. Expression and knockdown of cellular prion protein (PrPC) in differentiating mouse embryonic stem cells. Differentiation. 2011;81:68-77.
- Mattei V, Garofalo T, Misasi R, et al. Association of cellular prion protein with gangliosides in plasma membrane microdomains of neural and lymphocytic cells. Neurochem Res. 2002;27:743-749.
- Garofalo T, Matarrese P, Manganelli V, et al. Evidence for the involvement of lipid rafts localized at the ER-mitochondria associated membranes in autophagosome formation. Autophagy. 2016;12:917-935.