ABSTRACT
In the course of embryo implantation extensive interaction of the trophoblast with uterine tissue is crucial for adequate trophoblast invasion. This interaction is highly controlled, and it has been pointed out that a specific glycocode and changes in glycosylation may be important for successful implantation and maintenance of pregnancy. Both uterine and trophoblast cells have been shown to express cell surface glycoconjugates and sugar binding proteins, such as mucins (MUC) and galectins (gals). An increasing number of studies have investigated potential candidates interacting in this process. However, knowledge about the biochemical nature of the interactions and their importance for trophoblast cell function, and, consequently, for pregnancy outcome are still lacking. This review is aimed at deliberating the possibility that mucins, as heavily glycosylated proteins, might be among the functionally relevant galectin ligands in human trophoblast, based on both published data and our original research.
Introduction
The presence of MUC1 on luminal epithelium of the uterus has been widely accepted to direct blastocyst attachment to areas free of its hindering effect.Citation1 Expression of mucins has also been recorded on human trophoblast cells of fetal origin, from which they can be co-isolated with the endogenous lectin, gal-1.Citation2 On the other hand, gal-1 has been shown to influence adhesive, migratory and invasive behavior of trophoblast cells in vitro,Citation3 through interactions with glycoconjugates. This review is aimed at deliberating the possibility that mucins, as heavily glycosylated proteins, might be among the functionally relevant galectin ligands in human trophoblast.
Expression and functions of mucins
Some of the major components of surface epithelia in respiratory, gastrointestinal and reproductive tracts are heterogeneous highly glycosylated proteins known as mucins. Thus far, 20 mucin family members have been identified (MUC1-MUC20).Citation4 Although they are encoded by different genes, their protein structure shares some characteristic similarities.Citation5 Mature mucin protein is processed in the Golgi apparatus, where it can be glycosylated, sialylated and sulfated, typically in a cell-type specific manner.Citation6 According to their cellular localization mucins are classified into 2 distinct categories, membrane-bound or secreted mucins.Citation7,8 The secreted mucins are gel-forming components of viscoelastic mucus, which lubricates and protects underlying epithelia, with expression restricted to secretory organs and cell types. On the other hand, membrane-bound mucins belong to the group of type-I membrane anchored proteins, defined by the presence of a long extracellular domain with many sites for O-glycosylation and fewer for N-glycosylation. Their expression on the apical side of epithelial cells enables interaction with secreted mucins and anchors the mucus layer to the epithelium.Citation9 Secreted variants of membrane-bound mucins, can be released from the plasma membrane either by enzymatic cleavage or result from alternative splicing. Together with their protective role, membrane mucins are also recognized as sensors of the extracellular environment that can transmit signals, and as molecules involved in cell-cell and cell-matrix interactions.
Different tissues normally express unique combinations of mucins, but in neoplasia this pattern is frequently altered. Aberrant glycosylation is probably responsible for many differences between carcinomas and normal epithelial tissue.Citation10 In addition to their very complex functional roles in both normal and malignant cells, changes in both amounts and forms of mucins may influence cellular growth, differentiation, transformation, adhesion and invasion.Citation11-14 Considerable research data have shown that overexpression of MUC1 is associated with invasive and metastatic tumors of the gall bladder, colon, pancreas and oral epithelium.Citation14-16 Also, it was found that MUC3, i.e. its cysteine-rich domain, can promote cell migration, accelerate wound healing and inhibit apoptosis.Citation17 Therefore, mucins are hypothesized to contribute to tumor cell invasion by simultaneously disrupting existing interactions between neighboring cells (anti-adhesion) and establishing new ligands for interaction between the invading cell and adjoining cells (adhesion).Citation10
The human female reproductive tract, including ovaries, oviduct, uterus, cervix and vagina, expresses several different mucins.Citation18 The earliest studies focused on mucin expression in the uterus, especially on membrane-bound MUC1, because of its dominant presence in human endometrium.Citation19-21 MUC1 has characteristics of an integral membrane protein with a large extracellular domain, a transmembrane domain and a short cytoplasmic sequence.Citation8 The long extracellular domain contains a variable number of tandem repeats (VNTR) rich in serine and threonine, potential sites of glycosylation. Throughout the menstrual cycle MUC1 is expressed at the apical surface of both luminal and glandular epithelium () and is secreted into the uterine cavity, while some of the glycoepitopes are phase of cycle dependent.Citation19,20 MUC1-associated glycans in the endometrium constitute at least 40% of the mucin molecular mass. Using different antibodies, highly sulfated lactosaminoglycans and sialokeratan sulfate epitopes have been detected on endometrial MUC1.Citation1 Moreover, several sialoepitopes are expressed on endometrial MUC1, e.g. Sialyl-Tn antigen (STn, Neu5Acα2-6GalNAc) and the carbohydrate adhesion ligands, Sialyl-LewisX (SLeX) and Sialyl-LewisA (SLeA).Citation22,23 Endometrial MUC1 is also known to bear the Thomsen-Friedenreich antigen (TF antigen, Galβ1-3GalNAc).Citation24 Much evidence clearly points to hormonal regulation of MUC1 expression in the endometrium, with cyclically regulated expression of core protein and glycan sialylation, where maximum levels occur in the secretory phase of the menstrual cycle.Citation25 Specific MUC1 structures and the abundance of sialic acid and sulfate residues may subsequently inhibit interaction between the embryo and maternal apical adhesion molecules during implantation. Thus, the high density of MUC1 molecules must be lost from the implantation site if embryo implantation is to occur. Local loss of MUC1 could result from production of downregulating factors by blastocysts,Citation26 or binding by carbohydrate binding proteins (lectins) from the blastocyst cell surface specific for MUC1-associated glycans.Citation27 The latter possibility indicates that involvement of the selectin-adhesion mechanism may be critical for embryo implantation, implying the importance of lectin-carbohydrate recognition/binding in initial adhesive interactions.
Figure 1. Schematic representation of the localization of mucins and galectin-1 at the feto-maternal interface. Distribution of the mucins and gal-1 expressed at the site of trophoblast invasion during the first trimester of pregnancy, and in the anchoring villi that are attached to the decidua basalis. bm, basement membrane; CC, cell column; CT, cytotrophoblast; dNK, decidual natural killer cell; dSC, decidual stromal cell; EVT, extravillous trophoblast; GE, glandular epithelium; HC, Hofbauer cell; pbGC, placental bed giant cell; pF, placental fibroblast; pV, placental vessel; SA, spiral artery; ST, syncytiotrophoblast; UG, uterine gland.
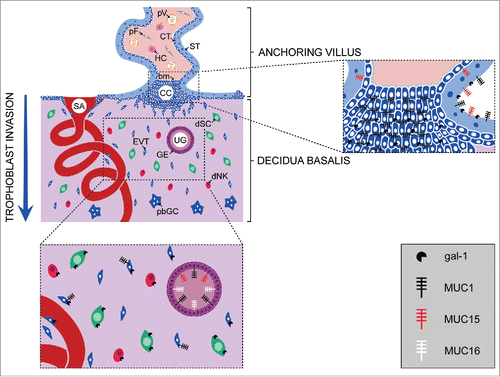
Mucins are thought to play important roles in many cellular processes, including cell signaling, cell proliferation and tumor progression, and also to mediate immune evasion.Citation10 Due to abundant glycosylation, mucins and MUC1 in particular, have either pro- or anti-adhesive properties and influence cell-cell and cell-extracellular matrix (ECM) interactions,Citation28-30 depending on the cell type. Thus, sialomucin CD34 inhibited adhesion of HEK293T cells, hematopoietic stem and progenitor cells,Citation31,32 but increased murine hematopoietic cell adhesion to human bone marrow stromal cells.Citation33 Another membrane-bound mucin, MUC16, promoted the formation of multicellular aggregates of ovarian carcinoma cells in vitro and may play a role in ovarian carcinoma progression.Citation34
Trophoblast mucins
Mucins are also expressed by the trophoblast. To date, only membrane-bound mucins have been investigated. Thus, at the protein level MUC1 and MUC15 (), as well as mRNA for MUC1, MUC3, MUC15 and MUC20 have been detected in human placenta.Citation35,36 The presence and localization of MUC1 was studied using different antibodies specific for various epitopes on the molecule. These antibodies detected either MUC1 epitopes within the VNTR region or MUC1-associated glycans, indicating the significance of glycosylation of the core protein.Citation35,36 MUC1 has been observed in human and macaque placenta, choriocarcinoma cell lines and isolated trophoblast, but findings regarding the distribution and staining intensity of this glycoprotein are discordant.Citation28,36-38 Using antibodies specific for underglycosylated and hypoglycosylated MUC1, Shyu et al. showed that expression of MUC1 increases with gestational age, with dominant expression by the syncytiotrophoblast (ST) and to a lesser extent by a subset of extravillous trophoblast (EVT) cells ().Citation36 Other distinct glycoepitopes carried by trophoblast MUC1, CA 15-3 and CA 19-9, were detected in extravillous trophoblast, while CA 19-9 was also present in surrounding decidual cells. Moreover, it has been shown that invasive trophoblast of the first and second trimester of pregnancy expresses CA 15-3 (Fitzgerald clone M411149), in keeping with the study of Shyu et al.,Citation36 regarding the intensity of staining (relatively weak) and the relative number of stained trophoblast cells. In contrast, using polyclonal anti-bovine submaxillar mucin antibodies (anti-BSM) we recently demonstrated moderate to strong MUC1/mucin(s) staining of first trimester placental villi (),Citation2 while Jeschke et al. reported strong MUC1 expression in human first and second trimester placenta.Citation37
Expression of MUC1 has also been observed in isolated trophoblast cells, as well as in JAr and BeWo choriocarcinoma cells.Citation2,37 The MUC1 staining pattern is consistent with plasma membrane/cytoplasmic localization in rhesus monkey trophoblast cells and in freshly isolated trophoblast.Citation2,28 Strong membrane staining was obtained using either antibodies specific for the cytoplasmic MUC1 domain or for carbohydrate MUC1 epitopes.Citation2 Kumar et al. recently detected the MUC1 extracellular domain in nuclear speckles and in spliceosomes in rhesus monkey trophoblast cells using 3 different antibodies specific for MUC1 VNTR.Citation39 Nucleus associated staining was also seen in JAr choriocarcinoma cells and in MCF7 breast cancer cells.Citation39 Clearly, the staining pattern strongly depends on the antibody used, and this must be considered in the light of variability of MUC1 glycosylation in different cells and the influence of glycosylation on epitope recognition by MUC1 extracellular domain specific antibodies.
Another studied member of the class of membrane-bound mucins is MUC15, which is abundantly expressed in placenta.Citation35 MUC15 is predominantly found at the apical ST membrane, while it is absent from EVT cells (). Interestingly, as previously shown for MUC1, MUC15 is differentially expressed in human placenta through gestation, with the highest levels at term, for both MUC15 protein and mRNA.Citation35
It is well known that there is tissue specific glycosylation of MUC1, despite almost identical transcripts.Citation40 For example, pancreatic MUC1 is larger and more glycosylated than that from breast or endometrium.Citation41,42 While endometrial MUC1 glycotopes have been the subject of many studies,Citation19,20,22,43 data regarding carbohydrates associated with placental mucin molecules are incomplete. Most of the results were obtained from histochemical studies and lectin-blotting, by combining antibodies specific for different mucin glycotopes with different plant lectins to decipher glycotope specificities. These data suggest the probable presence of short mucin-type O-glycans, such as TF and Tn (GalNAc) antigens. Some of these glycotopes terminate with sialic acid, mainly linked by the α2,3 bond and less by the α2,6 bond,Citation44 as indicated by binding of lectins from Maackia amurensis (MAA) and Sambucus nigra (SNA). Some of these glycans could vary during pregnancy or be engaged by their binding partners, which may further affect the functional properties of MUC1.
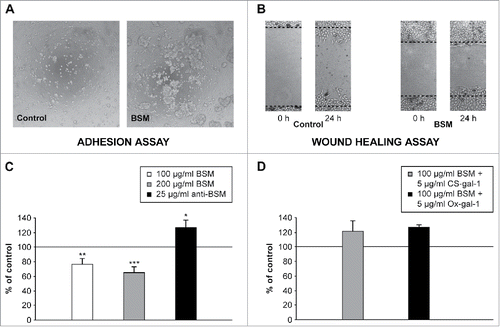
In recent years, the functional properties of MUC1/mucins expressed by the trophoblast have also been investigated. Adhesion of HTR-8/SVneo EVT cells to ECM components, such as collagen type IV, fibronectin and laminin was negatively affected by MUC1 overexpression.Citation45 In our study HTR-8/SVneo enhanced cell aggregation was also observed () when bovine submaxillary mucin (BSM), similar to MUC1 with respect to the presence of defined glycotopes such as TF and STn, was added to cell culture. Under similar culture conditions the presence of BSM reduced HTR-8/SVneo cell migration, which could be prevented by a specific antibody ().
Figure 2. Effect of BSM on adhesion and migration of HTR-8/SVneo extravillous trophoblast cells. (A) Cell adhesion after 2 h in the presence of BSM (100 µg/ml) in culture medium. A representative micrograph shows aggregation of trophoblast cells in the presence of BSM. (B) Representative images of the bidirectional migration of HTR-8/SVneo monolayers following wounding. Cells were grown with or without (control) BSM in culture media. Cell migration was reduced in the presence of BSM. (C, D) Statistical analyses of wound healing assays. (C) BSM (100 µg/ml and 200 µg/ml) reduced cell migration, which was prevented in the presence of anti-BSM antibodies (25 µg/ml). (D) Supplementation of culture media with 5 µg/ml of CS-gal-1 or Ox-gal-1 (2 molecular forms of biologically active recombinant human gal-1) prevented inhibition of HTR-8/SVneo cell migration by BSM (100 µg/ml). The data are expressed as a percentage of the value for the untreated control ± SEM, n = 6. *p < 0.05; **p < 0.01; ***p < 0.001. Method details available in Supplemental material.
In the macaque, EVT cell adhesion and transendothelial migration were mediated by MUC1 involving intercellular adhesion molecule-1 (ICAM-1).Citation28 However, the finding that endothelial ICAM-1 is one of the counter-receptors for trophoblast MUC1 does not exclude potential additional MUC1 ligands on the trophoblast or other uterine cell types. It has been suggested that both MUC1 and MUC15 could have active roles in trophoblast invasion, since overexpression of each suppressed invasion of choriocarcinoma cells.Citation35,36 Consistent with this finding, overexpression of MUC1 also reduced invasion of HTR-8/SVneo cells.Citation45 Since MUC1 overexpression inhibited β1-integrin activity and downstream signaling, invasion may be suppressed mainly by modulation of β1-integrin signaling.Citation45
Trophoblast galectin-1
Galectins are small soluble proteins defined by an affinity for β-galactosides and significant sequence similarity of the carbohydrate recognition domain (CRD) among family members.Citation46,47 Due to their capacity to modulate cellular functions, they are involved in diverse processes, including immunomodulation, cell differentiation and death, proliferation, adhesion, migration and invasion. Several lines of evidence suggest that human galectins play important roles in implantation, angiogenesis, maternal-fetal immunotolerance and placentation.Citation48-50 For example, gal-1 expression by the trophectoderm and inner cell mass of human preimplantation embryos, points to involvement in attachment to the uterine epithelium, even though gal-1 null mice implant normally.Citation51,52 Among the 16 human galectins, gal-1, -3, and -8 were detected in EVT differentiating along the invasion pathway.Citation3 Since they are expressed by the EVT of the anchoring villi, these galectins could be involved in the organization of the ECM, modulating cell adhesion through interaction with glycans of the cell column and the placental bed ECM.Citation3,53,54 Galectin-1 was the first to be isolated, purified and cloned from human placenta. It is immunolocalized in the syncytiotrophoblast, cytotrophoblast of middle and distal cell columns, and abundantly in the endometrium and decidua of early gestation ().Citation53,55,56 Galectin-1 is also expressed by isolated cytotrophoblast in culture and trophoblast cell lines.Citation3,53,57-59 This protein has an important role in maintenance of pregnancy in mice, with a high fetal loss rate in gal-1 deficient animals, preventable by treatment with recombinant gal-1.Citation48 In the human, in addition to its proposed involvement in immunomodulation leading to non-rejection of the fetus,Citation51 gal-1 level was found to be positively correlated with pregnancy success in vivo,Citation60 and contributes to trophoblast invasiveness in vitro.Citation3 Blocking the extracellular function of endogenous gal-1 of primary trophoblasts and HTR-8/SVneo extravillous cells reduced invasion substantially, while supplementation with recombinant gal-1 enhanced it.
Mucins and galectin-1
Specialized structural features of the mucin protein backbone and the abundant glycosylation lead to interaction with distinct protein partners. There are multiple ways for both secreted and membrane mucins to interact, with secreted, membrane bound and transmembane proteins, including mucins, some of which initiate signaling cascades or influence the availability of the proteins involved. Mucin-binding partners are found in the extracellular space in different tissues, most of them being detected in saliva.Citation61,62 However, as carbohydrate binding proteins, galectins are so far the only known extracellularly located mucin-binding partners of non-salivary origin.Citation63 Several biochemical studies have provided direct evidence of interaction between mucins and galectins. As shown by histochemistry, gal-1 binds mucin and epithelial surface glycocalyces of the gastrointestinal mucosa.Citation64 Glycans associated with both soluble and membrane-bound CA125 (MUC16) present in HeLa cells are specifically and preferentially recognized by gal-1, as opposed to gal-3.Citation65 Although the exact biological significance of this interaction is currently not known, it has been proposed that CA125 might be a factor regulating gal-1 export to the surface of these cells.Citation65 It has been shown that MUC1 and MUC16, both membrane-bound mucins, interact with gal-3 on the apical surface of ocular epithelial cells. This interaction is abrogated in the presence of competitive carbohydrate inhibitors of galectin binding, which leads subsequently to reduced gal-3 at the cell membrane.Citation66 Galectin-3 from the sera of cancer patients also interacted with MUC1 present on the cancer cell surface, i.e., with TF antigen.Citation67 This interaction promoted strong adhesion of tumor cells to the endothelial surface, thus enabling cancer metastasis.
Since gal-1 preferentially recognizes type I and type II N-acetyllactosamine residues on all complex N-linked and many O-linked glycoproteins, its direct connection with trophoblast invasion is probably a consequence of recognition and interaction with β-galactoside containing ligands on the cell surface or in the ECM.Citation68,69 It is well known that oncofetal fibronectin and laminin function as physiological ligands of gal-1 in human placenta,Citation70,71 but there are also data to support binding to TF antigen expressed on either MUC1 or other glycoproteins in the trophoblast.Citation72 Co-localization of both trophoblast mucin(s) and gal-1 at the plasma membrane of EVT cells suggested mutual interaction ().Citation2 Mucin(s) and gal-1 were co-isolated from trophoblast cells in culture, confirming that these molecules indeed interact in these cells. Furthermore, the molecular interaction between MUC1/mucin(s) and gal-1 was reduced in the presence of lactose, pointing to the relevance of gal-1 lectin activity.Citation2 Interestingly each of these proteins was shown to affect trophoblast cell invasion in vitro. Our own and other published data point to an inverse effect on this process, MUC1 decreasing itCitation45 and gal-1 inducing an increase.Citation3 It was interesting to speculate whether disturbing the balance between trophoblast mucin(s) and gal-1 would in any way affect trophoblast cell functional characteristics. Preliminary test results for cell migration under conditions in which both exogenous molecules were added to culture medium support the possibility that gal-1 and mucin(s) interact in such a way that the presence of gal-1 restores cell migration (), originally decreased by added BSM (). This suggests that a reduced presence of mucins, either by previous binding and thus diminished availability for other yet unspecified interactions, or by silencing, promotes the adhesive and invasive capacity of the trophoblast, since silencing MUC15 restored trophoblast cell migration/invasion,Citation35 while overexpression of MUC1 and MUC15 downregulated it.Citation35,36
Figure 3. Schematic representation of cellular effects resulting from interactions of gal-1 and MUC1. (A) Cytoplasmic gal-1 can be translocated into the nucleus, (B) where it can associate with ribonucleoproteins (RNP) and participate in spliceosome assembly, or it can accumulate in the cytoplasm (C) where it may interact with cytosolic binding partners. (D) Gal-1 is transported outside the cells via non-classical protein export. In the extracellular space (E) this lectin binds several ECM glycoproteins, and (F) can form homogeneous lattices at the cell surface and activate signaling cascades. (G) Gal-1 can bind to cell surface glycoconjugates and crosslink them with ECM, (H) or promote cell-cell adhesion. MUC1 may act as a binding partner of gal-1, in a carbohydrate-dependent manner. (I) A fragment of the cytoplasmic tail of MUC1 may be transported to the nucleus and regulate gene expression, (J) or might sequester some cytosolic proteins. (K) Binding of gal-1 to trophoblast MUC1 and crosslinking with other transmembrane glycoproteins could trigger signal transduction. (L) Hypothetical molecular interactions of exogenous recombinant human gal-1 (CS-gal-1 and Ox-gal-1) and MUC1-like molecules of BSM in trophoblast cell culture.
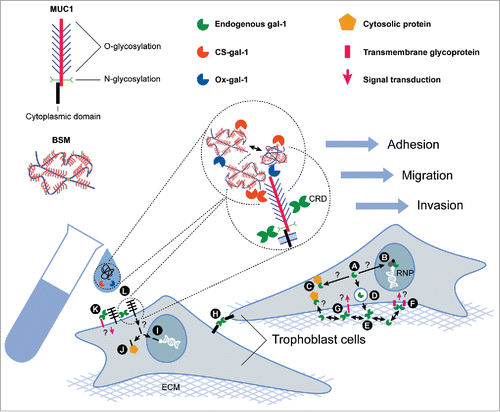
Based on the previously mentioned data, the potential cellular effects resulting from various possible interactions of trophoblast galectin-1 and MUC1 are diverse, depending on the cellular localization of this interaction, other participating molecules, or downstream targets. Translated gal-1 () can be translocated into the nucleus (), where it may associate with ribonucleoproteins (RNP), participate in spliceosome assembly, or it can accumulate in the cytoplasm where it may interact with cytosolic binding partners through protein-protein interactions (). Gal-1 is transported outside the cells via non-classical protein export (), where it is able to bind several ECM glycoproteins () and cell surface glycoconjugates (), crosslinking them () or forming homogeneous lattices () and thus activating signaling cascades, or promoting cell-cell adhesion (). MUC1 may act as a binding partner of gal-1 in a carbohydrate-dependent manner. Binding of dimeric gal-1 can crosslink trophoblast MUC1 and other transmembrane glycoproteins triggering signal transduction (). The cytoplasmic tail fragment of MUC1 may be transported to the nucleus and regulate gene expression (), or sequester some cytosolic proteins (). Based on our presented results () we hypothesize that exogenous MUC1-like molecules of the BSM could interfere with binding of endogenous trophoblast membrane bound or soluble gal-1 (), thus forming cell aggregates as shown in . In adherent cultures, however, addition of MUC1-like molecules of BSM could engage membrane gal-1, preventing it from binding other pro-migratory glycoprotein ligands. Co-addition of recombinant human gal-1 (CS-gal-1 and Ox-gal-1) provides larger amounts of binding molecules to engage the MUC1-like molecules of BSM (), preventing disturbance of pre-existing complexes and preserving cell migration, as shown in .
Conclusion
The data presented here have been selected with the aim of contributing to our understanding of the complex interactions between diverse proteins that are both associated with the trophoblast cell membrane, as well as present in the extracellular space, such as MUC1/mucins and gal-1. Individually, both proteins have been found to affect the adhesive and invasive properties of trophoblast cells in culture. Since these proteins co-isolate from human trophoblast cells, but their effects on the trophoblast seem to be opposed, the question arises how any change in their level would affect the adhesive and migratory functions of these cells. This is obviously a complex task to address, due to the shared specificity of galectins, of which at least 3 are expressed by extravillous trophoblast on the one hand, while multiple mucin (glyco)forms are likely to be present in these cells on the other. Interaction between MUC1/mucin(s) and galectin-1 may balance pro- and anti-migratory effects, while high expression of decidual gal-1 may shift the balance favoring migration/invasion, explaining trophoblast invasion in vivo. One way to further our understanding of this complex field would be to selectively silence or otherwise inhibit multiple of the proposed interacting proteins, in the context of the large number of other functionally relevant interactions of these molecules documented in the literature.
On the other hand, while the relevance of MUC1 glycosylation in tumor progression has been thoroughly studied, much less is known about trophoblast mucin glycosylation. A combination of several approaches is needed to provide insights into how glycan structures and probable changes during pregnancy affect functional properties of the trophoblast. Deciphering the trophoblast mucin glycocode would open up the possibility of identifying the exact nature of the interaction between gal-1 and MUC1/mucin(s). This might help explain the roles of trophoblast mucin and galectin-1 in vivo, since both individually and jointly affect trophoblast function in vitro.
Abbreviations
| BSM | = | bovine submaxillary mucin |
| CRD | = | carbohydrate recognition domain |
| EVT | = | extravillous trophoblast |
| ECM | = | extracellular matrix |
| gal | = | galectin |
| ICAM-1 | = | intercellular adhesion molecule-1 |
| MAA | = | Maackia amurensis lectin |
| MUC | = | mucin |
| SLeA | = | Sialyl-LewisA |
| SLeX | = | Sialyl-LewisX |
| SNA | = | Sambucus nigra lectin |
| ST | = | syncytiotrophoblast |
| Tn | = | Tn antigen (GalNAc) |
| STn | = | Sialyl-Tn antigen (Neu5Acα2-6GalNAc) |
| TF antigen | = | Thomsen–Friedenreich antigen (Galβ1-3GalNAc) |
| VNTR | = | variable number of tandem repeats. |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was funded by grant No. 173004 from the Ministry of Education, Science and Technological Development of the Republic of Serbia.
References
- Aplin JD, Hey NA, Graham RS. Human endometrial MUC1 carries keratan sulphate: characteristic glycoforms in the luminal epithelium at receptivity. Glycobiol 1998; 8:269-76; http://dx.doi.org/10.1093/glycob/8.3.269
- Bojić-Trbojević Ž, Jovanović Krivokuća M, Kolundžić N, Petronijević M, Vrzić- Petronijević S, Golubović S, Vićovac LJ. Galectin-1 binds mucin in human trophoblast. Histochem Cell Biol 2014; 142:541-53; PMID:24854997; http://dx.doi.org/10.1007/s00418-014-1229-7
- Kolundžić N, Bojić-Trbojević Ž, Kovačević T, Stefanoska I, Kadoya T, Vićovac LJ. Galectin-1 is part of human trophoblast invasion machinery - a functional study in vitro. PLoS One 2011; 6:e28514; PMID:Can't; http://dx.doi.org/10.1371/journal.pone.0028514
- Theodoropoulos G, Carraway KL. Molecular signaling in the regulation of mucins. J Cell Biochem 2007; 102:1103-16; PMID:17957706; http://dx.doi.org/10.1002/jcb.21539
- Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc Natl Acad Sci USA 2007; 104:16209-14; PMID:17911254; http://dx.doi.org/10.1073/pnas.0705984104
- Silverman HS, Sutton-Smith M, McDermott K, Heal P, Leir S-H, Morris HR, Hollingsworth MA, Dell A, Harris A. The contribution of tandem repeat number to the O-glycosylation of mucin. Glycobiol 2003; 13:265-77; http://dx.doi.org/10.1093/glycob/cwg028
- Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 2009; 9:874-85; PMID:19935676; http://dx.doi.org/10.1038/nrc2761
- Jonckheere N, Van Seuninge I. The membrane-bound mucins: how large O-glycoproteins play key roles in epithelial cancers and hold promise as biological tools for gene-based and immunotherapies. Crit Rev Oncog 2008; 14:177-96; PMID:19409062; http://dx.doi.org/10.1615/CritRevOncog.v14.i2-3.30
- Olson FJ, Bäckström M, Karlsson H, Burchell J, Hansson GC. A MUC1 tandem repeat reporter protein produced in CHO-K1 cells has sialylated core 1 O-glycans and becomes more densely glycosylated if coexpressed with polypeptide-GalNAc-T4 transferase. Glycobiol 2005; 15:177-91; http://dx.doi.org/10.1093/glycob/cwh158
- Hollingsworth MA, Swanson BJ. Mucins in cancer: Protection and control of the cell surface. Nat Rev Cancer 2004; 4:45-60; PMID:14681689; http://dx.doi.org/10.1038/nrc1251
- Quin RJ, McGuckin MA. Phosphorylation of the cytoplasmic domain of the MUC1 mucin correlates with changes in cell-cell adhesion. Int J Cancer 2000; 87:499-506; PMID:10918188; http://dx.doi.org/10.1002/1097-0215(20000815)87:4%3c499::AID-IJC6%3e3.0.CO;2-9
- Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and β-catenin in cell adhesion. J Biol Chem 1997; 272:12492-4; PMID:9139698; http://dx.doi.org/10.1074/jbc.272.19.12492
- Komatsu M, Jepson S, Arango ME, Carothers Carraway CA, Carraway KL. Muc4/sialomucin complex, an intramembrane modulator of ErbB2/HER2/Neu, potentiates primary tumor growth and suppresses apoptosis in a xenotransplanted tumor. Oncogene 2001; 20:461-70; PMID:11313977; http://dx.doi.org/10.1038/sj.onc.1204106
- Luttges J, Feyerabend B, Buchelt T, Pacena M, Kloppel G. The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am J Surg Pathol 2002; 26:466-71; PMID:11914624; http://dx.doi.org/10.1097/00000478-200204000-00008
- Nakamori S, Ota DM, Cleary KR, Shirotani K, Irimura T. MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology 1994; 106:353-61; PMID:7905449
- Kashiwagi H, Kijima H, Dowaki S, Ohtani Y, Tobita K, Tsukui M, Tanaka Y, Matsubayasi H, Tsuchida T, Yamazaki H et al. DF3 expression in human gallbladder carcinoma: significance for lymphatic invasion. Int J Oncol 2000; 16:455-9; PMID:10675475
- Ho SB, Dvorak LA, Moor RE, Jacobson AC, Frey MR, Corredor J, Polk DB, Shekels LL. Cysteine-rich domains of muc3 intestinal mucin promote cell migration, inhibit apoptosis, and accelerate wound healing. Gastroenter 2006; 131:1501-17; http://dx.doi.org/10.1053/j.gastro.2006.09.006
- Lagow E, DeSouza MM, Carson DD. Mammalian reproductive tract mucins. Hum Reprod Update 1999; 5:280-92; PMID:10465520; http://dx.doi.org/10.1093/humupd/5.4.280
- Hey NA, Graham RA, Seif MW, Aplin JD. The polymorphic epithelial mucin MUC1 in human endometrium is regulated with maximal expression in the implantation phase. J Clin Endocrinol Metab 1994; 78:337-42; PMID:8106621
- Hey NA, Li TC, Devine PI, Graham RA, Saravelos H, Aplin JD. MUC1 in secretory phase endometrium: expression in precisely dated biopsies and flushings from normal and recurrent miscarriage patients. Hum Reprod 1995; 10:2655-62; PMID:8567787
- Aplin JD, Hey NA, Li TC. MUC1 as a cell surface and secretory component of endometrial epithelium: reduced levels in recurrent miscarriage. Am J Reprod Immnunol 1996; 35: 261-6; http://dx.doi.org/10.1111/j.1600-0897.1996.tb00042.x
- Thor A, Viglione MJ, Murraro R, Ohuchi N, Schlom J, Gorstein F. Monoclonal antibody B72.3 reactivity with human endometrium: a study of normal and malignant tissues. Int J Gynecol Pathol 1987; 6:235-47; PMID:3429107; http://dx.doi.org/10.1097/00004347-198709000-00005
- Hey NA, Aplin JD. Sialyl-Lewis x and Sialyl-Lewis a are associated with MUC1 in human endometrium. Glycoconj J 1996; 13:769-79; PMID:8910004; http://dx.doi.org/10.1007/BF00702341
- Jeschke U, Walzel H, Mylonas I, Papadopoulos Shabani N, Kuhn K, Schulze S, Friese K, Karsten U, Anz D, et al. The human endometrium expresses the glycoprotein mucin-1 and shows positive correlation for Thomsen-Friedenreich epitope expression and galectin-1 binding. J Histochem Cytochem 2009; 57:871-81; PMID:19506091; http://dx.doi.org/10.1369/jhc.2009.952085
- Meseguer M, Pellicer A, Simón C. MUC1 and endometrial receptivity. Mol Hum Reprod 1998; 12:1089-98; http://dx.doi.org/10.1093/molehr/4.12.1089
- Meseguer M, Aplin JD, Caballero-Campo P, O'Connor JE, Martín JC, Remohí J, Pellicer A, Simón C. Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol Reprod 2001; 64:590-601; PMID:11159362; http://dx.doi.org/10.1095/biolreprod64.2.590
- Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, Yang ZQ, Kiessling LL, Rosen SD, Fisher SJ. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science 2003; 299:405-8; PMID:12532021; http://dx.doi.org/10.1126/science.1079546
- Thirkill TL, Cao T, Stout M, Blankenship TN, Barakat A, Douglas GC. MUC1 is involved in trophoblast transendothelial migration. Biochim Biophys Acta 2007; 1773:1007-14; PMID:17509701; http://dx.doi.org/10.1016/j.bbamcr.2007.04.006
- Hilkens J, Wesseling J, Vos HL, Storm J, Boer B, van der Valk SW, Maas MC. Involvement of the cell surface-bound mucin, episialin/MUC1, in progression of human carcinomas. Biochem Soc Trans 1995; 23:822-6; PMID:8654846; http://dx.doi.org/10.1042/bst0230822
- Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol 1995; 129:255-65; PMID:7698991; http://dx.doi.org/10.1083/jcb.129.1.255
- Drew E, Merzaban JS, Seo W, Ziltener HJ, McNagny KM. CD34 and CD43 inhibits mast cells adhesion and are required for optimal mast cell reconstitution. Immunity 2005; 22:43-57; PMID:15664158; http://dx.doi.org/10.1016/j.immuni.2004.11.014
- Ohnishi H, Sasaki H, Nakamura Y, Kato S, Ando K, Narimatsu H, Tachibana K. Regulation of cell shape and adhesion by CD34. Cell Adh Migr 2013; 7:426-33; PMID:24036614; http://dx.doi.org/10.4161/cam.25957
- Healy L, May G, Gale K, Grosveld F, Greaves M, Enver T. The stem cell antigen CD34 functions as a regulator of hemopoietic cell adhesion. Proc Natl Acad Sci USA 1995; 92:12240-4; PMID:8618877; http://dx.doi.org/10.1073/pnas.92.26.12240
- Giannakouros P, Comamala M, Matte I, Rancourt C, Piché A. MUC16 mucin (CA125) regulates the formation of multicellular aggregates by altering β-catenin signaling. Am J Canc Res 2014; 5:219-30
- Shyu MK, Lin MC, Shih JC, Lee CN, Huang J, Liao CH, Huang IF, Chen HY, Huang MC, Hsieh FJ. Mucin 15 is expressed in human placenta and suppresses invasion of trophoblast-like cells in vitro. Hum Reprod 2007; 22:2723-32; PMID:17720698; http://dx.doi.org/10.1093/humrep/dem249
- Shyu MK, Lin MC, Liu CH, Shih JC, Lee CN, Chen HY, Huang J, Huang MC, Hsieh FJ. MUC1 expression is increased during human placental development and suppresses trophoblast-like cell invasion in vitro. Biol Reprod 2008; 79:233-9; PMID:18417712; http://dx.doi.org/10.1095/biolreprod.108.067629
- Jeschke U, Richter DU, Hammer A, Briese V, Friese K, Karsten U. Expression of the Thomsen-Friedenreich antigen and its putative carrier protein mucin1 in the human placenta and in trophoblast cells in vitro. Histochem Cell Biol 2002; 117:219-26; PMID:11914919; http://dx.doi.org/10.1007/s00418-002-0383-5
- Bojić-Trbojević Ž, Krivokuća MJ, Vrzić-Petronijević S, Petronijević M, Vićovac LJ. Expression of tumor associated antigens CA 15-3 and CA 19-9 in trophoblast of the normal human placenta. Eur J Gynecol Oncol 2012; 33:281-4
- Kumar P, Lindberg L, Thirkill TL, Ji JW, Martsching L, Douglas GC. The MUC1 extracellular domain subunit is found in nuclear speckles and associated with spliceosomes. PLoS ONE 2012; 7:e42712; PMID:22905162; http://dx.doi.org/10.1371/journal.pone.0042712
- Lan MS, Batra SK, Qi WN, Metzgar RS, Hollingsworth MA. Cloning and sequencing of the human pancreatic tumor mucin cDNA. J Biol Chem 1990; 265:15294-9; PMID:2394722
- Ho JJ, Kim YS. Serological pancreatic tumor markers and MUC1 apomucin. Pancreas 1994; 9:674-91; PMID:7846010; http://dx.doi.org/10.1097/00006676-199411000-00002
- Aplin JD. Glycans as biochemical markers of human endometrial secretory differentiation. J Reprod Fertil 1991; 91:525-41; http://dx.doi.org/10.1530/jrf.0.0920525
- Hoadley ME, Seif MW, Aplin JD. Menstrual cycle-dependent expression of keratan sulphate in human endometrium. Biochem J 1990; 266:757-63; PMID:1691631; http://dx.doi.org/10.1042/bj2660757
- Paszkiewicz-Gadek A, Porowska H, Sredzinska K. Expression of MUC1 mucin in full-term pregnancy human placenta. Adv Med Sci 2008; 53:54-8; PMID:18614437; http://dx.doi.org/10.2478/v10039-008-0017-9
- Shyu MK, Chen CW, Lin NY, Liao WC, Chen CH, Lin CJ, Huang HC, Lee JJ, Huang MJ, Tseng GF, et al. MUC1 expression is elevated in severe preeclamptic placentas and suppresses trophoblast cell invasion via β1-integrin signaling. J Clin Endocrinol Metab 2011; 96:3759-67; PMID:21917866; http://dx.doi.org/10.1210/jc.2011-1368
- Barondes SH, Cooper DNW, Gitt MA, Leffler H. Galectins: structure and function of a large family of animal lectins. J Biol Chem 1994; 269:20807-10; PMID:8063692
- Kasai K, Hirabayashi J. Galectins: a family of animal lectins that decipher glycocodes. J Biochem 1996; 119:1-8; PMID:8907168; http://dx.doi.org/10.1093/oxfordjournals.jbchem.a021192
- Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, et al. A pivotal role for galectin-1 in fetomaternal interface. Nature Med 2007; 13:1450-7; PMID:18026113; http://dx.doi.org/10.1038/nm1680
- Blois SM, Conrad ML, Freitag N, Barrientos G. Galectins in angiogenesis: consequences for gestation. J Reprod Immunol 2015; 108:33-41; PMID:25622880; http://dx.doi.org/10.1016/j.jri.2014.12.001
- Than NG, Romero R, Kim CJ, McGowen MR, Papp Z, Wildman DE. Galectins: guardians of eutherian pregnancy at the maternalfetal interface. Trends Endocrinol Metab 2012; 23:23-31; PMID:22036528; http://dx.doi.org/10.1016/j.tem.2011.09.003
- Tirado-González I, Freitag N, Barrientos G, Shaikly V, Nagaeva O, Strand M, Kjellberg L, Klapp BF, Mincheva-Nilsson L, Cohen M, et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol Hum Reprod 2013; 19:43-53; PMID:Can't; http://dx.doi.org/10.1093/molehr/gas043
- Poirier F, Robertson EJ. Normal development of mice carrying null mutation in the gene encoding the L14 S-type lectin. Development 1993; 119:1229-36; PMID:8306885
- Vićovac LJ, Janković M, Čuperlović M. Galectin-1 and -3 in cells of the first trimester placental bed. Hum Reprod 1998; 13:730-5; PMID:9572443; http://dx.doi.org/10.1093/humrep/13.3.730
- Kolundžić N, Bojić-Trbojević Z, Radojčić LJ, Petronijević M, Vićovac LJ. Galectin-8 is expressed by villous and extravillous trophoblast of the human placenta. Placenta 2011; 32:909-11; PMID:Can't; http://dx.doi.org/10.1016/j.placenta.2011.07.087
- Maqui E, van den Brule FA, Castronovo V, Foidart JM. Changes in the distribution pattern of galectin-1 and galectin-3 in human placenta correlates with the differentiation pathways of trophoblasts. Placenta 1997; 18:433-9; PMID:9250706; http://dx.doi.org/10.1016/S0143-4004(97)80044-6
- von Wolff M, Wang X, Gabius HJ, Strowitzki T. Galectin fingerprinting in human endometrium and decidua during the menstrual cycle and in early gestation. Mol Hum Reprod 2005; 11:189-94; PMID:15681515; http://dx.doi.org/10.1093/molehr/gah144
- Bojić-Trbojević Ž, Janković M, Vićovac LJ. Influence of IGF-I on adhesion, proliferation, and galectin-1 production in JAr and Jeg-3 choriocarcinoma cell lines. Arch Onc 2005; 13:7-10; http://dx.doi.org/10.2298/AOO0501007B
- Bojić-Trbojević Ž, Božić M, Vićovac LJ. Steroid hormones modulate galectin-1 in the trophoblast HTR-8/SVneo cell line. Arch Biol Sci 2008; 60:11-23; http://dx.doi.org/10.2298/ABS0801011B
- Ćujić D, Bojić-Trbojević Ž, Kolundžić N, Kadoya T, Vićovac LJ. Molecular forms of galectin-1 from human placenta and trophoblast cells. J Serb Chem Soc 2015; 80:159-69; http://dx.doi.org/10.2298/JSC140428091C
- Liu AX, Jin F, Zhang WW, Zhou TH, Zhou CY, Yao WM, Qian YL, Huang HF. Proteomic analysis on the alteration of protein expression in the placental villous tissue of early pregnancy loss. Biol Reprod 2006; 75:414-20; PMID:16738225; http://dx.doi.org/10.1095/biolreprod.105.049379
- Iontcheva I, Oppenheim FG, Troxler RF. Human salivary mucin MG1 selectively forms heterotypic complexes with amylase, proline-rich proteins, statherin, and histatins. J Dent Res 1997; 76:734-43; PMID:9109822; http://dx.doi.org/10.1177/00220345970760030501
- Bruno LS, Li X, Wang L, Soares RV, Siqueira CC, Oppenheim FG, Troxler RF, Offner GD. Two-hybrid analysis of human salivary mucin MUC7 interactions. Biochim Biophys Acta 2005; 1746:471-5; http://dx.doi.org/10.1016/j.bbamcr.2005.08.007
- Senapati S, Das S, Batra S. Mucin-interacting proteins: from function to therapeutics. Trends Biochem Sci 2009; 35:236-45; PMID:19913432; http://dx.doi.org/10.1016/j.tibs.2009.10.003
- Wasano K, Hirakawa Y. Recombinant galectin-1 recognizes mucin and epithelial cell surface glycocalyces of gastrointestinal tract. J Histochem Cytochem 1997; 45:275-83; PMID:9016316; http://dx.doi.org/10.1177/002215549704500212
- Seelenmayer C, Wegenhingel S, Lechner J, Nickel W. The cancer antigen CA125 represents a novel counter receptor for galectin-1. J Cell Sci 2003; 116: 1305-18; PMID:12615972; http://dx.doi.org/10.1242/jcs.00312
- Argüeso P, Guzman-Araguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem 2009; 284:23037-45; PMID:19556244; http://dx.doi.org/10.1074/jbc.M109.033332
- Yu LG, Andrews N, Zhao Q, McKean D, Williams JF, Connor LJ, Gerasimenko OV, Hilkens J, Hirabayashi J, Kasai K, et al. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J Biol Chem 2007; 282:773-81; PMID:17090543; http://dx.doi.org/10.1074/jbc.M606862200
- Abbott WM, Hounsell EF, Feizi T. Further studies of oligosaccharide recognition by the soluble 13 kDa lectin of bovine heart muscle. Ability to accommodate the blood-group-H and -B-related sequences. Biochem J 1988; 252:283-7; PMID:3421906; http://dx.doi.org/10.1042/bj2520283
- Sparrow CP, Leffler H, Barondes SH. Multiple soluble beta-galactoside-binding lectins from human lung. J Biol Chem 1987; 262:7383-90; PMID:2438278
- Ozeki Y, Matsui T, Yamamoto Y, Funahashi M, Hamako J, Titani K. Tissue fibronectin is an endogenous ligand for galectin-1. Glycobiol 1995; 5:255-61; http://dx.doi.org/10.1093/glycob/5.2.255
- Zhou Q, Cummings RD. L-14 lectin recognition of laminin and its promotion of in vitro cell adhesion. Arch Biochem Biophys 1993; 300:6-17; PMID:8380972; http://dx.doi.org/10.1006/abbi.1993.1002
- Jeschke U, Karsten U, Wiest I, Schulze S, Kuhn C, Friese K, Walzel H. Binding of galectin-1 (gal-1) to the Thomsen-Friedenreich (TF) antigen on trophoblast cells and inhibition of proliferation of trophoblast tumor cells in vitro by gal-1 or an anti-TF antibody. Histochem Cell Biol 2006; 126:437-44; PMID:16607538; http://dx.doi.org/10.1007/s00418-006-0178-1
