Abstract
Haematopoietic stem and progenitor cells (HSPCs) can self-renew and differentiate in any blood cell type throughout life and thereby sustain the entire blood system. To do so, HSPCs had been shown to seed, in a multi-step process, intermediate haematopoietic niches before colonizing the adult marrow. While HSPC birth had been thoroughly characterized in the past, both in mammals and in zebrafish, how perivascular niches could host HSPCs and sustain their expansion was poorly understood. In an article published in the last issue of Cell, Tamplin et al.Citation1 elegantly exploited the many advantages provided by the zebrafish embryo to describe how endothelium remodeling in the perivascular niche, referred to as “cuddling,” favors HSPCs colonization and expansion.
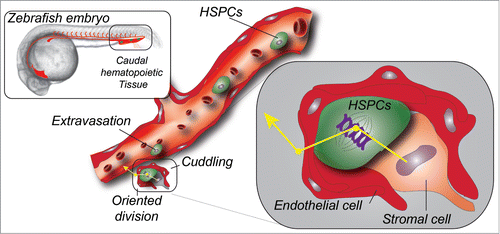
Stem cells are capable to balance self-renewal and differentiation to allow mature cells to be generated while preserving the stem cell pool intact. This phenomenon requires optimal stem cell niches whose composition and mechanism of formation has been widely studied. HSPCs are primitive precursors of all blood cell lineages. They emerge from the embryonic and haemogenic dorsal aorta, through a process called endothelial haematopoietic transition.Citation2-4 Live imaging had shown that they extrude from the dorsal wall of the haemogenic endothelium, travel through the underlying mesenchyme, and enter the circulation to eventually colonize an intermediate haematopoietic niche. Precise knowledge about this last step was still lacking. Indeed, although it was well described that HSPCs had to transit through this intermediate niche before colonizing the adult marrow, we had only few clues as to how HSPCs were hosted in such perivascular niches. This is an important step for HSPCs expansion that precedes seeding of bones and kidney, in mammals and zebrafishCitation5 respectively. In the adult marrow niche, endothelialCitation6 and mesenchymal,Citation7 cells are instrumental for establishing a proper niche for HSPCs maintenance and differentiation.Citation8 Whether and how perivascular niches function in intermediate haematopoietic tissue, the caudal haematopoietic tissue (CHT) in zebrafish and the fetal liver (FL) in mammals, is less well characterized.
In this study, Tamplin et al. start by first establishing a highly specific zebrafish transgenic line that allows dynamic tracking of HSPCs at high-resolution, from their birth (through budding from the haemogenic endothelium) to the extravasation and seeding of the CHT. More precisely, they used a regulatory element from the first intron of the mouse Runx1 locus, a well-established HSPCs marker,Citation2 to drive fluorescent protein expression. This allowed them to precisely track HSPCs over time in the living zebrafish embryo and to confirm their self-renewal ability by long-term transplantation. Using live microscopic imaging at high spatial and temporal resolution over extended periods of time of the CHT, they could observe that HSPCs lodging in the perivascular niche was accompanied by a striking endothelial remodeling, which they name endothelial cuddling. At this stage, it remains unclear how HSPCs extravasate and especially what drives this highly controlled extravasation in specific regions of the CHT, though additional experiments suggest that this early event was driven by the CXCR4/CXCL12 axis. Through use of correlative and light microscopy (CLEM) in the zebrafish embryo they were able to accurately dissect the anatomy of these perivascular cuddling niches, which they could also image in explants of murine fetal livers. CLEM makes use of existing anatomical landmarks of the developing zebrafish embryo to retrieve regions of interest previously imaged using live microscopy.Citation9,10 Ultrastructural details obtained through CLEM imaging confirmed that perivascular pockets localized close to the caudal artery were mostly composed of 5–6 endothelial cells, and of stromal cells that in some cases tightly wrapped the HSPCs. Using another zebrafish transgenic line allowing to observe mesenchymal stromal cells in the CHT, they could confirm that HSPCs lodging occurred close to CXCL12-producing stromal cells and showed quantitatively that stromal cells were instrumental in orienting stem cell division by, very likely, providing a “polarizing signal” that drives mitosis and promotes subsequent entry of daughter cells into the circulation to colonize the kidney. Finally, they conducted a chemical genetic screen in order to identify regulators of CHT niche colonization by HSPCs. These studies revealed that lycorine, a natural alkaloid extracted from plants with potential anti-inflammatory and anti-cancer properties, was a potent stimulator of CHT colonization by HSPCs. Remarkably, transient treatment of embryos with this compound led to a substantial increase of the stem cell pool in the adult. Although the precise mechanism of action of lycorine is not described, the drug functions in early stages as indicated by the increased number of seeded sites in the CHT.
Altogether, this study makes elegant usage of a combination of genetics, live imaging, electron microscopy and functional stem cell transplantation assays to characterize early HSPC colonization and expansion, and to demonstrate that perivascular cells cuddle HSPC in intermediate haematopoietic tissues. Some aspects, however, remain unclear. What guides HSPCs extravasation in specific areas of the CHT in zebrafish? Do HSPCs carry active molecular partners to trigger endothelial remodeling? How is the contribution of stromal cells orchestrated and especially what is the nature of the polarizing signal? Finally, why would HSPCs need such a niche to sustain their expansion? The authors suggest that such niches could provide entrapment, in addition to protection, of HSPCs and thereby favor the concentration of local growth factors and signaling molecules that could drive their directional division and adult marrow seeding. Interestingly, these structures are reminiscent of recently described hemospheres in the adult bone marrow,Citation11 suggesting that cuddling may be a common feature of functional haematopoietic niches. Although some elements remain unclear, this study provides a giant leap in our understanding of stem cell niches and provides fertile ground for assessing niche interactions in the adult marrow. This important step could provide useful information to the field of regenerative medicine, where understanding the key mechanisms driving niche function could be translated for therapeutic use.
References
- Tamplin OJ, et al. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 2015; 160:241-52; PMID:25594182; http://dx.doi.org/10.1016/j.cell.2014.12.032
- Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 2010; 464:112-5; PMID:20154732; http://dx.doi.org/10.1038/nature08761
- Bertrand JY, et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 2010; 464:108-11; PMID:20154733; http://dx.doi.org/10.1038/nature08738
- Boisset JC, et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 2010; 464:116-20; PMID:20154729; http://dx.doi.org/10.1038/nature08764
- Traver D, et al. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol 2003; 4:1238-46; PMID:14608381; http://dx.doi.org/10.1038/ni1007
- Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005; 121:1109-21; PMID:15989959; http://dx.doi.org/10.1016/j.cell.2005.05.026
- Méndez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010; 466:829-34; PMID:20703299; http://dx.doi.org/10.1038/nature09262
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014; 505:327-334; PMID:24429631; http://dx.doi.org/10.1038/nature12984
- Goetz JG, Monduc F, Schwab Y, Vermot J. Using correlative light and electron microscopy to study zebrafish vascular morphogenesis. Methods Mol Biol 2015; 1189:31-46; PMID:25245685; http://dx.doi.org/10.1007/978-1-4939-1164-6_3
- Goetz J G, et al. Endothelial cilia mediate low flow sensing during zebrafish vascular development. Cell Rep 2014; 6:799-808; PMID:24561257; http://dx.doi.org/10.1016/j.celrep.2014.01.032
- Wang L, et al. Identification of a clonally expanding haematopoietic compartment in bone marrow. EMBO J 2013; 32:219-230; PMID:23188081; http://dx.doi.org/10.1038/emboj.2012.308