abstract
Embryo implantation and subsequent placentation require a fine balanced fetal-maternal cross-talk of hormones, cytokines and chemokines. Amongst the group of chemokines, CX3CL1 (also known as fractalkine) has recently attracted attention in the field of reproductive research. It exists both as membrane-bound and soluble isoforms. On the basis of current experimental evidence, fractalkine is suggested to regulate adhesion and migration processes in fetal-maternal interaction at different stages of human pregnancy. Expressed by uterine glandular epithelial cells, predominantly during the mid-secretory phase of the menstrual cycle, fractalkine appears to prime the blastocyst for forthcoming implantation. After implantation, fractalkine is suggested to regulate invasion of extravillous trophoblasts by altering their expression profile of adhesion molecules. With onset of perfusion of the intervillous space at the end of first trimester, fractalkine present at the apical microvillous plasma membrane of the syncytiotrophoblast may mediate close interaction of placental villi with circulating maternal blood cells.
Introduction
Embryo implantation into the maternal decidua and subsequent placentation are mandatory steps for successful human pregnancy. These essential steps require a well-orchestrated interaction between fetal and maternal tissues and comprise a fine balanced cross-talk of hormones, cytokines and chemokines. Chemokines represent a special heterogeneous family of 8-15 kDa low molecular mass cytokines that promote migration of leukocytes and thereby mediate inflammation and modulate immune responses.Citation1,2 Moreover, chemokines play important key roles in several physiologic and pathologic aspects of human reproduction, including menstruation, ovulation, implantation, cervical ripening, preterm labor, and endometriosis.Citation3 According to the number and spacing of the first two cysteine residues in a conserved cysteine structural motif, chemokines are classified into four subclasses.Citation4 These four subclasses are referred to as C, CC, CXC, and CX3C, where C is a cysteine and X any amino-acid residue. A unique member of the CX3C subclass is CX3CL1 (also referred as fractalkine), which is encoded on chromosome 16, and derives from non-haemopoietic cells.Citation5,6 Fractalkine is synthesized as transmembrane molecule, but can be shed by a disintegrin and metalloprotease (ADAM)10 and ADAM17 into a soluble isoform.Citation7-9 Depending on whether it is cleaved or not, fractalkine mediates different steps of leukocyte recruitment. Full length fractalkine represents a 373 amino acid transmembrane molecule, comprising an extracellular N-terminal domain (residues 1-76), a mucin-like stalk (77-317), a transmembrane α-helix (318-336) and a short cytoplasmic tail (337-373).Citation10,11 While the soluble form has chemoattractive activity, the membrane-bound form promotes flow resistant adhesion of leukocytes to endothelial or epithelial cells via its corresponding G protein-coupled, 7-transmembrane receptor CX3CR1, which is expressed on natural killer (NK) cells, CD3+ T-cells and a majority of CD14+ monocytes.Citation5,12
Fractalkine and its receptor CX3CR1 are expressed in several reproductive tissues, including ovaries, Fallopian tubes, uterus and testis.Citation5,13,14 Mice lacking fractalkine are fertile and do not show any developmental defects, which most likely excludes a fundamental functional role of the chemokine/receptor duo in reproduction at least in mice.Citation15,16 This is not surprising given the redundancy between chemokines and their receptors. However, accumulating evidence suggests that dysregulation of fractalkine expression is associated with a number of pregnancy complications. In pregnancies complicated by diabetes mellitus, upregulated placental fractalkine was suggested to contribute to increased placental villous microvessel density.Citation17 Moreover, increased release of soluble fractalkine was detected in perfusion fluids of perfused placental cotyledons in response to lipopolysaccharide and hypoxia,Citation18 indicating upregulation of fractalkine in the placental endothelium under pro-inflammatory conditions. In line with this assumption, increased fractalkine expression was shown in human amnionic epithelial cells in pregnancies complicated by chorioamnionitis.Citation19 Recently, increased placental fractalkine expression and a trend towards elevated levels of circulating soluble fractalkine was shown in patients with severe early-onset preeclampsia.Citation20,21
This review will focus on putative roles of fractalkine in several aspects of fetal-maternal interaction at different stages of human pregnancy.
The role of fractalkine in blastocyst attachment and implantation
In human, the first step of blastocyst implantation – the so-called apposition – takes place around day 6 to 7 postcoitus (p.c.). At this stage, the implanting blastocyst is composed of approximately 250 cells, most of which comprise the outer wall surrounding the blastocyst cavity and the inner cell mass.Citation22 This outer wall consists of mononucleated trophoblast cells (also referred to as trophectoderm cells). Implantation is initiated by attachment of the apical plasma membranes of the trophoblasts to the apical plasma membranes of the uterine epithelium. This process has been discussed as paradoxic phenomenon, since apical plasma membranes of epithelia are described as normally nonadhesive.Citation22 Nevertheless, adhesiveness of the trophectoderm cells of the blastocyst and the apical plasma membrane of the uterine epithelium is assured for a short phase described as the implantation window. At that time, uterine epithelial cells become highly secretory and release numerous regulatory molecules into the uterine lumen, where they can affect the blastocyst even prior to attachment.Citation23 Fractalkine may be part of the molecular cocktail released by uterine epithelial cells. This assumption is based on immunohistochemical analysis of human endometrium, showing fractalkine maximally expressed in the luminal and glandular uterine epithelium during the mid-secretory phase of the menstrual cycle.Citation13 These data suggest that released uterine fractalkine, as part of a secreted chemokine cocktail, may prime the blastocyst for attachment through activation of various chemokine receptors that have been detected in human blastocysts.Citation24
However, whether or not the fractalkine/CX3CR1 interaction directly mediates attachment of the blastocyst is not known. Since appropriate human specimens are rare and usually poorly preserved, current knowledge on molecular and micro-anatomical processes taking place in the human implantation window are very limited. However, there are considerable differences between mammalian species in the type of implantation and placentation.Citation25 Nevertheless, our understanding of the earliest stages of implantation is mainly based on animal models, most of which have the initial steps of apposition and attachment, although the step of invasion is not a feature of implantation in all species. For example, in sheep, elongation of the trophoblast on day 11, is followed by immobilization of the embryo in the uterine lumen at day 14, and induction of implantation at day 15.Citation26 However, there is no invasion: instead there is fusion of trophectodermal and endometrial epithelial cells to form specialized trinucleate cells.Citation27 Gene expression analysis of ewe conceptuses at the stages of elongation, immobilization and implantation showed increasing fractalkine levels during elongation with peak expression on day 14, suggesting specific upregulation at the time when the first contacts between trophoblast and uterus are established.Citation28 Fractalkine-mediated attachment of the conceptus to the uterine epithelium implies epithelial expression of the receptor CX3CR1, but this was not examined in this study. Compared with ruminants, human embryos have three phases of implantation, including apposition, adhesion and invasion. During apposition an active cytokine and growth factor dialogue is established between the blastocyst and the decidua. This dialogue induces upregulation of adhesive molecules on the surface of both and enables adhesion. In human, while fractalkine is produced by endometrial epithelium, CX3CR1 expression was detected only in the uterine glandular epithelium whereas luminal epithelial cells were consistently negative for CX3CR1 expression at all stages of the cycle.Citation13 CX3CR1 was also present on invasive trophoblast cells, although trophectoderm was not available for analysis. It is therefore still not known whether there is direct involvement of the fractalkine/CX3CR1 axis in blastocyst attachment. However, available data certainly suggests involvement of fractalkine in the process of trophoblast invasion.
The role of fractalkine in trophoblast invasion
Trophoblast invasion is a key event during implantation and placentation, which is not only responsible for further invasion of the blastocyst itself but also for anchorage of the developing placenta and remodelling of uteroplacental arteries to adapt to pregnancy.Citation22 During very early stages of implantation, trophoblasts of the implanting embryonic pole of the blastocyst show increased proliferation which results in a double-layered trophoblast. By fusion of neighbouring mononucleated trophoblasts of the outer layer directly facing the decidua, the so-called syncytiotrophoblast is formed: this is the trophoblast type responsible for enzymatic cleavage of the extracellular matrix of the developing decidua, enabling progression of trophoblast invasion until day 14 p.c.Citation22 Thereafter, proliferating and migrating cytotrophoblasts reach the trophoblastic shell via syncytiotrophoblast trabeculae and finally penetrate the decidual stroma. Thus, very early invasion processes are driven by the syncytiotrophoblast, whereas from day 15 p.c. mononucleated cytotrophoblasts – so-called extravillous trophoblasts (EVT) – invade the uterine tissue as far as to the inner third of the myometrium. In this context it should be noted that the vast majority of studies on trophoblast invasion, describe advanced implantation processes using mononucleated trophoblast cell lines. However, expression analyses and functional studies suggest a functional role of the fractalkine/CX3CR1 axis in stimulating the migratory and invasive behaviour of extravillous trophoblasts.
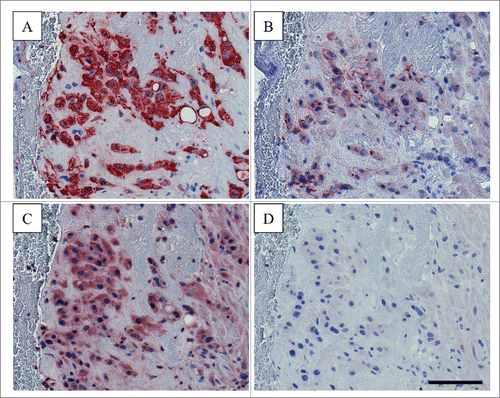
Noteworthy, expression patterns of fractalkine and CX3CR1 seem to vary between different types of trophoblasts. While fractalkine expression has been detected in primary term trophoblasts and several trophoblast cell lines such as JEG-3, AC1M-32 and AC1M-88 as well as differentiated BeWo,Citation29-31 CX3CR1 is differentially expressed throughout different types of trophoblasts. Immunohistochemistry of first-trimester human implantation sites detected CX3CR1 in endovascular EVT, which invade uteroplacental vessels. In contrast, no CX3CR1 expression was detected in interstitial EVT, which migrate through the decidual stroma, but do not invade blood vessels.Citation30 Differential CX3CR1 expression was also shown for trophoblast cell lines. While Jeg-3 and AC1M-88 express the receptor, both undifferentiated and differentiated BeWo cells lack CX3CR1 expression.Citation29,30 Thus, fractalkine may function on well-defined subpopulations of invading trophoblasts, depending on their CX3CR1 expression profile. Accordingly, AC1M-88 trophoblasts, abundantly expressing CX3CR1, show enhanced migration in response to recombinant fractalkine.Citation30 Besides uterine glandular epithelial cells, also decidualized stromal cells, uterine natural killer cells and macrophages in decidualized zones were shown to express fractalkine,Citation13 which may act on invading trophoblasts in a paracrine manner. This assumption is substantiated by experiments using specific neutralizing antibodies to fractalkine, which significantly attenuated migration of AC1M-88 trophoblasts in response to conditioned culture medium from primary endometrial epithelial cells.Citation30 Since some trophoblast cell lines show both fractalkine and CX3CR1 expression, it is tempting to speculate on autocrine functions of the chemokine/receptor duo, as shown for granulosa cells in preovulatory follicles,Citation32 fibroblast-like synoviocytes,Citation33 and rat aortic smooth muscle cells.Citation34 Indeed, our preliminary immunohistochemistry showed both fractalkine and CX3CR1 expression in invading extravillous trophoblasts in postpartum decidua sections from a human hysterectomy ().
Figure 1. Immunohistochemical staining for fractalkine and CX3CR1 in human postpartum decidua Serial postpartum decidua sections (5 µm) obtained from human hysterectomy were stained for (A) extravillous trophoblast marker HLA-G (using clone 4H84, BD Pharmingen, 0.25 µg/ml working concentration), (B) fractalkine (using monoclonal anti-human CX3CL1/fractalkine antibody, clone 81513, R&D Systems, 1 µg/ml), C) CX3CR1 (using polyclonal anti-CX3CR1 antibody C8354, Sigma-Aldrich, 2 µg/ml) and (D) Negative Control for Rabbit IgG Ab-1 (Neomarkers, Thermo Scientific, 2 µg/ml). Staining was performed using the UltraVision Large Volume Detection System HRP Polymer Kit (Thermo Fisher Scientific) as previously described.Citation21,29,38 Invading extravillous trophoblasts were positive for fractalkine and CX3CR1. Scale bar represents 100 µm.
Recently, a regulatory role of fractalkine on trophoblast adhesion has been suggested and may explain observed effects in migration studies. Treatment of AC1M-88 trophoblasts with recombinant fractalkine significantly increased adhesion to fibronectin. This increased adhesion properties of fractalkine treated trophoblasts are based on altered gene expression of adhesion molecules and extracellular matrix components, as shown by oligo-array and qPCR analysis.Citation31 Importantly, another chemokine (CCL14, also known as HCC-1), examined in the same study, also stimulating adhesion, but regulated different adhesion and extracellular matrix components.
The role of fractalkine in the perfused intervillous space
Due to endovascular trophoblast plugs within the lumen of invaded spiral arteries, perfusion of the intervillous space with maternal blood is not established prior to the end of the first trimester.Citation35,36 In this period only a combination of maternal blood plasma and secretory products of uterine glands, passes the endovascular trophoblast plugs as an ultrafiltrate and circulates through the intervillous space. This way, nutrients, growth factors and cytokines are provided to, but maternal blood cells are kept away from developing placental villi in first trimester.Citation37 Thus, although fractalkine is present at the apical microvillous plasma membrane of the syncytiotrophoblast in human first trimester placental villi,Citation38 direct interaction between the syncytiotrophoblast and maternal CX3CR1 expressing cells can be excluded at this stage of pregnancy. However, soluble fractalkine from placental villi and eroded uterine glands, which are connected with the intervillous space by the enlarging syncytiotrophoblast from approximately day 17 post-conception throughout the first trimester,Citation39,40 may be continuously released into the intervillous space, i.e. maternal plasma, where it can interfere with maternal CX3CR1 expressing cells by an endocrine route. Besides endocrine actions, autocrine/paracrine signalling by syncytiotrophoblast derived soluble fractalkine may be considered, since weak CX3CR1 staining has been detected in the villous trophoblast layer of human first trimester placenta.Citation30 CX3CR1 expression in the villous trophoblast compartment declines until it is completely absent at term,Citation17,41 probably excluding autocrine effects with progressing pregnancy.
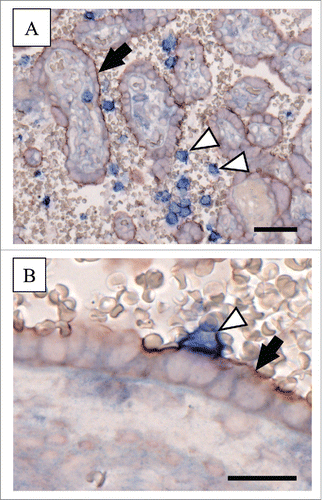
At the end of the first trimester of human pregnancy, dissolution of endovascular trophoblast plugs enables onset of maternal blood flow into the intervillous space and gives rise to direct physical interaction between maternal circulating blood cells and the placental syncytiotrophoblast.Citation37,42 This is regulated by cytokines, chemokines and adherence molecules. Amongst the panel of adhesion molecules, membrane-bound fractalkine has recently been suggested as another candidate enabling stable interaction between CX3CR1 expressing maternal leukocytes and the syncytiotrophoblast. This assumption is based on adhesion assays, showing impaired adherence of the monocyte cell line THP-1 to villous trophoblasts after pre-incubation of THP-1 with human recombinant fractalkine and silencing of CX3CR1 expression.Citation29 Our preliminary immunohistochemical double staining of term placenta for fractalkine and CX3CR1 substantiates previous cell culture findings by occasionally showing tight contact of CX3CR1 expressing maternal blood cells and the syncytiotrophoblast (). Since adhesion of circulating maternal leukocytes to syncytiotrophoblast does not seem to be a prevalent event in healthy pregnancy, potential mechanisms preventing exaggerated binding of CX3CR1 expressing cells to the syncytiotrophoblast may exist. In this context, specific glycans, (like sialyl Lewis X and Lewis a) on glycosylated proteins such as human chorionic gonadotropin (hCG), have recently been suggested to prevent maternal leukocyte adhesion to trophoblast.Citation43 However, the small proportion of circulating maternal leukocytes, which finally adhere to the syncytiotrophoblast may fulfil physiological functions. This has been suggested for adhering monocytes, which may be involved in trophoblast renewal.Citation44 In this process, membrane-bound fractalkine could mediate detection and elimination of aged trophoblast areas by adhering monocytes. Beside this highly speculative hypothesis, another attractive hypothesis has recently been proposed, suggesting a bidirectional monocyte-trophoblast interaction, enabling trophoblasts to attract and prime monocytes to release a particular set of cytokines supporting their growth and survival.Citation45
Figure 2. Immunohistochemical double staining for fractalkine and CX3CR1 in human term placenta Human term placenta sections (5 µm) were stained with the MultiVision Polymer Detection System (Thermo Scientific), using monoclonal anti-human CX3CL1/fractalkine antibody (clone 81513, R&D Systems, 1 µg/ml) and polyclonal anti-CX3CR1 antibody (C8354, Sigma-Aldrich, 0.5 µg/ml) as previously described.Citation21,29,38 (A) Immunohistochemical double staining of human term placenta localized fractalkine at the apical microvillous plasma membrane of the syncytiotrophoblast (red staining; arrow), whereas CX3CR1 was detected on circulating maternal blood cells (blue staining; arrowheads) in the intervillous space. (B) Tight contact of the fractalkine positive syncytiotrophoblast (arrow) and a CX3CR1 expressing maternal blood cell (arrowhead) was occasionally observed and suggest fractalkine/CX3CR1-mediated fetal-maternal interaction. Scale bars in Aand Brepresent 50 µm and 20 µm, respectively.
In contrast to healthy pregnancy, fractalkine-mediated adhesion of circulating maternal leukocytes to the syncytiotrophoblast may be affected under inflammatory conditions in pregnancy pathologies, such as gestational diabetes mellitus (GDM) or preeclampsia. Indeed, placental fractalkine expression is upregulated in severe early onset preeclampsia, and recent data from human first trimester placental explant experiments showed increased expression and release of placental fractalkine in response to TNF-α.Citation21 Upregulated placental fractalkine may not only enhance leukocyte adhesion to the syncytiotrophoblast, but could also induce their activation, which has been suggested to contribute to the progression of preeclampsia.Citation46 Activation of leukocytes during their uteroplacental passage has been shown by increased adhesion molecules and complement related factors on neutrophils and monocytes in samples from uterine veins compared to samples from antecubital veins in patients with severe preeclampsia.Citation46 However, enhanced fractalkine-mediated leukocyte adhesion to the syncytiotrophoblast may also occur in other pregnancy pathologies, such as chronic intervillositis, which is associated with massive intervillous monocyte recruitment.Citation47 In this context, tightly adherent maternal mononuclear leukocytes have been proposed to facilitate transmission of cell-associated infectious pathogens across the placental barrier either by mediating direct infection of the trophoblast or by transmigration of infected cells into the villous stroma.Citation44,47 Finally, placental fractalkine may contribute to transplacental cancer transmission. Previous one-sided ex vivo placenta perfusion studies with fluorescence labeled T cell leukemia cell lines showed adhesion bridges formed between cells and the syncytiotrophoblast, enabling transmigration of cells through the villous trophoblast layer.Citation48
Conclusion
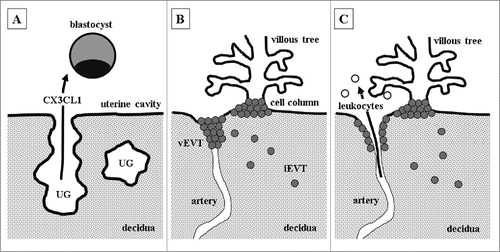
Accumulating experimental evidence suggests fractalkine to be involved in the fetal-maternal cross-talk, regulating a number of critical steps required for successful pregnancy. Fractalkine as part of a molecule cocktail secreted by uterine glands may prime the blastocyst for implantation (). After implantation, fractalkine may participate in regulating trophoblast invasion into the uterine wall to guarantee proper remodelling of uteroplacental arteries (). With dissolution of endovascular trophoblast plugs and perfusion of the intervillous space at the end of first trimester, fractalkine may mediate interaction between circulating maternal blood cells and the syncytiotrophoblast (). Thus, depending on the stage of pregnancy, fractalkine may mediate adhesion and migration processes of not only fetal but also maternal cells, at the fetal-maternal interface.
Figure 3. Potential roles of fractalkine at different stages of pregnancy (A) Uterine glands (UG) secrete fractalkine (CX3CL1), which may prime the blastocyst for adhesion to the uterine epithelium and subsequent implantation. (B) After implantation, extravillous trophoblasts (EVT) detach from cell columns and start to invade as interstitial extravillous trophoblasts (iEVT) the decidua. Endovascular extravillous trophoblasts (vEVT) invade uteroplacental arteries and plug them. Fractalkine, either released by decidualized stromal cells, uterine natural killer cells, macrophages or EVT itself, may enhance invasion of vEVT in a paracrine and autocrine manner. (C) Dissolution of endovascular trophoblast plugs at the end of first trimester enables maternal blood flow into the intervillous space. Placental fractalkine, located on the surface of syncytiotrophoblast, mediates adhesion of maternal leukocytes to placental villi.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are indebted to Astrid Blaschitz and Monika Siwetz for their assistance with immunohistochemistry.
Funding
M. Gauster was supported by funds of the Oesterreichische Nationalbank (Oesterreichische Nationalbank, Anniversary Fund, project number: 16513) and the Austrian Science Fund (FWF): P23859-B19. LAS is supported by an NHMRC of Australia Fellowship (#1022028). All work at the Hudson Institute is supported by the Victorian Government's Infrastructure Support funding.
References
- Steinke JW, Borish L. 3. cytokines and chemokines. J Allergy Clin Immunol 2006; 117:S441-5; PMID:16455343; http://dx.doi.org/ S0091-6749(05)01584-8 [pii].
- Raman D, Sobolik-Delmaire T, Richmond A. Chemokines in health and disease. Exp Cell Res 2011; 317:575-89; PMID:21223965; http://dx.doi.org/10.1016/j.yexcr.2011.01.005
- Garcia-Velasco JA, Arici A. Chemokines and human reproduction. Fertil Steril 1999; 71:983-93; PMID:10360897; http://dx.doi.org/ S0015-0282(99)00120-X [pii].
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity 2000; 12:121-7; PMID:10714678.
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature 1997; 385:640-4; PMID:9024663; http://dx.doi.org/10.1038/385640a0
- Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature 1997; 387:611-7; PMID:9177350; http://dx.doi.org/10.1038/42491
- Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J Biol Chem 2001; 276:37993-8001; PMID:11495925; http://dx.doi.org/10.1074/jbc.M106434200
- Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R, Matthews V, et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood 2003; 102:1186-95; PMID:12714508; http://dx.doi.org/10.1182/blood-2002-12-3775
- Hundhausen C, Schulte A, Schulz B, Andrzejewski MG, Schwarz N, von Hundelshausen P, Winter U, Paliga K, Reiss K, Saftig P, et al. Regulated shedding of transmembrane chemokines by the disintegrin and metalloproteinase 10 facilitates detachment of adherent leukocytes. J Immunol 2007; 178:8064-72; PMID:17548644.
- Umehara H, Bloom ET, Okazaki T, Nagano Y, Yoshie O, Imai T. Fractalkine in vascular biology: From basic research to clinical disease. Arterioscler Thromb Vasc Biol 2004; 24:34-40; PMID:12969992; http://dx.doi.org/10.1161/01.ATV.0000095360.62479.1F
- Jones BA, Beamer M, Ahmed S. Fractalkine/CX3CL1: A potential new target for inflammatory diseases. Mol Interv 2010; 10:263-70; PMID:21045240; http://dx.doi.org/10.1124/mi.10.5.3; 10.1124/mi.10.5.3
- Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 1997; 91:521-30; PMID:9390561
- Hannan NJ, Jones RL, Critchley HO, Kovacs GJ, Rogers PA, Affandi B, Salamonsen LA. Coexpression of fractalkine and its receptor in normal human endometrium and in endometrium from users of progestin-only contraception supports a role for fractalkine in leukocyte recruitment and endometrial remodeling. J Clin Endocrinol Metab 2004; 89:6119-29; PMID:15579768; http://dx.doi.org/10.1210/jc.2003-031379
- Huang S, Zhao P, Yang L, Chen Y, Yan J, Duan E, Qiao J. Fractalkine is expressed in the human ovary and increases progesterone biosynthesis in human luteinised granulosa cells. Reprod Biol Endocrinol 2011; 9:95, 7827-9-95; PMID:21718473; http://dx.doi.org/10.1186/1477-7827-9-95
- Cook DN, Chen SC, Sullivan LM, Manfra DJ, Wiekowski MT, Prosser DM, Vassileva G, Lira SA. Generation and analysis of mice lacking the chemokine fractalkine. Mol Cell Biol 2001; 21:3159-65; PMID:11287620; http://dx.doi.org/10.1128/MCB.21.9.3159-3165.2001
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 2000; 20:4106-14; PMID:10805752
- Szukiewicz D, Kochanowski J, Pyzlak M, Szewczyk G, Stangret A, Mittal TK. Fractalkine (CX3CL1) and its receptor CX3CR1 may contribute to increased angiogenesis in diabetic placenta. Mediators Inflamm 2013; 2013:437576; PMID:23956503; http://dx.doi.org/10.1155/2013/437576; 10.1155/2013/437576
- Szukiewicz D, Kochanowski J, Mittal TK, Pyzlak M, Szewczyk G, Cendrowski K. CX3CL1 (fractalkine) and TNFalpha production by perfused human placental lobules under normoxic and hypoxic conditions in vitro: The importance of CX3CR1 signaling. Inflamm Res 2014; 63:179-89; PMID:24270813; http://dx.doi.org/10.1007/s00011-013-0687-z; 10.1007/s00011-013-0687-z
- Szukiewicz D, Kochanowski J, Mittal TK, Pyzlak M, Szewczyk G, Cendrowski K. Chorioamnionitis (ChA) modifies CX3CL1 (fractalkine) production by human amniotic epithelial cells (HAEC) under normoxic and hypoxic conditions. J Inflamm (Lond) 2014; 11:12, 9255-11-12. eCollection 2014; PMID:24851083; http://dx.doi.org/10.1186/1476-9255-11-12
- Stepanian A, Benchenni S, Beillat-Lucas T, Omnes S, Defay F, Peynaud-Debayle E, Baron G, Le Querrec A, Dreyfus M, Salomon L, et al. Search for an association between V249I and T280M CX3CR1 genetic polymorphisms, endothelial injury and preeclampsia: The ECLAXIR study. PLoS One 2009; 4:e6192; PMID:19587779; http://dx.doi.org/10.1371/journal.pone.0006192; 10.1371/journal.pone.0006192
- Siwetz M, Dieber-Rotheneder M, Cervar-Zivkovic M, Kummer D, Kremshofer J, Weiss G, Herse F, Huppertz B, Gauster M. Placental fractalkine is up-regulated in severe early-onset preeclampsia. Am J Pathol 2015; PMID:25769431; http://dx.doi.org/ S0002-9440(15)00088-7 [pii].
- Benirschke K, Kaufmann P, Baergen RN. Pathology of the human placenta. In: Fifth Edition ed. New York, NY: Springer, 2006:42-49.
- Dimitriadis E, Nie G, Hannan NJ, Paiva P, Salamonsen LA. Local regulation of implantation at the human fetal-maternal interface. Int J Dev Biol 2010; 54:313-22; PMID:19757390; http://dx.doi.org/10.1387/ijdb.082772ed
- Dominguez F, Galan A, Martin JJ, Remohi J, Pellicer A, Simon C. Hormonal and embryonic regulation of chemokine receptors CXCR1, CXCR4, CCR5 and CCR2B in the human endometrium and the human blastocyst. Mol Hum Reprod 2003; 9:189-98; PMID:12651900
- Bazer FW, Spencer TE, Johnson GA, Burghardt RC, Wu G. Comparative aspects of implantation. Reproduction 2009; 138:195-209; PMID:19502456; http://dx.doi.org/10.1530/REP-09-0158
- Guillomot M. Cellular interactions during implantation in domestic ruminants. J Reprod Fertil Suppl 1995; 49:39-51; PMID:7623329.
- Wooding FB. Role of binucleate cells in fetomaternal cell fusion at implantation in the sheep. Am J Anat 1984; 170:233-50; PMID:6465051; http://dx.doi.org/10.1002/aja.1001700208
- Cammas L, Reinaud P, Dubois O, Bordas N, Germain G, Charpigny G. Identification of differentially regulated genes during elongation and early implantation in the ovine trophoblast using complementary DNA array screening. Biol Reprod 2005; 72:960-7; PMID:15616222; http://dx.doi.org/10.1095/biolreprod.104.034801.
- Siwetz M, Sundl M, Kolb D, Hiden U, Herse F, Huppertz B, Gauster M. Placental fractalkine mediates adhesion of THP-1 monocytes to villous trophoblast. Histochem Cell Biol 2015; 143(6):565-74; PMID:25566740; http://dx.doi.org/10.1007/s00418-014-1304-0
- Hannan NJ, Jones RL, White CA, Salamonsen LA. The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface. Biol Reprod 2006; 74:896-904; PMID:16452465; http://dx.doi.org/10.1095/biolreprod.105.045518
- Hannan NJ, Salamonsen LA. CX3CL1 and CCL14 regulate extracellular matrix and adhesion molecules in the trophoblast: potential roles in human embryo implantation. Biol Reprod 2008; 79:58-65; PMID:18367676; http://dx.doi.org/10.1095/biolreprod.107.066480; 10.1095/biolreprod.107.066480
- Zhao P, De A, Hu Z, Li J, Mulders SM, Sollewijn Gelpke MD, Duan EK, Hsueh AJ. Gonadotropin stimulation of ovarian fractalkine expression and fractalkine augmentation of progesterone biosynthesis by luteinizing granulosa cells. Endocrinology 2008; 149:2782-9; PMID:18292196; http://dx.doi.org/10.1210/en.2007-1662
- Sawai H, Park YW, He X, Goronzy JJ, Weyand CM. Fractalkine mediates T cell-dependent proliferation of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheum 2007; 56:3215-25; PMID:17907166; http://dx.doi.org/10.1002/art.22919
- Chandrasekar B, Mummidi S, Perla RP, Bysani S, Dulin NO, Liu F, Melby PC. Fractalkine (CX3CL1) stimulated by nuclear factor kappaB (NF-kappaB)-dependent inflammatory signals induces aortic smooth muscle cell proliferation through an autocrine pathway. Biochem J 2003; 373:547-58; PMID:12729461; http://dx.doi.org/10.1042/BJ20030207
- Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the boyd collection revisited. Am J Obstet Gynecol 1999; 181:718-24; PMID:10486489; http://dx.doi.org/ S0002937899005669 [pii].
- Hustin J, Schaaps JP. Echographic [corrected] and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am J Obstet Gynecol 1987; 157:162-8; PMID:3300349.
- Huppertz B, Berghold VM, Kawaguchi R, Gauster M. A variety of opportunities for immune interactions during trophoblast development and invasion. Am J Reprod Immunol 2012; 67:349-57; PMID:22593844
- Siwetz M, Blaschitz A, Kremshofer J, Bilic J, Desoye G, Huppertz B, Gauster M. Metalloprotease dependent release of placenta derived fractalkine. Mediators Inflamm 2014; 2014:839290; PMID:24771984; http://dx.doi.org/10.1155/2014/839290
- Burton GJ, Jauniaux E, Charnock-Jones DS. Human early placental development: Potential roles of the endometrial glands. Placenta 2007; 28 Suppl A:S64-9; PMID:17349689; http://dx.doi.org/10.1016/j.placenta.2007.01.007
- Hempstock J, Cindrova-Davies T, Jauniaux E, Burton GJ. Endometrial glands as a source of nutrients, growth factors and cytokines during the first trimester of human pregnancy: a morphological and immunohistochemical study. Reprod Biol Endocrinol 2004; 2:58; PMID:15265238; http://dx.doi.org/10.1186/1477-7827-2-58
- Joerink M, Rindsjo E, van Riel B, Alm J, Papadogiannakis N. Placental macrophage (hofbauer cell) polarization is independent of maternal allergen-sensitization and presence of chorioamnionitis. Placenta 2011; 32:380-5; PMID:21419483; http://dx.doi.org/10.1016/j.placenta.2011.02.003; 10.1016/j.placenta.2011.02.003
- Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol 2000; 157:2111-22; PMID:11106583; http://dx.doi.org/ S0002-9440(10)64849-3 [pii].
- Jeschke U, Toth B, Scholz C, Friese K, Makrigiannakis A. Glycoprotein and carbohydrate binding protein expression in the placenta in early pregnancy loss. J Reprod Immunol 2010; 85:99-105; PMID:20299109; http://dx.doi.org/10.1016/j.jri.2009.10.012; 10.1016/j.jri.2009.10.012
- Xiao J, Garcia-Lloret M, Winkler-Lowen B, Miller R, Simpson K, Guilbert LJ. ICAM-1-mediated adhesion of peripheral blood monocytes to the maternal surface of placental syncytiotrophoblasts: Implications for placental villitis. Am J Pathol 1997; 150:1845-60; PMID:9137107.
- Grasso E, Paparini D, Hauk V, Salamone G, Leiros CP, Ramhorst R. Differential migration and activation profile of monocytes after trophoblast interaction. PLoS One 2014; 9:e97147; PMID:24849800; DOI: 10.1371/journal.pone.0097147
- Mellembakken JR, Aukrust P, Olafsen MK, Ueland T, Hestdal K, Videm V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension 2002; 39:155-60; PMID:11799095
- Labarrere CA, Bammerlin E, Hardin JW, Dicarlo HL. Intercellular adhesion molecule-1 expression in massive chronic intervillositis: Implications for the invasion of maternal cells into fetal tissues. Placenta 2014; 35:311-7; PMID:24631282; http://dx.doi.org/10.1016/j.placenta.2014.02.006
- Schamberger S, Weber M, Backsch C, Sonnemann J, Markert UR. Establishment of a one-sided ex vivo human placenta perfusion model to assess adhesion and invasion behavior of T cell leukemia cell lines. Leuk Lymphoma 2013; 54:1811-3; PMID:23240910; http://dx.doi.org/10.3109/10428194.2012.758844
