ABSTRACT
Chemokine network is central to the innate and adaptive immunity and entails a variety of proteins and membrane receptors that control physiological processes such as wound healing, angiogenesis, embryo growth and development. During early pregnancy, the chemokine network coordinates not only the recruitment of different leukocyte populations to generate the maternal-placental interface, but also constitutes an additional checkpoint for tissue homeostasis maintenance. The normal switch from a pro-inflammatory to an anti-inflammatory predominant microenvironment characteristic of the post-implantation stage requires redundant immune tolerance circuits triggered by key master regulators. In this review we will focus on the recruitment and conditioning of maternal immune cells to the uterus at the early implantation period with special interest on high plasticity macrophages and dendritic cells and their ability to induce regulatory T cells. We will also point to putative immunomodulatory polypeptides involved in immune homeostasis maintenance at the maternal-placental interface.
KEYWORDS:
Introduction
Pregnancy implies a modulation of the maternal immune response associated with a variety of cellular processes to ensure trophoblast growth and invasion, uterine quiescence, vascularization and tissue remodeling at the maternal-placental interface. The induction and maintenance of tolerance is provided by maternal immune cells differentially activated and selectively recruited to the uterus at different stages. A highly coordinated chemokine network underlies not only different leukocyte subset recruitment but also orchestrates unique mechanisms of immunomodulation.Citation1-5 Trophoblast cells have a central role in the control of maternal immune homeostasis through modulating uterine NK cell activity, decidual macrophage polarization to an alternative profile, tolerogenic dendritic cell (DC) differentiation and induction of regulatory T cells (Tregs).Citation6-8
Following the inflammatory period characteristic of implantation, an anti-inflammatory and immune tolerant microenvironment is induced to sustain immune homeostasis and successful pregnancy. Many redundant mechanisms trigger immune tolerance through key master immune regulators of different nature, such as cytokines, hormones, lectins and immune polypeptides, all of them displaying pivotal immunotolerance mechanisms.
In this review we will focus on the recruitment and conditioning of main subsets of immune-maternal cells at the early implantation period with special interest on macrophages and dendritic cells due to their immunological plasticity and ability to induce regulatory T cells. We will discuss the chemokine network involved in their recruitment toward the maternal-placental interface; and whether chemokines among other locally synthesized immune polypeptides contribute to maintain the homeostasis in the pregnant uterus.
Chemokines and other chemoattractants at early pregnancy
Chemokines are central to innate and adaptive immunity and they control physiological processes such as wound healing and angiogenesis, as well as embryo growth and development.Citation9,10 During early pregnancy, the chemokine network coordinates not only the recruitment of different leukocyte populations to generate the maternal-placental interface but it also constitutes an additional checkpoint for tissue homeostasis maintenance, even in the presence of threats and infection.Citation11-13
Basically, these chemo-attractant cytokines and their receptors are classified according to their structure or expression. The first classification is based on the cystein-conserved positions: CXC, CC, CX3C and C groups of the α, β, γ and δ-chemokines.Citation14,15 The second one, correspond to inflammatory or induced upon T-cell activation, and constitutive chemokines that fulfill housekeeping functions and/or participate in constitutive leukocyte trafficking.Citation9,15,16
The chemokine receptors are named according to the chemokine structure (CXCR for CXC or CCR for CC chemokines) and belong to the super-family of G protein coupled receptors. The switch from receptors for constitutive chemokines to receptors for inflammatory chemokines changes the migratory properties of leukocyte populations.Citation17-19
This system is highly promiscuous since a single chemokine can interact with multiple receptors.Citation10 In fact, chemokine receptors signaling could be regulated by several adaptors that mediate the internalization and signal transduction by forming a dynamic bond with spatial and temporal plasticity (chemosynapse).Citation20-22
Interestingly, non-signaling-chemokine receptors (decoy receptors) were described which do not elicit conventional signaling responses, but display inflammatory and immunomodulatory effects. Until now, 3 decoy receptors were described, chemokine decoy receptor (D6), Duffy antigen receptor for chemokines (DARC) and chemocentryx decoy receptor (CCX CKR). The expression of decoy receptors is mainly restricted to placental cells and endothelial cells of lymphatic afferent vessels in skin, gut and lung.Citation23,24
Among other factors of protein nature synthesized at the maternal-placental interface that have proved chemotaxis properties associated to their immunomodulatory role TGF-β1, hCG, vasoactive intestinal peptide or Galectin 1 are suitable examples.
Hence, TGF-β1 reversibly regulates chemotaxis of DCs via regulation of chemokine receptor expression.Citation25 The treatment of immature DCs with TGF-β1 resulted in increased expression of several chemokines associated with a chemotactic migratory response; whereas it induced down-regulation of CCR1, CCR3, CCR5, CCR6 and CXCR-4 on mature DCs, and chemotaxis to their respective ligands.Citation25 Another example is hCG, a very first hormone produced by trophoblast cells, that acts as a chemoattractant and selectively recruits Tregs at the human fetal–maternal interface.Citation26
The vasoactive intestinal peptide (VIP) is a pleiotropic peptide targeting multiple cell types at the maternal interface with suppressant/tolerant activity. It binds to G protein coupled receptors VPAC1 and VPAC2 with potent immunomodulatory and trophic effects on adult and embryonic tissues.Citation27-37 At the maternal-placental interface, VIP localize in first and third trimester placenta in the syncytium and extravillous cytotrophoblast cells.Citation38 VIP dose-dependently stimulated hCG production from human primary cultured trophoblast cells as well as choriocarcinoma cell line JEG-3.Citation39,40
Finally, Galectin-1 is a lectin with a central immunoregulatory role in the post-implantation period.Citation41 Gal1 is mainly evolutionarily conserved and expressed in invasive extravillous trophoblast cells of human first trimester and term placenta. It is regulated by progesterone and pro-inflammatory cytokines thus limiting T cell viability, dampening the secretion of Th1-type cytokines and favoring the expansion of CD4+CD25+FoxP3+ Treg cells.Citation42 In fact, mice lacking Gal1 (Lgals1−/−) showed higher rates of fetal loss and the administration of recombinant Gal1 prevents fetal loss and restores tolerance in vivo.Citation8
Physiology of the chemokine network: Relevance to their role in pregnancy
The classical physiologic function associated with chemokine is the recruitment of immune cells in inflammation or homeostatic conditions, depending on the synthesis and release of different sets of chemokines.Citation43 In this way, chemokines direct the trafficking of immune cells through the circulation in blood, lymph vessels and tissues. Particularly, they mediate the generation of the maternal-placental interface through the interaction between decidual stromal cells, trophoblast cells and the selective recruitment of maternal and fetal leukocytes.
Trophoblast cells actively recruit immune cells through chemokine production and can also condition their functional profile. Mei-Rong Du et al. have investigated differential expression patterns of chemokines and their receptors and they determined that primary trophoblasts express CXCR4 and CXCR6.Citation14 In fact, trophoblasts secrete high levels of CXCL12, CXCL16 and CCL24 ( and ).
Figure 1. Chemokines, as multifunctional molecules, contributes to the generation of the maternal-placental interface through the interaction between: decidual stromal cells, trophoblast cells and the selective recruitment of maternal and fetal leukocytes.
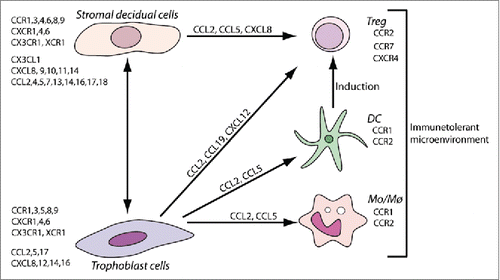
Regarding the decidual stromal cells, CCR2, CCR5 and CCR10 are highly expressed as well CCL2 and CCL13 (the ligands of CCR2), and CCL28 (the ligand of CCR10).Citation14 Liang Ren et al. explored the role of CXCR4-mediated signal transduction on primary cultured decidual stromal cells.Citation44 The decidualization process increases CXCR4 in response to CXCL12 secreted by human first-trimester trophoblast cells, and this increase could be effectively abolished by CXCL12 or CXCR4 neutralizing antibody.Citation45 This chemokine/chemokine receptor pair contributes to additional reproductive biology processes, such as uterine NK cell recruitment, placentation, implantation and embryogenesis.Citation46-51
Despite CXCR4 is expressed by trophoblast and decidual stromal cells, the CXCL12/CXCR4 ligand/receptor pair might present various effects on different cells via different signaling pathways. It was proposed that CXCL12 produced by trophoblast cells has a cascade pathway which promotes cytotrophoblast proliferation and invasion, while it enhances MMP activity in decidual stromal cells, cooperating in the formation of the maternal–fetal immune milieu. This is a suitable example to show the relevance of the cell type involved on the multiple effects displayed by the same chemokine-chemokine receptor axis.
Another interesting point is that decidual endometrial cells could regulate the access to the fetal-maternal interface. Nancy et al. demonstrated that decreased chemoattraction of T cells to the decidua occurs in order to support a tolerogenic response.Citation52 They demonstrated that the epigenetic silencing associated with methylation of T cell-attracting inflammatory chemokines genes through repressive histone marks in decidual stromal cells impaired the accumulation of maternal effector T cells within the decidua.Citation52 This new insight suggests an active role of the decidua controlling the migration of maternal T cells toward the implantation site. In this sense, trophoblast cells secrete cytokines that regulate the function and differentiation of decidual immune cells and also might induce epigenetic changes in stromal decidual cells modulating their capacity to produce chemokines responsible for T cell recruitment. Under pathologic conditions, for example following recognition of pathogen associated molecular patterns (PAMPs) expressed on bacteria, virus, parasite or fungi, this balance could be broken and the same stromal decidual cells might be involved in the local recruitment and activation of effector T cells, generating a threat for pregnancy.Citation50-53
Since chemokines are multifunctional molecules, they also contribute to 3 crucial processes to sustain embryo implantation: angiogenesis, wound healing and embryogenesis.
Regarding the chemokines' angiogenic properties, the CXC family can be further subdivided by the presence or absence of the ELR motif, a Glu-Leu-Arg conserved sequence at the NH2 terminus. The presence of the ELR motif in CXCL1-3 and CXCL5-8, which bind to CXCR1 and CXCR2, allows angiogenic effects.Citation56 Also the CXCL12-CXCR4 axis displays angiogenic properties during embryogenesis.Citation57,58
Pointing to the wound healing process, chemokines play an integral part in directing wound closure and healing.Citation59-61 Epithelial cells express chemokine ligands and receptors involved in several steps in the reconstitution of the epithelial barrier as cell migration, extracellular matrix deposition, cellular adhesion and proliferation.Citation62-64 CXCL12, CXCL1, and CXCL8, guide these steps of re-epithelialization in the skin however their relevance at the maternal-placental interface is unclear.Citation64,65
Differential migration and activation profile of maternal monocytes/macrophages
Monocytes/macrophages at the maternal-placental interface coordinate opposite demands. On one hand, they produce suppressor cytokines and wound healing mediators that provide immune tolerance signals to dampen pro-inflammatory signals and to suppress the response to allogeneic fetal antigens; on the other, macrophages can be activated into the classic inflammatory profile in response to ascending and blood-borne pathogens.Citation66
Several evidences point that macrophage responses are determined by the kind of stimulus and the specific micro-environmental conditions in which cells were differentiated prior to their activation.Citation67,68 Analysis of microarray gene expression indicates that macrophages isolated from decidua during the first trimester, display an alternative activation profile, also know as M2 phenotype, with upregulation of CCL18, DC-SIGN, mannose receptor C type-1, fibronectin 1 and insulin-like growth factor-1.Citation69
When we explored maternal CD14+ cell profile, isolated from fertile women, after interaction with first trimester trophoblast cell line Swan-71, we observed the induction of an alternative activated phenotype based on an increased expression of CD16, a marker associated with a regulatory monocyte profile, and also the expression of CD39, a surface enzyme that was proposed as self-limiting for macrophage pro-inflammatory activation based on its rapid catabolism of endogenous ATP into adenosine.Citation67,70 At the same time, macrophages did not modulate the expression of the co-stimulatory molecule CD80. Their phenotypic profile was accompanied by changes in CD14+ cell functional profile with increased IL-10 synthesis but not of pro-inflammatory cytokine production as IL-12, IL-1β and TNFα.Citation71 Also decidual macrophages secrete high level of CCL2 that recruit additional macrophages into the decidua potentiating this loop.Citation11
An interesting point is that under bacterial or viral PAMP stimulation, trophoblast cells restrain early monocyte migration depending on the type of PAMP stimulus.Citation71 For example, they restrict monocytes migration toward trophoblast cells in the presence of bacterial PAMP (LPS or PGN) with a decrease in the expression of CCR1, CCR5, CCL5 and CXCL8 on the CD14+ cells, while in the presence of a viral PAMP (poly[I:C]), this decrease was not observed.Citation71
On the other hand, the modulation of chemoattractant signals upon trophoblast-monocyte interaction is bidirectional: not only monocytes modulate chemokines and their receptor expression in the context of the maternal-placental interaction, but also trophoblasts modulate them after culture with monocytes in the presence of PAMPs stimuli.Citation71 Therefore, trophoblast cells are able to attract and condition monocytes to produce and secrete a particular set of cytokines and chemokines, supporting their growth and survival. At the earliest phases of an infection, the selective modulation of chemokines and their receptor expression by trophoblast cells would represent one of the first steps to control leukocyte trafficking to avoid potential tissue damage.
In addition, trophoblast cells secrete multiple target molecules as VIP with the ability to induce a regulatory/suppressant activation profile in macrophages.Citation33,72,73 In human and murine macrophages, VIP increases IL-10 synthesis and reduces IL-12, TNF-α and inducible nitric oxide synthase activity through both VPACs. In an in vitro model, we could observed that trophoblast cell conditioned media increased CD14+ cell migration and the effect was further induced when trophoblast cells were pretreated with VIP, due to VIP increases CCL2, CCL3, CCL5 and CXCL−8 mRNA expression in trophoblast cells.Citation74
Differential migration and activation profile of dendritic cells
Conventional DC are specialized antigen presenting cells with a critical role in the generation of the maternal-fetal interface.Citation75-78 In fact, during implantation, the depletion of uterine DC (uDC) results in a severe impairment of the implantation process, leading to embryo resorption in allogeneic or syngeneic pregnancies.Citation79 Consistent with these observations, using a transgenic mouse model that allows conditional ablation of uDC, Plaks and colleagues observed an impaired proliferation and differentiation of the decidua, even in the absence of embryos. These changes are associated with disrupted angiogenesis characterized by reduced vascular expansion and attenuated maturation, associated to sFlt1 (FMS-like tyrosine kinase 1) and TGF-β1 modulation.Citation80
After implantation, the immunoregulatory properties of uDC are more evident and constitute a unique and complex system of cells able to commit between tolerogenic and immunogenic responses. The uDC comprise about 1–2% of the immune cells in the decidua and are scattered through both the decidua basalis (in which trophoblast cells are infiltrated) and the decidua parietalis. Kammerer et al. described the presence of subpopulations of DC in decidual tissue during the early phase of human pregnancy which seem to participate in the protection of the allogeneic fetus in a mouse pregnancy model.Citation81-83
Using in vitro models we could oberve that those DC differentiated from CD14+ cells from fertile women and co-cultured with trophoblast cells prevented the up-regulation of CD83 expression, a maturation marker after a challenged with LPS.Citation84 In fact, they did not modulate the expression of CD86, CD40 and HLA-DR suggesting that DCs remained in a semi-mature state.Citation84 The modulation of phenotipic markers also was accompained by an increase in the production of IL-10 and a reduction in the IL-12p70 and TNF-α production.Citation84
From a functional point of view, those DC pre-cultured with trophoblast cells and challenged with LPS, suppressed the allogeneic response and were able to induce Tregs.
Finally, trophoblast cells appeared to be responsible of recruiting DC, as evidenced by a significant increase in the frequency of DC that migrated toward conditioned media from Swan-71 cells.Citation84
Taking together all these data suggest that the ‘education’ that trophoblast cells impose to DCs might induce an alternative activation pattern and, in the present in vitro model, they acquired a functional profile compatible with a suppressor-tolerogenic profile.
Induction and migration of maternal tregs cells
The initial pro-inflammatory response characteristic of implantation is actively modulated to a predominant anti-inflammatory and tolerogenic profile at early gestation, and Treg cells are essential for this immune switch. The polarizing microenvironment will determine the chemokine receptor profile on Treg cells and therefore might direct them to appropriate tissue sites to regulate the immune response. Naturally, the induced Treg cells (iTregs) upregulate the expression of chemokine receptors that are also expressed by effector T cells favoring the co localization with effector T cells and then displaying their suppressive functions.Citation9
Recently, Teles et al. have demonstrated using 2-photon imaging that Foxp3+ cells accumulated in the mouse uterus during the receptive phase of the estrus cycle. In vivo depletion of Tregs using Foxp3.DTR-based models prior to pairing, impaired implantation inducing a hostile microenvironment associated with the recruitment of effector T cells, uterine inflammation and fibrosis. In fact, CCR7 interfered with accumulation of Tregs in the uterus and implantation indicating that homing of Tregs to the uterus was mediated by CCR7.Citation82
Endometrial stromal cells under the decidualization program differentiate into epithelioid decidual cells and secrete diverse mediators, contributing to the generation of a local immune response supporting the nidation of a semiallogenic fetus.Citation83,84
Using an in vitro model of decidualization with a human endometrial stromal cell line (HESC) stimulated with P4 and LPS simulating the inflammatory response during implantation, we observed that P4 increased VIP intracellular production in a concentration dependent manner.Citation71 LPS increased CCL2, CCL5 and CXCL8 expression evaluated by RTqPCR in HESC cells and VIP by itself did not have a significant effect on cytokine production by HESCs; however, the combination of LPS and VIP further enhanced LPS-induced CCL5 expression.Citation71 Getting insight into the VIP effects on this mechanism, we observed that VIP antagonist prevented the increase of CCL5 expression mediated by P4 and LPS, suggesting that CCL5 induction involved a VIP-mediated pathway.Citation71
Since CCL5 contributes to the induction and the recruitment of Tregs we determined if HESC cells have the ability to attract iTregs.Citation16 Previously, we developed an in vitro differentiation model of iTregs from naïve CD45RA+CCR7+ obtained from peripheral blood mononuclear cells isolated from fertile women and we performed migration assays using a multi-chamber system. The condition media (CM) from HESC cells increased the frequency of Foxp3+ cells and VIP antagonist prevented the recruitment of iTregs. Moreover, addition of anti-RANTES neutralizing Ab to the CM from HESC treated with P4 and LPS also was able to prevent iTregs migration suggesting that CCL5 participates in the specific recruitment of iTregs toward HESC cells.Citation71
During the implantation period, CCL5 produced by human endometrium it has the potential to act in an autocrine manner by the differential expression of its receptors CCR1, CCR3, and CCR5.Citation85,86 CCL5 also is produced by human endometrial T-infiltrated lymphocytes, CD4+ and CD8+, which production increased in the presence of physiological P4 concentrations.Citation85
At later pregnancy stages, the Foxp3+ cell subset suffer an expansion and using a Rag-1−/− model of cell transfer Tregs, Teles et al. demonstrated that natural Tregs (thymic origin), are needed for pregnancy establishment and induced Tregs (iTregs) contributes to the Treg pool in the periphery.Citation90
Trophoblast cells not only contribute to iTreg cell differentiation, but also selectively recruit them. We demonstrated that Swan-71 cells secreted VIP and their co-culture with maternal PBMC significantly increased the frequency of maternal CD4+CD25+FoxP3+ cells. This increase was prevented by an anti TGFβ Ab and VIP antagonist, suggesting that VIP could have an active role in the immunoregulatory through a mechanism involving TGFβ1.
Migration assays performed in transwell systems with conditioned media from first trimester trophoblast HTR-8 or Swan-71 cell lines doubled Foxp3+ cell recruitment compared to the positive control of human serum.Citation91,92 In fact, the frequency of Foxp3+ cells migrated toward trophoblast cells in the presence of a bacterial or viral stimulus increased and CCL4, CCL5, CXCL1 and CXCL8 secretion by trophoblast cells further recruited iTregs.Citation93
Finally, Treg-trafficking is bidirectional. Mold et al. demonstrated that maternal alloantigens promote the development of tolerogenic fetal Treg cells in utero. Maternal cells migrate toward the fetal lymph nodes, inducing the development of fetal Tregs that suppress fetal anti-maternal immunity and persist at least until early adulthood.Citation94-96 Consistently, Tilburgs et al. presented evidence of a selective migration of fetus specific CD4+CD25bright Treg cells to decidua basalis and parietalis that suppress fetus specific and non specific responses.Citation97,98
Finally, in the non-obese diabetic mice, VIP treatment of mice at gestational day 6,5 switches the Tregs/Th17 ratio leading to tolerance. VIP also reduces Th17/Th1 and Th1/Th2 ratios,Citation99 induces Foxp3+ regulatory T cells and, in the presence of TGF-β.Citation100
Challenges for chemokine targeting
Approaches to targeting chemokine signaling require a careful analysis of the balance of chemokines and their receptors that belong to super family of GPCRs. One of available examples of attempts to block chemokine signaling through receptor inhibition is the AMD3100 (Plerixafor), which blocks localized CXCL12 signaling through CXCR4 stem cells in bone marrow allowing cells to enter the circulation.Citation101 This finding allowed AMD3100 to be proposed for autologous stem cell transplantation applied to patients with leukemia or multiple myeloma for short term.Citation102 Another novel approach involves the CCR5 inhibitor drugs. CCR5 is an HIV co-receptor and its inhibitor UK-427857 (Maraviroc) has been approved for patients resistant to conventional therapies.Citation103 Beyond the use of small molecule inhibitors, several neutralizing antibodies against chemokine receptors have been tested in early-phase clinical trials, including an antibody designed for blocking CCR2 activity in a variety of inflammatory diseases.Citation104,105
Alternate strategies include the generation of inhibitors that antagonize chemokine ligands instead of receptor chemokines. Novel targeting of chemokine ligands involve the characterization of conserved sites present in all chemokines as the sulfotyrosine binding pocket.Citation106 This strategy gives potential versatility to targeting either individual chemokines or entire subgroups of ligands. Citation107
Conclusion
The selective recruitment and activation of the different cell populations at the maternal-placental interface induce an immunological environment at the uterus and deciduas and represents a rich area of research for understanding the regulation of the immune system in order to a better understanding of pregnancies complications, cancer, infections or autoimmunity.
When the signaling of chemokines presents a dysregulation, the chemokine inhibition or the systemic blockade of chemokine receptor signaling as therapies might generate side effects; therefore the real challenge is to restore the physiologic gradient concentrations of chemokines.
Table 1. Expression of most relevant chemokines and chemokine receptors on different subpopulations involved in the generation of the maternal-fetal interfase.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by the National Agency of Sciences and Technology ANPCyT (PICT 2011-0144, 2013-1632, 2014-0657) and University of Buenos Aires (UBACyT 2012-2015 and 2014-2017) to CPL and to RR.
References
- Redman CWG, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol 2010; 63:534-43; PMID:20331588; http://dx.doi.org/10.1111/j.1600-0897.2010.00831.x
- Daher S, de Arruda Geraldes Denardi K, Blotta MHSL, Mamoni RL, Reck APM, Camano L, Mattar R. Cytokines in recurrent pregnancy loss. J Reprod Immunol 2004; 62:151-7; PMID:15288190; http://dx.doi.org/10.1016/j.jri.2003.10.004
- Clark DA, Yu G, Arck PC, Levy GA, Gorczynski RM. MD-1 is a Critical Part of the Mechanism Causing Th1-Cytokine-Triggered Murine Fetal Loss Syndrome. Am J Reprod Immunol 2003; 49:297-307; PMID:12854734; http://dx.doi.org/10.1034/j.1600-0897.2003.00045.x
- Kwak-Kim J, Yang KM, Gilman-Sachs A. Recurrent pregnancy loss: a disease of inflammation and coagulation. J Obstet Gynaecol Res 2009; 35:609-22; PMID:19751318; http://dx.doi.org/10.1111/j.1447-0756.2009.01079.x
- Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med 2006; 203:2165-75; PMID:16923853;http://dx.doi.org/10.1084/jem.20061022
- Yoshinaga K. Research on Blastocyst Implantation Essential Factors (BIEFs). Am J Reprod Immunol 2010; 63:413-24; PMID:20455874; http://dx.doi.org/10.1111/j.1600-0897.2010.00853.x
- Molvarec A, Blois SM, Stenczer B, Toldi G, Tirado-Gonzalez I, Ito M, Shima T, Yoneda S, Vásárhelyi B, Rigó J, et al. Peripheral blood galectin-1-expressing T and natural killer cells in normal pregnancy and preeclampsia. Clin Immunol 2011; 139:48-56; PMID:21292557; http://dx.doi.org/10.1016/j.clim.2010.12.018
- Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med 2007; 13:1450-7; PMID:18026113; http://dx.doi.org/10.1038/nm1680
- Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol 2008; 9:970-80; PMID:18711434; http://dx.doi.org/10.1038/ni.f.213
- Raman D, Sobolik-Delmaire T, Richmond A. Chemokines in health and disease. Exp Cell Res 2011; 317:575-89; PMID:21223965;http://dx.doi.org/10.1016/j.yexcr.2011.01.005
- Rozner AE, Dambaeva SV, Drenzek JG, Durning M, Golos TG. Modulation of cytokine and chemokine secretions in rhesus monkey trophoblast co-culture with decidual but not peripheral blood monocyte-derived macrophages. Am J Reprod Immunol 2011; 66:115-27; PMID:21276119;http://dx.doi.org/10.1111/j.1600-0897.2010.00979.x
- Kallikourdis M, Andersen KG, Welch KA, Betz AG. Alloantigen-enhanced accumulation of CCR5+ “effector” regulatory T cells in the gravid uterus. Proc Natl Acad Sci U S A 2007; 104:594-9; PMID:17197426; http://dx.doi.org/10.1073/pnas.0604268104
- Fraccaroli L, Alfieri J, Larocca L, Calafat M, Mor G, Leirós CP, Ramhorst R. A potential tolerogenic immune mechanism in a trophoblast cell line through the activation of chemokine-induced T cell death and regulatory T cell modulation. Hum Reprod 2009; 24:166-75; PMID:18824472;http://dx.doi.org/10.1093/humrep/den344
- Du M-R, Wang S-C, Li D-J. The integrative roles of chemokines at the maternal-fetal interface in early pregnancy. Cell Mol Immunol 2014; 11:438-48; PMID:25109684; http://dx.doi.org/10.1038/cmi.2014.68
- Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol 2000; 18:217-42; PMID:10837058;http://dx.doi.org/10.1146/annurev.immunol.18.1.217
- Fraccaroli L, Alfieri J, Leiros CP, Ramhorst R. Immunomodulatory effects of chemokines during the early implantation window. Front Biosci (Elite Ed) 2009; 1:288-98; PMID:19482646
- Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol 2001; 2:102-7; PMID:11175801; http://dx.doi.org/10.1038/84205
- Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol 2001; 2:123-8; PMID:11175804; http://dx.doi.org/10.1038/84219
- Sohy D, Yano H, de Nadai P, Urizar E, Guillabert A, Javitch JA, Parmentier M, Springael J-Y. Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J Biol Chem 2009; 284:31270-9; PMID:19758998; http://dx.doi.org/10.1074/jbc.M109.054809
- Neel NF, Barzik M, Raman D, Sobolik-Delmaire T, Sai J, Ham AJ, Mernaugh RL, Gertler FB, Richmond A. VASP is a CXCR2-interacting protein that regulates CXCR2-mediated polarization and chemotaxis. J Cell Sci 2009; 122:1882-94; PMID:19435808; http://dx.doi.org/10.1242/jcs.039057
- Raman D, Neel NF, Sai J, Mernaugh RL, Ham A-JL, Richmond AJ. Characterization of chemokine receptor CXCR2 interacting proteins using a proteomics approach to define the CXCR2 “chemosynapse”. Methods Enzymol 2009; 460:315-30; PMID:19446732; http://dx.doi.org/10.1016/S0076-6879(09)05215-X
- Raman D, Sai J, Neel NF, Chew CS, Richmond A. LIM and SH3 protein-1 modulates CXCR2-mediated cell migration. PLoS One 2010; 5:e10050; PMID:20419088; http://dx.doi.org/10.1371/journal.pone.0010050
- Borroni EM, Bonecchi R, Buracchi C, Savino B, Mantovani A, Locati M. Chemokine decoy receptors: new players in reproductive immunology. Immunol Invest 2008; 37:483-97; PMID:18716935; http://dx.doi.org/10.1080/08820130802191318
- Wessels JM, Linton NF, van den Heuvel MJ, Cnossen SA, Edwards AK, Croy BA, Tayade C. Expression of chemokine decoy receptors and their ligands at the porcine maternal-fetal interface. Immunol Cell Biol 2011; 89:304-13; PMID:20680026; http://dx.doi.org/10.1038/icb.2010.95
- Sato K, Kawasaki H, Nagayama H, Enomoto M, Morimoto C, Tadokoro K, Juji T, Takahashi TA. TGF- 1 Reciprocally Controls Chemotaxis of Human Peripheral Blood Monocyte-Derived Dendritic Cells Via Chemokine Receptors. J Immunol 2000; 164:2285-95; PMID:10679062; http://dx.doi.org/10.4049/jimmunol.164.5.2285
- Schumacher A, Brachwitz N, Sohr S, Engeland K, Langwisch S, Dolaptchieva M, Alexander T, Taran A, Malfertheiner SF, Costa S-D, et al. Human Chorionic Gonadotropin Attracts Regulatory T Cells into the Fetal-Maternal Interface during Early Human Pregnancy. J Immunol 2009; 182:5488-97; PMID:19380797; http://dx.doi.org/10.4049/jimmunol.0803177
- Said SI. The discovery of VIP: initially looked for in the lung, isolated from intestine, and identified as a neuropeptide. Peptides 2007; 28:1620-1; PMID:17719695; http://dx.doi.org/10.1016/j.peptides.2007.06.007
- Lara-Marquez M, O'Dorisio M, O'Dorisio T, Shah M, Karacay B. Selective gene expression and activation-dependent regulation of vasoactive intestinal peptide receptor type 1 and type 2 in human T cells. J Immunol 2001; 166:2522-30; PMID:11160313; http://dx.doi.org/10.4049/jimmunol.166.4.2522
- Martinez C, Delgado M, Abad C, Gomariz RP, Ganea D, Leceta J. Regulation of VIP production and secretion by murine lymphocytes. J Neuroimmunol 1999; 93:126-38; PMID:10378876; http://dx.doi.org/10.1016/S0165-5728(98)00216-1
- Li J-M, Southerland L, Hossain MS, Giver CR, Wang Y, Darlak K, Harris W, Waschek J, Waller EK. Absence of vasoactive intestinal peptide expression in hematopoietic cells enhances Th1 polarization and antiviral immunity in mice. J Immunol 2011; 187:1057-65; PMID:21677142; http://dx.doi.org/10.4049/jimmunol.1100686
- Lodde BM, Mineshiba F, Wang J, Cotrim AP, Afione S, Tak PP, Baum BJ. Effect of human vasoactive intestinal peptide gene transfer in a murine model of Sjogren's syndrome. Ann Rheum Dis 2006; 65:195-200; PMID:15975969; http://dx.doi.org/10.1136/ard.2005.038232
- Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med 2001; 7:563-8; PMID:11329057; http://dx.doi.org/10.1038/87887
- Gonzalez-Rey E, Delgado M. Vasoactive intestinal peptide and regulatory T-cell induction: a new mechanism and therapeutic potential for immune homeostasis. Trends Mol Med 2007; 13:241-51; PMID:17467339; http://dx.doi.org/10.1016/j.molmed.2007.04.003
- Yadav M, Goetzl EJ. Vasoactive intestinal peptide-mediated Th17 differentiation: an expanding spectrum of vasoactive intestinal peptide effects in immunity and autoimmunity. Ann N Y Acad Sci 2008; 1144:83-9; PMID:19076367; http://dx.doi.org/10.1196/annals.1418.020
- Jimeno R, Gomariz RP, Gutiérrez-Cañas I, Martínez C, Juarranz Y, Leceta J. New insights into the role of VIP on the ratio of T-cell subsets during the development of autoimmune diabetes. Immunol Cell Biol 2010; 88:734-45; PMID:20309012; http://dx.doi.org/10.1038/icb.2010.29
- Rosignoli F, Torroba M, Juarranz Y, García-Gómez M, Martinez C, Gomariz RP, Pérez-Leirós C, Leceta J. VIP and tolerance induction in autoimmunity. Ann N Y Acad Sci 2006; 1070:525-30; PMID:16888219; http://dx.doi.org/10.1196/annals.1317.073
- Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells that prevent acute graft-versus-host disease while maintaining the graft-versus-tumor response. Blood 2006; 107:3787-94; PMID:16418327; http://dx.doi.org/10.1182/blood-2005-11-4495
- Graf AH, Hütter W, Hacker GW, Steiner H, Anderson V, Staudach A, Dietze O. Localization and distribution of vasoactive neuropeptides in the human placenta. Placenta 1996; 17:413-21; PMID:8899870; http://dx.doi.org/10.1016/S0143-4004(96)90023-5
- Marzioni D, Fiore G, Giordano A, Nabissi M, Florio P, Verdenelli F, Petraglia F, Castellucci M. Placental expression of substance P and vasoactive intestinal peptide: evidence for a local effect on hormone release. J Clin Endocrinol Metab 2005; 90:2378-83; PMID:15623814; http://dx.doi.org/10.1210/jc.2004-1512
- Deutsch PJ, Sun Y, Kroog GS. Vasoactive intestinal peptide increases intracellular cAMP and gonadotropin-alpha gene activity in JEG-3 syncytial trophoblasts. Constraints posed by desensitization. J Biol Chem 1990; 265:10274-81; PMID:1693918
- Blidner AG, Rabinovich GA. “Sweetening” pregnancy: galectins at the fetomaternal interface. Am J Reprod Immunol 2013; 69(4):369-82; PMID:23406009
- Ramhorst RE, Giribaldi L, Fraccaroli L, Toscano MA, Stupirski JC, Romero MD, Durand ES, Rubinstein N, Blaschitz A, Sedlmayr P, et al. Galectin-1 confers immune privilege to human trophoblast: implications in recurrent fetal loss. Glycobiology 2012; 22:1374-86; PMID:22752006;http://dx.doi.org/10.1093/glycob/cws104
- Barker JN, Sarma V, Mitra RS, Dixit VM, Nickoloff BJ. Marked synergism between tumor necrosis factor-alpha and interferon-gamma in regulation of keratinocyte-derived adhesion molecules and chemotactic factors. J Clin Invest 1990; 85:605-8; PMID:2105343; http://dx.doi.org/10.1172/JCI114481
- Ren L, Liu Y-Q, Zhou W-H, Zhang Y-Z. Trophoblast-derived chemokine CXCL12 promotes CXCR4 expression and invasion of human first-trimester decidual stromal cells. Hum Reprod 2012; 27:366-74; PMID:22114110; http://dx.doi.org/10.1093/humrep/der395
- Zhou W-H, Du M-R, Dong L, Yu J, Li D-J. Chemokine CXCL12 promotes the cross-talk between trophoblasts and decidual stromal cells in human first-trimester pregnancy. Hum Reprod 2008; 23:2669-79; PMID:18687671; http://dx.doi.org/10.1093/humrep/den308
- McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol 1999; 213:442-56; PMID:10479460; http://dx.doi.org/10.1006/dbio.1999.9405
- Dominguez F, Pellicer A, Simon C. The Chemokine Connection: Hormonal and Embryonic Regulation at the Human Maternal-Embryonic Interface—A Review. Placenta 2003; 24:S48-S55; PMID:14559030; http://dx.doi.org/10.1016/S0143-4004(03)00134-6
- Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R, Katz G, Haimov-Kochman R, Fujii N, Yagel S, et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16- human natural killer cells. Blood 2003; 102:1569-77; PMID:12730110; http://dx.doi.org/10.1182/blood-2003-02-0517
- Santoni A, Carlino C, Gismondi A. Uterine NK cell development, migration and function. Reprod Biomed Online 2008; 16:202-10; PMID:18284874; http://dx.doi.org/10.1016/S1472-6483(10)60575-5
- Kumar A, Kumar S, Dinda AK, Luthra K. Differential expression of CXCR4 receptor in early and term human placenta. Placenta 2004; 25:347-51; PMID:15028427; http://dx.doi.org/10.1016/j.placenta.2003.10.003
- Wu X, Jin L-P, Yuan M-M, Zhu Y, Wang M-Y, Li D-J. Human first-trimester trophoblast cells recruit CD56brightCD16- NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol 2005; 175:61-8; PMID:15972632; http://dx.doi.org/10.4049/jimmunol.175.1.61
- Nancy P, Tagliani E, Tay C-S, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 2012; 336:1317-21; PMID:22679098; http://dx.doi.org/10.1126/science.1220030
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006; 12:1065-74; PMID:16892062; http://dx.doi.org/10.1038/nm1452
- Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 2004; 5:266-71; PMID:14758358; http://dx.doi.org/10.1038/ni1037
- Silasi M, Mor G. Decidual stromal cells as regulators of T-cell access to the maternal-fetal interface. Am J Reprod Immunol 2012; 68:279-81; PMID:22935072; http://dx.doi.org/10.1111/aji.12006
- Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem 1995; 270:27348-57; PMID:7592998; http://dx.doi.org/10.1074/jbc.270.45.27348
- Heidemann J, Ogawa H, Rafiee P, Lügering N, Maaser C, Domschke W, Binion DG, Dwinell MB. Mucosal angiogenesis regulation by CXCR4 and its ligand CXCL12 expressed by human intestinal microvascular endothelial cells. Am J Physiol Gastrointest Liver Physiol 2004; 286:G1059-68; PMID:14764445; http://dx.doi.org/10.1152/ajpgi.00417.2003
- Salcedo R, Oppenheim JJ. Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation 2003; 10:359-70; PMID:12851652; http://dx.doi.org/10.1080/mic.10.3-4.359.370
- Nanney LB, Mueller SG, Bueno R, Peiper SC, Richmond A. Distributions of melanoma growth stimulatory activity of growth-regulated gene and the interleukin-8 receptor B in human wound repair. Am J Pathol 1995; 147:1248-60; PMID:7485389
- Ishida Y, Gao J-L, Murphy PM. Chemokine receptor CX3CR1 mediates skin wound healing by promoting macrophage and fibroblast accumulation and function. J Immunol 2008; 180:569-79; PMID:18097059; http://dx.doi.org/10.4049/jimmunol.180.1.569
- Inokuma D, Abe R, Fujita Y, Sasaki M, Shibaki A, Nakamura H, McMillan JR, Shimizu T, Shimizu H. CTACK/CCL27 accelerates skin regeneration via accumulation of bone marrow-derived keratinocytes. Stem Cells 2006; 24:2810-6; PMID:16931770; http://dx.doi.org/10.1634/stemcells.2006-0264
- Moyer RA, Wendt MK, Johanesen PA, Turner JR, Dwinell MB. Rho activation regulates CXCL12 chemokine stimulated actin rearrangement and restitution in model intestinal epithelia. Lab Invest 2007; 87:807-17; PMID:17572689; http://dx.doi.org/10.1038/labinvest.3700595
- Dwinell MB, Johanesen PA, Smith JM. Immunobiology of epithelial chemokines in the intestinal mucosa. Surgery 2003; 133:601-7; PMID:12796725; http://dx.doi.org/10.1067/msy.2003.143
- Smith JM, Johanesen PA, Wendt MK, Binion DG, Dwinell MB. CXCL12 activation of CXCR4 regulates mucosal host defense through stimulation of epithelial cell migration and promotion of intestinal barrier integrity. Am J Physiol Gastrointest Liver Physiol 2005; 288:G316-26; PMID:15358596; http://dx.doi.org/10.1152/ajpgi.00208.2004
- Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol 2001; 69:513-21; PMID:11310836
- Nagamatsu T, Schust DJ. The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol 2010; 63:460-71; PMID:20163399; http://dx.doi.org/10.1111/j.1600-0897.2010.00813.x
- Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TRDJ, Reedquist KA, Tak PP, Baeten DLP. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods 2012; 375:196-206; PMID:22075274; http://dx.doi.org/10.1016/j.jim.2011.10.013
- Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013; 229:176-85; PMID:23096265; http://dx.doi.org/10.1002/path.4133
- Gustafsson C, Mjösberg J, Matussek A, Geffers R, Matthiesen L, Berg G, Sharma S, Buer J, Ernerudh J. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One 2008; 3:e2078; PMID:18446208; http://dx.doi.org/10.1371/journal.pone.0002078
- Abrahams VM, Mor G. Toll-like receptors and their role in the trophoblast. Placenta 2005; 26:540-7; PMID:15993703; http://dx.doi.org/10.1016/j.placenta.2004.08.010
- Grasso E, Paparini D, Hauk V, Salamone G, Leiros CP, Ramhorst R. Differential migration and activation profile of monocytes after trophoblast interaction. PLoS One 2014; 9:e97147; PMID:24849800; http://dx.doi.org/10.1371/journal.pone.0097147
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958-69; PMID:19029990; http://dx.doi.org/10.1038/nri2448
- Delgado M, Pozo D, Martinez C, Leceta J, Calvo JR, Ganea D, Gomariz RP. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit endotoxin-induced TNF-alpha production by macrophages: in vitro and in vivo studies. J Immunol 1999; 162:2358-67; PMID:9973516
- Paparini D, Grasso E, Calo G, Vota D, Lauricella AM, Quintana I, Ramhorst R, Leiros CP. VIP enhances apoptotic cell phagocytosis by monocyte/macrophages in an in vitro model of immune-trophoblast interaction. Placenta 2015;36:495; http://dx.doi.org/10.1016/j.placenta.2015.01.468
- Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol 2002; 20:621-67; PMID:11861614; http://dx.doi.org/10.1146/annurev.immunol.20.100301.064828
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol 2003; 21:685-711; PMID:12615891; http://dx.doi.org/10.1146/annurev.immunol.21.120601.141040
- Ardavín C, Amigorena S, Reis e Sousa C. Dendritic cells: immunobiology and cancer immunotherapy. Immunity 2004; 20:17-23; http://dx.doi.org/10.1016/S1074-7613(03)00352-2
- Laskarin G, Kämmerer U, Rukavina D, Thomson AW, Fernandez N, Blois SM. Antigen-presenting cells and materno-fetal tolerance: an emerging role for dendritic cells. Am J Reprod Immunol 2007; 58:255-67; PMID:17681042; http://dx.doi.org/10.1111/j.1600-0897.2007.00511.x
- Pollard JW. Uterine DCs are essential for pregnancy. J Clin Invest 2008; 118:3832-5; PMID:19033651
- Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest 2008; 118:3954-65; PMID:19033665
- Kämmerer U, Schoppet M, McLellan AD, Kapp M, Huppertz HI, Kämpgen E, Dietl J. Human decidua contains potent immunostimulatory CD83(+) dendritic cells. Am J Pathol 2000; 157:159-69; http://dx.doi.org/10.1016/S0002-9440(10)64527-0
- Blois S, Alba Soto CD, Olmos S, Chuluyan E, Gentile T, Arck PC, Margni RA. Therapy with dendritic cells influences the spontaneous resorption rate in the CBA/J x DBA/2J mouse model. Am J Reprod Immunol 2004; 51:40-8; PMID:14725565; http://dx.doi.org/10.1046/j.8755-8920.2003.00120.x
- Zarnani AH, Moazzeni SM, Shokri F, Salehnia M, Dokouhaki P, Ghods R, Mahmoodi AR, Jeddi-Tehrani M. Microenvironment of the feto-maternal interface protects the semiallogenic fetus through its immunomodulatory activity on dendritic cells. Fertil Steril 2008; 90:781-8; PMID:17482607; http://dx.doi.org/10.1016/j.fertnstert.2007.01.102
- Salamone G, Fraccaroli L, Gori S, Grasso E, Paparini D, Geffner J, Pérez Leirós C, Ramhorst R. Trophoblast cells induce a tolerogenic profile in dendritic cells. Hum Reprod 2012; 27:2598-606; PMID:22718280; http://dx.doi.org/10.1093/humrep/des208
- Teles A, Schumacher A, Kühnle M-C, Linzke N, Thuere C, Reichardt P, Tadokoro CE, Hämmerling GJ, Zenclussen AC. Control of uterine microenvironment by foxp3(+) cells facilitates embryo implantation. Front Immunol 2013; 4:158; PMID:23801995; http://dx.doi.org/10.3389/fimmu.2013.00158
- Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol 2003; 178:357-72; PMID:12967329; http://dx.doi.org/10.1677/joe.0.1780357
- Mesiano S, Wang Y, Norwitz ER. Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reprod Sci 2011; 18:6-19; PMID:20889955; http://dx.doi.org/10.1177/1933719110382922
- Ramhorst R, Patel R, Corigliano A, Etchepareborda JJ, Fainboim L, Schust D. Induction of maternal tolerance to fetal alloantigens by RANTES production. Am J Reprod Immunol 2006; 56:302-11; PMID:17076674; http://dx.doi.org/10.1111/j.1600-0897.2006.00430.x
- Ramhorst R, Gutiérrez G, Corigliano A, Junovich G, Fainboim L. Implication of RANTES in the modulation of alloimmune response by progesterone during pregnancy. Am J Reprod Immunol 2007; 57:147-52; PMID:17217369; http://dx.doi.org/10.1111/j.1600-0897.2006.00458.x
- Teles A, Thuere C, Wafula PO, El-Mousleh T, Zenclussen ML, Zenclussen AC. Origin of Foxp3(+) cells during pregnancy. Am J Clin Exp Immunol 2013; 2:222-33; PMID:24179730
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res 1993; 206:204-11; PMID:7684692; http://dx.doi.org/10.1006/excr.1993.1139
- Straszewski-Chavez SL, Abrahams VM, Alvero a B, Aldo PB, Ma Y, Guller S, Romero R, Mor G. The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta 2009; 30:939-48; PMID:19766308; http://dx.doi.org/10.1016/j.placenta.2009.08.007
- Ramhorst R, Fraccaroli L, Aldo P, Alvero AB, Cardenas I, Leirós CP, Mor G. Modulation and recruitment of inducible regulatory T cells by first trimester trophoblast cells. Am J Reprod Immunol 2012; 67:17-27; PMID:21819477; http://dx.doi.org/10.1111/j.1600-0897.2011.01056.x
- Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee T-H, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 2008; 322:1562-5; PMID:19056990; http://dx.doi.org/10.1126/science.1164511
- Mjösberg J, Berg G, Jenmalm MC, Ernerudh J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol Reprod 2010; 82:698-705; PMID:20018909; http://dx.doi.org/10.1095/biolreprod.109.081208
- Mjösberg J, Svensson J, Johansson E, Hellström L, Casas R, Jenmalm MC, Boij R, Matthiesen L, Jönsson J-I, Berg G, et al. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17beta-estradiol. J Immunol 2009; 183:759-69; PMID:19535629; http://dx.doi.org/10.4049/jimmunol.0803654
- Tilburgs T, Roelen DL, van der Mast BJ, de Groot-Swings GM, Kleijburg C, Scherjon SA, Claas FH. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol 2008; 180:5737-45; PMID:18390759; http://dx.doi.org/10.4049/jimmunol.180.8.5737
- Tilburgs T, Schonkeren D, Eikmans M, Nagtzaam NM, Datema G, Swings GM, Prins F, van Lith JM, van der Mast BJ, Roelen DL, et al. Human decidual tissue contains differentiated CD8+ effector-memory T cells with unique properties. J Immunol 2010; 185:4470-7; PMID:20817873; http://dx.doi.org/10.4049/jimmunol.0903597
- Jimeno R, Leceta J, Martínez C, Gutiérrez-Cañas I, Pérez-García S, Carrión M, Gomariz RP, Juarranz Y. Effect of VIP on the balance between cytokines and master regulators of activated helper T cells. Immunol Cell Biol 2012; 90:178-86; PMID:21445087; http://dx.doi.org/10.1038/icb.2011.23
- Hauk V, Azzam S, Calo G, Gallino L, Paparini D, Franchi A, Ramhorst R, Leirós CP. Vasoactive intestinal Peptide induces an immunosuppressant microenvironment in the maternal-fetal interface of non-obese diabetic mice and improves early pregnancy outcome. Am J Reprod Immunol 2014; 71:120-30; PMID:24405265; http://dx.doi.org/10.1111/aji.12167
- Hendrix CW, Flexner C, MacFarland RT, Giandomenico C, Fuchs EJ, Redpath E, Bridger G, Henson GW. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother 2000; 44:1667-73; PMID:10817726; http://dx.doi.org/10.1128/AAC.44.6.1667-1673.2000
- Plerixafor: AMD 3100, AMD3100, JM 3100, SDZ SID 791. Drugs R D 2007; 8:113-9; PMID:17324009; http://dx.doi.org/10.2165/00126839-200708020-00006
- Abel S, Back DJ, Vourvahis M. Maraviroc: pharmacokinetics and drug interactions. Antivir Ther 2009; 14:607-18; PMID:19704163; http://dx.doi.org/10.3851/IMP1297
- Horuk R. Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov 2009; 8:23-33; PMID:19079127; http://dx.doi.org/10.1038/nrd2734
- Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology 2009; 136:1182-97; PMID:19249397; http://dx.doi.org/10.1053/j.gastro.2009.02.001
- Veldkamp CT, Seibert C, Peterson FC, De la Cruz NB, Haugner JC, Basnet H, Sakmar TP, Volkman BF. Structural basis of CXCR4 sulfotyrosine recognition by the chemokine SDF-1/CXCL12. Sci Signal 2008; 1:ra4; PMID:18799424; http://dx.doi.org/10.1126/scisignal.1160755
- Roy I, Evans DB, Dwinell MB. Chemokines and chemokine receptors: update on utility and challenges for the clinician. Surgery 2014; 155:961-73; PMID:24856117; http://dx.doi.org/10.1016/j.surg.2014.02.006
