ABSTRACT
Numerous cell adhesion molecules, extracellular matrix proteins and axon guidance molecules participate in neuronal network formation through local effects at axo-dendritic, axo-axonic or dendro-dendritic contact sites. In contrast, neurotrophins and their receptors play crucial roles in neural wiring by sending retrograde signals to remote cell bodies. Semaphorin 3A (Sema3A), a prototype of secreted type 3 semaphorins, is implicated in axon repulsion, dendritic branching and synapse formation via binding protein neuropilin-1 (NRP1) and the signal transducing protein PlexinAs (PlexAs) complex. This review focuses on Sema3A retrograde signaling that regulates dendritic localization of AMPA-type glutamate receptor GluA2 and dendritic patterning. This signaling is elicited by activation of NRP1 in growth cones and is propagated to cell bodies by dynein-dependent retrograde axonal transport of PlexAs. It also requires interaction between PlexAs and a high-affinity receptor for nerve growth factor, toropomyosin receptor kinase A. We propose a control mechanism by which retrograde Sema3A signaling regulates the glutamate receptor localization through trafficking of cis-interacting PlexAs with GluA2 along dendrites; this remote signaling may be an alternative mechanism to local adhesive contacts for neural network formation.
Introduction
The formation of functional neuronal networks requires precise temporal and spatial regulation of axonal and dendritic maturation. Processes of neural network formation are governed by a coupling between activities and cell-to-cell contacts that utilizes numerous proteins including cell adhesion molecules, extracellular matrix proteins and axon guidance molecules, such as ephrins, semaphorins and netrins,Citation1,2 which are tightly regulated both spatially and temporally during development.Citation3,4 The membrane association of both Ephs and ephrins enables their unique ability to transduce signals bidirectionally into Eph-expressing cells (forward signaling) and ephrin-expressing cells (reverse signaling) upon cell–cell contact. Ephrin-A and ephrin-B transduce distinct reverse signals upon interaction with their Eph partners acting as ligands and participate in postsynaptic formation.Citation7 In Caenorhabditis elegans, Netrin/2NC-6 is involved in synaptogenesis. In the postsynaptic neuron RIA, the netrin receptor UNC-40 plays a conventional axon guidance role, directing outgrowth of the RIA process ventrally toward the glia. In the presynaptic neuron AIY, UNC-40 cell-autonomously promotes assembly of presynaptiC-terminals in the vicinity of the netrin/2NC-6-expressing glial cell endfeet. In this situation, UNC-6 may act as a short-range signaling molecule, creating microenvironments that promote synaptogenesis.Citation2 In either case, specific sets of proteins control synaptogenesis through mechanisms that require local cell–cell contact. In addition to neuronal wiring, axon guidance molecules and their receptors preset at synapses in the adult nervous system also influence synaptic plasticity in learning and memory.Citation5,6
The remote communication of neurotrophin signaling from axon terminals to neuron cell bodies is also crucial for neuron survival and plasticity.Citation8-15 When released by postsyaptic targets, neurotrophins bind their receptors toropomyosin receptor kinases (Trks) on nerve terminals. Activated Trks signal locally within distal axons or growth cones and retrogradely through axons to cell bodies in order to promote gene expression and survival, and regulate synaptic strength and plasticity.Citation9,13
Here we review recent findings highlighting the novel functions and mechanisms of activity-dependent remote signaling by semaphorin 3A (Sema3A), a semaphorin superfamily protein, in dendritic development and synaptic assembly.
Background: Dendritic development regulated by semaphorins
More than 20 semaphorin proteins have been identified and are categorized into 8 subfamily classes.Citation16 Among semaphorin superfamily proteins, Sema3A is one of the secreted forms of type 3 semaphorin and one of the most characterized axon guidance molecules.Citation17 Sema3A repulses axons through the co-receptor protein neuropilin-1 (NRP1)Citation18 and plexinAs (PlexAs).Citation19 Although the receptor complexes of the type 3 semaphorins remain incompletely understood, Sema3C and Sema3F are known to exert their biological actions through receptor complexes including NRP2.Citation20 NRPs and PlexAs are ligand-binding and signal-transducing subunits of class 3 semaphorin receptor complexes, respectively. During target cell selection and synaptogenesis in mouse brains, NRP1 and NRP2 are expressed by septo-hippocampal neurons during postnatal stages. In the target hippocampus, pyramidal and granule cells express Sema3E and Sema3A, and cholinergic septo-hippocampal fibers terminate onto Sema3E and Sema3A-expressing pyramidal and granule cells. However, GABAergic septo-hippocampal fibers preferentially terminate onto Sema3C-positive interneurons. The expression patterns of semaphorins and preferential target selections suggest that these secreted type 3 semaphorins play a role in target selection and synaptogenesis in higher vertebrates.Citation21 More specifically, an initial study on Drosophila semaphorin mutants revealed that a transmembrane type semaphorin Sema-1a is part of a bi-directional signaling system that leads to the formation of the adult giant fiber synapse.Citation22
The type 3 semaphorins have been reported to play diverse functions in dendritic branching and synaptogenesis.Citation23-27 The first description of the role of Sema3A in dendrite morphogenesis was provided by slice overlay experiments. Sema3A was found to be a major component of this diffusible signal, and cortical neurons appeared to transduce this signal through NRP1 to direct the extension of apical and basal dendrites.Citation25,26 Morphological analysis of neurons in neocortical slices from sema3A-deficient (Sema3A−/−) mice cultured and transfected with green fluorescence protein expression vector revealed reductions in dendritic length and branching in these mice; furthermore, increases in dendritic length and branching occurred after the addition of exogenous Sema3A in wild-type neurons.Citation23 In cultured cortical neurons, Sema3A induced clustering of both postsynaptic density-95 (PSD-95) and presynaptic synapsin I. When treated with Sema3A, the cluster size of PSD-95 was enhanced, and extensive colocalization of PSD-95 and NRP1 or actin-rich protrusion was observed. Consistently, Golgi-labeled cortical neurons in layer V, but not layer III, showed a lowered density of synaptic bouton-like structure on basal dendrites in Sema3A−/− mice. These findings suggest that the Sema3A signaling pathway plays an important role in promoting dendritic branching and spine maturation. However, the type3 semaphorins could be a negative regulator of spine development, synaptic structure and synaptic transmission.Citation27-29 Kolodkin and his colleagues demonstrated that the Sema3F−/−, Nrp2−/− and PlexA3−/− mice, exhibited increased dentate gyrus (DG) granule cell (GC) and cortical layer V pyramidal neuron spine number and size as well as aberrant spine distribution.Citation27 In dissociated neurons in vitro, Sema3F promotes loss of spines and excitatory synapses. In Nrp2−/− brain slices, cortical layer V and DG GCs exhibit increased miniature excitatory postsynaptic current frequency. Consistent with the previous findings,Citation23,24 Tran et al. showed that the Sema3A-NRP1/2lexA4 signaling cascade promotes basal dendritic arborization in layer V cortical neurons. Mice homozygous for a knock-in mutation that expressed a NRP1 protein incapable of binding to Sema3A (Nrp1Sema−) phenocopied embryonic neuronal defects observed in Nrp1−/− mice and exhibited reduced growth and branching of layer V cortical neuron basal dendritic arborization. Acute application of Sema3A to wild-type brain slices promoted an increase in growth and branching of basal dendritic arbors. However, Tran et al. reported that the Sema3A signaling did not influence spine morphogenesis or distribution.Citation27 The Golgi-labeled Nrp1Sema− and Sema3A−/− mouse brains did not exhibit spine density defects along apical dendrites of cortical layer V neurons. In addition, analysis of adult PlexA3−/− and PlexA4−/− mutant brains revealed that apical dendrite spine morphology was not altered in PlexA4−/−. In cultured wild-type postnatal day 5 DG neurons, Sema3F, when treated for 2 or 6 h, decreased the average number of puncta exhibiting colocalization of presynaptic vGlut1 and postsynaptic PSD-95, but Sema3A treatment had no effect. These findings appear to be in contrast to our previous findings on cultured cortical neurons and layer V cortical pyramidal neurons in wild-type and Sema3A−/− mice.Citation24,30 Experimental differences could account for the discrepancies; Tran et al. showed that Sema3A, when treated for 2 or 6 h, had no effect on the average number of puncta exhibiting colocalization of presynaptic vGlut1 and postsynaptic PSD-95, but in our study the increase in the cluster size of PSD-95 was observed in cultured cortical neurons treated with Sema3A for 24 h.Citation24 Discrepancies might also be explained by differences in the breeding conditions because learning and novel sensory experience greatly influence spine formation and elimination in the mouse cortex.Citation31
The Sema3A signaling cascade involved in axonal, dendritic and synaptic development
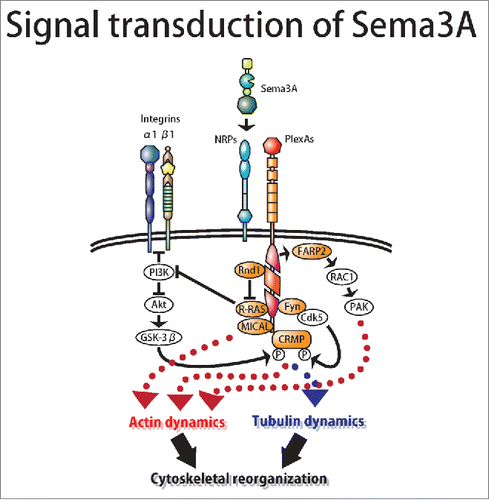
The principal receptors for semaphorins are plexins, which are transmembrane proteins that are divided into 4 classes (A–D) based on structure.Citation32,33 The plexin extracellular region contains several different motifs and domains, including a divergent sema domain, whereas the intracellular region always contains a GTPase-activating protein (GAP) domain. Unlike type 3 semaphorins, most semaphorins bind directly to plexins,Citation34 but subclass-specific signaling for semaphorin/plexin has been reported. At least 3 signaling pathways for Sema3A have been reported ().Citation34-38 First, the plexin RasGAP inactivates ligand-binding–induced R-Ras inactivation.Citation37 PlexinA GAP activity is regulated by FERM, RhoGEF and pleckstrin domain protein 2 (FARP2)-mediated Rac1 activation. After Sema3A stimulation, FARP2 dissociates from plexinA1 and activates Rac1 in neuronal growth cones. Active Rac1 facilitates the association of Rnd1, a small GTPase, with PlexA1 and may modulate actin dynamics through the sequential activation of p21-activated kinase, LIM kinase 1 and coffilin.Citation39,40 Second, Rnd1-PlexAs interactions stimulate PlexAs GAP activity toward R-Ras by releasing or terminating inhibitory interactions within the plexin cytoplasmic region.Citation41,42 The subsequent decrease in the amount of active R-Ras triggers the inactivation of phosphatidylinositol-3-OH kinase (PI3K), which inhibits β1 integrin signaling. The inactivation of PI3K also inactivates the v-akt murine thymoma viral oncogene homolog Akt. Plexin-induced inhibition of PI3K-Akt signaling prevents the inactivation of the serine/2hreonine kinase glycogen synthase kinase-3β (GSK-3β), thus promoting the phosphorylation and inactivation of CRMP2. Third, Sema3A activates Src type tyrosine kinase Fyn, thereby leading to sequential phosphorylation of CRMP2 by Cdk5 and GSK3β to regulate axon guidance and dendritic development.Citation36 Cdk5 primarily phosphorylates CRMP1 and CRMP2 at Ser522, and GSK3β subsequently phosphorylates Thr509 and Thr514 of CRMP2. CRMP2 binds the tubulin heterodimer and promotes microtubule assembly at the plus end, and the phosphorylation of CRMP2 at Ser522 reduces its affinity to tubulin.Citation43-45 The lowered affinity of phosphorylated CRMPs to tubulin may be responsible for Sema3A-induced axon repulsion or growth cone collapse.Citation36,45,46 As substrates, CRMPs also physically interact with the intracellular molecule interacting with CasL (MICAL), and transduce Sema3A signaling.Citation47,48 Another possible mechanism for Sema3A-induced cytoskeletal reorganization is the regulation of the function of Filamin-A, an actin-binding protein. The C. elegans CRMP1 homolog UNC-33 was shown to interact with FLN-1, a Filamin-A ortholog. CRMP1 binds both the actin-binding domain and the last immunoglobulin-like repeat of Filamin-A. Through their interacting residues, alanine mutants of Filamin-A or CRMP1 suppressed the Sema3A repulsion in neurons. In contrast, a phospho-mimicking mutant CRMP1(Ser522Asp) enhanced the Sema3A response. Furthermore, CRMP1(Ser522Asp) weakened the F-actin gelation crosslinked by Filamin-A. These findings indicate that phosphorylated CRMP1 may remove Filamin-A from the actin cytoskeleton to facilitate its remodeling.Citation49
Figure 1. Signaling model of Sema3A. The plexin RasGAP inactivates ligand-binding–induced R-Ras inactivation.Citation42 PlexinA GAP activity is regulated by FERM, RhoGEF and pleckstrin domain protein 2 (FARP2)-mediated Rac1 activation. After Sema3A stimulation, FARP2 dissociates from plexinA1 and activates Rac1 in neuronal growth cones. Active Rac1 facilitates the association of Rnd1, a small GTPase, with PlexA1 and may modulate actin dynamics through the sequential activation of p21-activated kinase, LIM kinase 1 and coffilin.Citation44,45 Rnd1-PlexAs interactions stimulate PlexAs GAP activity toward R-Ras by releasing or terminating inhibitory interactions within the plexin cytoplasmic region.Citation46,47 Plexin-induced inhibition of PI3K-Akt signaling prevents the inactivation of the serine/2hreonine kinase glycogen synthase kinase-3β (GSK-3β), thus promoting the phosphorylation and inactivation of CRMP2. Sema3A also activates Src type tyrosine kinase Fyn, thereby leading to sequential phosphorylation of CRMP2 by Cdk5 and GSK3β to regulate axon guidance and dendritic development.Citation41 Cdk5 phosphorylates CRMP1 and CRMP2 at Ser522, and GSK3β subsequently phosphorylates Thr509 and Thr514 of CRMP2.
Ion channel–coupled intracellular transport elicited by Sema3A
CRMPs are related to UNC33, and a mutant worm carrying unc33 showed abnormalities in the form and number of microtubules, the basic cytoskeletal components for axonal transport. Whether Sema3A has any effects on axonal transport was examined by using computer-assisted video-enhanced differential interference contrast microscopy.Citation50,51 Sema3A was found to induce both anterograde and retrograde axonal transport in cultured chick dorsal root ganglion (DRG) neurons.Citation51 Sema3A enhances the rate and the number of fast anterograde and retrograde axonal transport of organelles, including mitochondrial, lysosomal and other membranous organelles.Citation51 Sema3A seems to induce axonal transport through NRP1 located at axonal growth conesCitation52 because it induces axonal transport when locally applied to axonal growth cones but not elsewhere. Such a polarized responsiveness to Sema3A is similarly observed in hippocampal neurons.Citation53 Importantly, tetrodotoxin, which blocks voltage-dependent sodium channels, suppressed the acceleration of axonal transport but not the growth cone collapse induced by Sema3A.Citation54,55 Likewise, K252a, a tropomyosin receptor kinase A (TrkA) inhibitor, or TrkA knockdown suppressed the Sema3A-induced acceleration of axonal transport but did not affect the growth cone collapse (see below).Citation51 These findings suggest that Sema3A is active in at least 2 distinct pathways and axonal transport is not just a secondary effect of growth cone collapse. The signaling pathways of Sema3A involved in growth cone collapse and axonal transport may share some elements, including extracellular calcium ions.Citation55,56 Sema3A-induced growth cone collapse was inhibited by hanatoxin (a voltage-gated K+ or Ca2+ channel blocker),Citation57 voltage-dependent L-type Ca2+ channel blockers such as nimodipine, and a reduction in extracellular Ca2+.Citation54,56,58-60 Sema3A enhances intracellular Ca2+ in the growth cones,Citation54,56,60,61 through which Sema3A can enhance both endocytosis and exocytosis.Citation60,62 Sema3A-induced growth cone collapse or repulsive turning was shown to be mediated through Ca2+-triggered clathrin-mediated endocytosis.Citation60 Sema3A-induced growth cone collapse is suppressed by introduction of GSK3β mutants, GSK3β-R96A, L128A, or K85M, into DRG neurons, indicating that unprimed and primed substrates are involved in stimulating endocytosis of the growth cone plasma membrane.Citation63 Either Axin-1 or β-catenin RNAi knockdown suppresses the internalization of venus-tagged Sema3A. In addition, local treatment by monodancylcadaverin, an inhibitor of clathrin-dependent endocytosis, at the distal axon suppressed growth cone and distal axon distribution of venus-tagged Sema3A.Citation53 These findings further suggest active and physiological roles of the semaphorin in stimulating endocytosis of the growth cone plasma membrane,Citation54,55 and the Sema3A receptor complex is retrogradely transported through tetrodotoxin- and nimodipine-sensitive signaling along the axon. However, as described above, tetrodotoxin suppressed the acceleration of axonal transport but not the growth cone collapse induced by Sema3A.Citation54,55 Thus calcium ions are involved in regulating both growth cone morphology and axonal transport, but sodium ions are involved only in Sema3A-induced axonal transport.Citation53,54
The coupling mechanism upon Sema3A stimulation between Ca2+ channels and Na+ channels is unknown. Although several L-type Ca2+ channel blockers suppress Sema3A-induced axonal transport,Citation54,55 the voltage-dependent Ca2+ channels that mediate Sema3A response may include R-type Ca2+ channels, as demonstrated in Xenopus spinal neurons,Citation64 and in embryonic day 15 (E15) mouse DRG.Citation65 Indeed, despite minor effect, nimodipine suppresses R-type Ca2+ channels, and R-type Ca2+ channels contribute to fast synaptic excitation and action potentials in guinea pig myenteric neurons.Citation66 Thus it is possible that Sema3A activates the R-type Ca2+ channels in the growth cones, thereby leading to opening the Na+ channels in the growth cones and/2r axons.
How Sema3A activates or interacts with Ca2+ channels? One plausible mechanism is that Sema3A signaling may involve the interaction between CRMP2 and Cav2.2, a voltage-gated calcium channel belonging to the Cav2 family.Citation67,68 The Cav2 family includes channels containing α1A, α1B and α1E, which mediate P/2 type, N-type, and R-type Ca2+ currents, respectively.Citation69 Sema3A activates Cdk5, which phosphorylates CRMP2 at Ser522.Citation36,70 The in vitro phosphorylated CRMP2 at Ser522 was shown to enhance its interaction with Cav2.2.Citation67 This enhanced interaction between CRMP2 and Cav2.2 was associated with an activation of Cav2.2. Indeed, overexpression of wild-type CRMP2 caused increase in Ca2+ current, while overexpression of CRMP2S522A, a non-phosphorylated CRMP2 mutant, did not increase currents above levels in hippocampal neurons.Citation67 These findings suggest that Sema3A activates the voltage-gated Ca2+ channel(s) through its enhanced interaction with the phosphorylated CRMP2 by Cdk5.
Sema3A regulates dendritic localization of AMPA glutamate receptors
Sema3A appears to induce axonal transport through an intracellular signaling pathway. However, the physiological function of the Sema3A-induced response has not been settled yet. To elucidate the functional implications of Sema3A-induced transport, the effect of Sema3A on dendritic transport was examined in cultured hippocampal neurons, which possess well-differentiated axonal and dendritic domains. Sema3A induced dendritic transport as well as axonal transport, with the peak effect on dendrites lagging behind that on axons.Citation53 Furthermore, Sema3A that was locally applied to axonal growth cone induced axonal and dendritic transport. This finding suggests that local signaling of Sema3A at the axonal growth cones may be propagated to cell body and/or dendrites, thereby inducing dendritic transport. It was previously shown that when Sema3A is added to cultured cortical neurons, synapsin I, a presynaptic marker, and postsynaptic density-95 (PSD95), a postsynaptic marker protein, immunoreactivity is concentrated in puncta at dendrites.Citation24 In mature neurons, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)-type glutamate receptors are concentrated in synaptic clusters associated with dendrites,Citation71 and altering the level of AMPA receptor activity has been shown to play an important role in dendritic patterning and synapse maturation and formation.Citation72,73 Therefore Sema3A may have some effects on the dendritic localization of glutamate receptors. As shown by immunocytochemistry, Sema3A enhanced the immunostaining of GluA2 in MAP2-positive dendrites. Interestingly, however, Sema3A did not change the levels of GluA1 and GluN1 at an early stage of 3 d in vitro (3DIV) culture of neurons from mouse E18. Thus Sema3A selectively induces the dendritic localization of the GluA2-containing AMPA-receptor complex. The sites of action of Sema3A to induce an increase in the dendritic GluA2 localization might be the axonal growth cone, dendritic growth cone or other cell regions. Although the local application of Sema3A to axonal growth cones can induce axonal and dendritic transport, NRP-1, PlexAs or both are necessary for biased extension of the dendrites independently of axon polarization during development in Xenopus and chick embryos.Citation74,75 To determine the site of Sema3A action, a microfluidic system was employed in a hippocampal neuron culture. When Sema3A was locally applied to axonal areas, but not to somatodendritic areas, it increased the immunostaining of GluA2 in the dendrites, suggesting that Sema3A signaling propagates from the axonal growth cone and induces the dendritic localization of GluA2.Citation53 If the site of Sema3A action is localized in the axonal growth cone area, how was this retrograde Sema3A signaling propagated to cell bodies and/or dendrites? One possible mechanism is a dynein-dependent retrograde axonal transport. A siRNA knockdown of dynein heavy chain suppressed Sema3A-induced increase in GluA2 immunostaining in dendrites. In addition, overexpression of dynamitin, a subunit of the dynactin complex, which dissociates the dynactin complex, suppressed the Sema3A-induced localization of GluA2. These findings suggest that dynein-dependent retrograde transport is involved in Sema3A-induced dendritic localization of GluA2.
In addition to local growth cone, ion-related signals are also involved in Sema3A signaling in the axon. Nimodipine and tetrodotoxin suppressed Sema3A-induced axonal transport in DRG neurons, and they suppressed axon and dendritic transport and dendritic localization of GluA2 in hippocampal neurons. The local application of nimodipine or tetrodotoxin to distal axons suppressed retrograde transport of Venus-tagged Sema3A (Venus-Sema3A) that had been exogenously applied to the distal axons. Following the local application of Venus-Sema3A or the application combined with the ion channel blockers in the microfluidic chamber, we observed that Venus-Sema3A could be visualized as punctate signals from the distal axon leading up to the somatodendrite. Nimodipine and tetrodotoxin both suppressed Venus-Sema3A signals along the proximal axons. Sema3A consistently facilitated axonal transport of PlexA4, which was suppressed in neurons treated with either nimodipine or tetrodotoxin. The activation of ion-related signal evoked by KCl similarly enhanced the axonal transport and dendritic localization of GluA2. However, unlike Sema3A, KCl equally enhanced the immunostaining of both GluA1 and GluA2. Furthermore, the KCl-induced increase in GluA2 immunostaining was observed only when KCl stimulation occurred in the somatodendritic area, clearly indicating that the sites of action of Sema3A and KCl differed.Citation55 In summary, a dynein-dependent retrograde transport coupled with ion channels is probably essential to maintaining active, fast and specific signal propagation ().
Figure 2. A model for retrograde Sema3A signaling that drives AMPA receptor subunit GluA2 to dendrites. After activation of receptor complex for Sema3A, Ca2+ channels and ryanodine receptors are activated, which trigger intracellular Ca2+ mobilization, which subsequently induce clathrin-dependent endocytosis of the receptor complex Sema3A/2lexA4/2RP1/2rkA in the growth cone. The transport may be coupled with intracellular Ca2+ channels as well as voltage-dependent Ca2+ and Na+ channels at the plasma membrane. The Sema3A/2lexA4/2RP1/2rkA complex is then transported through interaction between PlexA4, TrkA and dynein motor protein toward the somatodendritic region. PlexA4 interacts with GluA2 through its extracellular IPT-domain in the somatodendritic region, then escorts it to the distal dendrites.
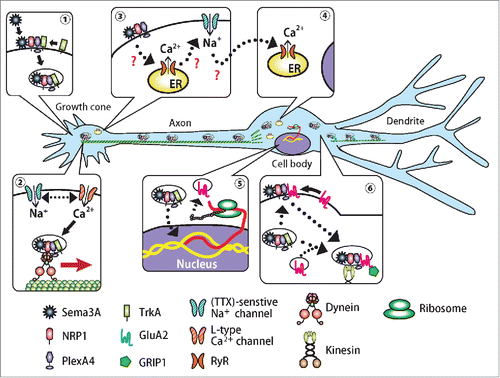
Local protein synthesis in dendrites could occur in response to various kinds of stimulus.Citation6 It is therefore possible that local protein synthesis of GluA2 in dendrites explains its localization in dendrites. In fact, mRNAs of GluA1 and A2, targets of a mRNA-binding protein, fragile X mental retardation protein (FMRP), are localized in dendrites of hippocampal neurons.Citation76 Since Sema3A induces local translation in hippocampal neurons through FMRP, it may stimulate local translation of GluA2 via regulating FMRP in dendrites.Citation77 However, when applied to the somatodendritic region, the protein synthesis inhibitor, cycloheximide only partially suppressed the increase of GluA2 levels in dendrites. This finding suggests that upon Sema3A stimulation, both newly generated and pre-existing GluA2 were transported along dendrites to distal dendrites. GluA2 is transported from the cell body to dendrites by the glutamate receptor interacting protein 1 (GRIP1)/kinesin superfamily protein 5 (KIF5) complex.Citation78,79 siRNA knockdown and dominant negative experiments revealed that GRIP1 and KIF5 were required for Sema3A-induced dendritic localization of GluA2, suggesting that Sema3A enhances the transport of GluA2 and localizes the receptor to dendrites (). Consistently, through the use of fluorescence recovery after photobleaching, Sema3A was revealed to enhance the dendritic transport of enhanced green fluorescent protein (EGFP)-tagged GRIP1 and GluA2, and the sites of this action were localized in axonal growth cones.Citation53
The retrograde signaling for Sema3A through trafficking of PlexAs
Neurotrophins induce the retrograde axonal transport of their active signal components to transmit survival signals from distal axons to cell bodies and/or dendrites.Citation8,11,12,14,80 Therefore, Sema3A may possibly induce the retrograde transport of signaling components of both itself and its receptor complex. Monodansylcadaverine, an inhibitor of clathrin-dependent endocytosis, suppressed the axonal distribution of Venus-Sema3A applied at distal axons. These findings suggest that Sema3A is internalized at distal axons and then retrogradely transported along the axons. PlexA4, a signal-transducing component of Sema3A, may also be a component of the signaling complex for Sema3A. The knockdown of PlexA4 suppressed Sema3A-enhanced immunostaining of GluA2 in dendrites. Sema3A induced a retrograde axonal transport and then a dendritic transport of PlexA4. Furthermore, when Venus-Sema3A was similarly applied at distal axons, the punctate signals of NRP1 or PlexA4 were double positive with those of Venus-Sema3A in MAP2-negative axons. These findings suggest that the Sema3A/2RP1/2lexA4 complex is formed in axons and then transported to somatodendrites. Does PlexA4 play a role in dendritic transport of GluA2? Owing to the large intracellular domain, it is possible that PlexA4 may interact with GluA2 and then escort it to distal dendrites. An immunoprecipitation assay revealed that GluA2 was associated with PlexA4. Unexpectedly, the 3 extracellular immunoglobulin-like Plexin-transcription-factor (IPT) domains of PlexA4 interacted with GluA2 but not with NRP1. The next questions were whether endogenous PlexA4 interacted with GluA2, and if this interaction was required for dendritic localization of GluA2 in hippocampal neurons in vitro and in vivo. To visualize protein interactions in fixed cells, we performed the immunostaining using in situ proximity ligation assay technology.Citation81 In this assay system, when PlexA4 interacts directly with GluA2, red signals are observed because of the proximity of the corresponding secondary antibodies. In the presence of Sema3A, PlexA4-GluA2 interacting signals were first detected in the cell body. The signal clusters accumulated in the cell body and then dispersed to dendrites. To address whether the interaction between PlexA4 and GluA2 is necessary for dendritic distribution of GluA2, we generated PlexA1-IPT, which interacted with GluA2, but not NRP1. Overexpression of PlexA1-IPT suppressed the Sema3A-induced increase in the immunostaining intensity of GluA2, and the number of PlexA4-GluA2 interacting signals in cultured hippocampal neurons was indeed suppressed in both soma and dendrites. The suppression by the extracellular IPT domain of PlexA1 suggests that PlexA may be a cis-interacting partner of GluA2 at the IPT domain. Because clathrin- and dynamin-mediated endocytosis of GluA2 mainly occurs at the somatodendritic plasma membrane, the Sema3A-induced interaction between PlexA and GluA2 might occur in the somatodendritic area. In fact, when locally applied to the somatodendritic but not to axonal field, the soluble form of PlexA1-IPT (sPlexA1-IPT) attenuated the Sema3A-enhanced immunostaining of GluA2 in dendrites. These findings suggest that PlexA-GluA2 interaction in the somatodendritic region is required for Sema3A to induce dendritic localization of GluA2. Furthermore, in sema3A-deficient mice, GluA2 and GRIP1 were retained in the stratum pyramidale and stratum radiatum, the layers containing cell bodies and proximal dendrites of pyramidal neurons, respectively. Meanwhile, in the hippocampus of P0 wild-type mice, GluA2 and GRIP1 were evenly distributed in the stratum lacunosum-moleculare, stratum pyramidale and stratum radiatum. Introduction of PlexA1-IPT by in utero electroporation induced accumulation of GluA2 at the apical side of the perinuclear region of the cell body, indicating that the overexpression of PlexA1-IPT disrupted the distribution of GluA2 in the distal dendritic domain in vivo. In normal development, the dendritic localization of GluA2 may contribute to dendritic branching and spine formation. The glutamatergic transmission converging on Ca2+ signaling plays an important role in dendritic development.Citation82 In addition, GluA2 regulated dendritic development by recruiting N-cadherin through direct interaction.Citation83 In fact, the disruption of the PlexA-GluA2 interaction by the introduction of PlexA1-IPT also induced proximal bifurcation phenotype in the apical dendrites of CA1 hippocampal neurons, a similar phenotype to that seen in sema3A-deficient mice.Citation53,84 These findings suggest that the PlexA4-GluA2 interaction is essential for Sema3A to regulate not only dendritic localization of GluA2 but also proper dendritic patterning in vitro and in vivo (). In addition to serving as a Sema3A signal-transducing receptor, PlexAs may function as a novel regulator of glutamate receptor localization.
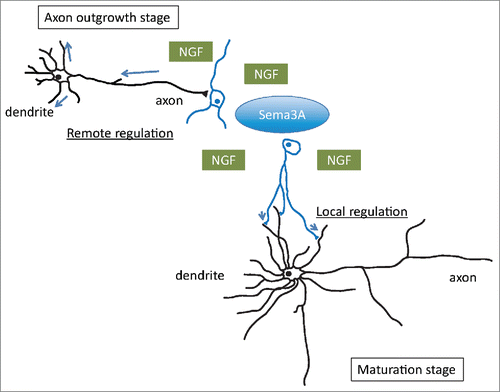
These findings strongly suggest the axonal growth cone as a site of Sema3A action responsible for dendritic localization of GluA2 at an early stage (3DIV) of neurons from E18 rat embryos. However, the site of action may be likely to localize in the somatodendritic regions, especially in mature neurons possessing a long axon and multiple dendrites. In addition, ontogenic alterations in the localizations of NRPs and PlexAs may occur during neuronal development (). Indeed, NRP1 was observed on basal and apical dendritic processes at 18DIV of primary mouse cortical neurons from E14.5 mouse embryos.Citation27 Aberrant dendritic or spine morphology and spine density in P21 and adult DG GCs of NRPs and PlexAs mutant mice further support the role of these dendritic semaphorin receptors in dendritic branching and spine morphogenesis. Sema3A may be secreted from non-neuronal cells such as astrocytes and smooth muscle cells as well as pre- and/or postsynaptic neurons. Sema3A protein is localized in dendrites of cultured hippocampal neurons, and it may act in an autocrine manner.Citation84 The role of Sema3A secreted from these cellular components in neuronal development remains an open question.
Figure 3. Crosstalk between Sema3A and NGF. The axonal growth cone as a site of Sema3A action responsible for dendritic localization of GluA2 at an early embryonic stage. The site of action may also localize in the somatodendritic regions, especially in mature neurons possessing a long axon and multiple dendrites. Determining neurite extension or retraction and cell survival or death might depend on the balance between the levels of NGF and Sema3A signaling that are initiated.
Dendritic patterning regulated by retrograde signaling—Crosstalk between semaphorins and neurotrophins
To examine the physiological relevance of Sema3A-induced axonal transport, we first monitored axonal transport of NRPs and PlexA4, Fyn and TrkA before and after Sema3A stimulation. Sema3A accelerated anterograde and retrograde transport of NRP1, PlexA and Fyn in cultured chick DRG neurons. Sema3A did not show any effect on the transport of NRP2, the receptor component for Sema3F, thereby suggesting cargo-selective transport upon Sema3A stimulation (data not shown). Surprisingly, however, the transport of TrkA, a high-affinity receptor for nerve growth factor (NGF), was enhanced in response to Sema3A. This finding implies that TrkA might be involved in Sema3A-induced axonal transport and provides important clues to the underlying mechanism. How was TrkA involved in Sema3A signaling? Because PlexA4 mediates growth cone Sema3A signaling and acts as a cis-interacting receptor with GluA2 to escort GluA2 to distal dendrites, the effect of Sema3A on dendritic localization of GluA2 was investigated in TrkA knocked down neurons. The knockdown of TrkA attenuated Sema3A-enhanced immunostaining of GluA2 in dendrites, thereby suggesting that TrkA mediates the Sema3A retrograde signal that regulates the dendritic localization of GluA2. In fact, PlexA4 was found to interact with TrkA. Our analysis of TrkA mutants revealed that a TrkA mutant lacking the region spanning from C1 to Ig1 did not interact with PlexA4, thereby indicating that the TrkA Ig1 domain is essential for the interaction between TrkA and PlexA4. Furthermore, after Sema3A stimulation, PlexA4 and TrkA colocalization increased in the growth cone and thereafter increased in distal axons, suggesting that Sema3A induces formation of the PlexA4-TrkA complex in the growth cone and retrograde transport of the complex along the axon. Our study revealed that the kinase activity of TrkA is required for Sema3A-induced retrograde transport. The NGF-TrkA signaling cascade involves the phosphorylation of phospholipase C, gamma 1 (PLCg1), Akt and P44/22 mitogen-activated protein kinase (MAPK) by TrkA.Citation14,15,80,85 The crosstalk between Sema3A and neurotrophin signaling is further supported by the finding that the level of phosphorylated Akt increased after Sema3A application, and that the application of LY294002, an inhibitor of PI3K-dependent Akt phosphorylation, to distal axons suppressed the Sema3A-induced increase in GluA2 immunostaining in dendrites. These findings indicate that TrkA plays a crucial role in mediating Sema3A-induced retrograde signaling by interacting with PlexA4, thus providing a novel mode of crosstalk between the 2 signaling pathways of NGF and Sema3A.Citation86 Interestingly, the level of phosphorylated Akt was increased at the early phase (5 min), but decreased 30 min after exposure to Sema3A. Thus signaling mechanisms for the initial Akt response might differ from those for the late-phase response to Sema3A. Sema3A was reported to decrease Akt phosphorylation and induce apoptosis in cultured phodocytes.Citation87 This biphasic action of Sema3A might be related to induction of either neurite outgrowth or inhibition.Citation23,24,88
The involvement of TrkA may well be consistent with a property of Sema3A-induced axonal transport, the dependence on voltage-dependent ion channels.Citation54,55 Neurotrophins and their receptors participate in activity-dependent neuronal plasticity. For example, brain-derived neurotrophic factor (BDNF) has been implicated in hippocampal long-term potentiation. In CA pyramidal neurons, BDNF was shown to evoke membrane depolarization through rapid activation of the sodium channel Nav1.9.Citation89 Intracellular Ca2+ release may also be involved in the retrograde Sema3A signaling. Calcium stored within the endoplasmic reticulum of neurons represents a source of signal Ca2+ that is released upon activation of either the InsP3 receptors or the ryanodine receptors.Citation90 NGF induces endocytosis of TrkA through clathrin-mediated pathways, and its subsequent downstream pathways include the Ras-MAP kinase pathway, PI3 kinase and the PLCg1 pathways and may perhaps also include retrograde Ca2+ waves emanating from activated TrkA receptors.Citation10,11 In addition to TrkA, Sema3A activated ryanodine receptors in both the growth cones and soma, although the propagation of membranous depolarization and Ca2+ waves from growth cone to cell body were not detectable. However, tetrodotoxin suppressed the Sema3A-induced activation of ryanodine receptors in the soma, but not in the growth cones. This finding suggests that Ca2+ signaling is probably propagated from the growth cones toward the cell bodiesCitation54 ().
Distinct but overlapping roles of neurotrophins and semaphorins
As developing axons traverse long distances toward their final targets, they respond to growth and guidance molecules that are derived from intermediate and/or final target cells. Since TrkA plays a crucial role in mediating Sema3A signaling, Sema3A and NGF signaling pathways converge via TrkA, and this convergence may allow finely tuned reciprocal regulation of neurite outgrowth, dendritic branching and synapse maturation. For example, interaction between Sema3A and neurotrophins could be an important determinant of growth cone behavior to axon guidance cues in cultured chick DRG neurons.Citation91 The DRG growth cones cultured with NGF or BDNF were more resistant to Sema3A-induced growth cone collapse than those cultured with a lower concentration of NGF. Sema3A attenuated TrkA-mediated growth signaling, while overexpression of constitutively active TrkA blocks Sema3A-induced axon growth inhibition.Citation93 A series of experiments using several kinase inhibitors suggested that protein kinase A activity is involved in NGF modulation of Sema3A-induced growth cone collapse, while protein kinase G activity is directly involved in Sema3A-induced growth cone collapse. Sema3A induces activation of Akt, favoring signaling for cell survival.Citation11 In addition, p75 neurotrophin receptor (p75NTR), the low affinity NGF receptor, also interacted with NRP1 and PlexA4, and modulated Sema3A activity.Citation92 Thus determining neurite extension or retraction and cell survival or death might depend on the balance between the levels of NGF and Sema3A signaling that are initiated ().
The pathophysiological significance of semaphorins, neurotrophins and their receptors remains largely unknown. A highly phosphorylated form of CRMP2 was identified as a component of the neurofibrillary tangles associated with the disintegration of microtubules in the brains of patients affected with Alzheimer disease.Citation94 To elucidate the significance of CRMP2 phosphorylation in the pathogenesis of Alzheimer disease, CRMP2 phosphorylation-deficient knock-in (crmp2ki/ki) mice were generated in which the serine residue at 522 of the protein was replaced with alanine. Intracerebroventricular injection of Aβ25-35 peptide, a neurotoxic fragment of Aβ protein, in wild-type mice increased hippocampal phosphorylation of CRMP2. Behavioral assessment revealed that the injection of the Aβ25-35 peptide caused impairment of both cognitive function and long-term potentiation in wild-type animals, but not in crmp2ki/ki mice. This finding indicates that CRMP2 phosphorylation is required for the acute effect of Aβ to be manifest.Citation95 Sema3A might be involved in the pathogenesis of the disease. Immunohistochemical studies revealed that Sema3A accumulates in the cytoplasm of the CA1 hippocampal pyramidal neurons during the progression of Alzheimer disease.Citation96 This accumulation may reflect hyperactivation of the Sema3A signaling pathway, which could induce neuronal degeneration and loss since Sema3A can induce neuronal cell death. Interestingly, increasing concentrations of NGF, but not other neurotrophins, block Sema3A-induced cell death.Citation88 In Alzheimer disease, early and predominant loss of NGF-sensitive basal forebrain cholinergic neurons is the major functional basis for cognitive impairment.Citation97 Such disruption in the balance between Sema3A and NGF may possibly cause a bias toward cell death signaling pathways, further accelerating the neurodegenerative process in the brain affected by Alzheimer disease. Further studies using more physiologically relevant disease model animals will be required.
The involvement of TrkA in Sema3A signaling provides a novel mode of crosstalk between 2 signaling pathways of the axon guidance molecules NGF and Sema3A.Citation88,91,98 The crosstalk between Sema3A and NGF signaling may explain the effects of Sema3A on branching and dendritic spine maturation.Citation13,23,24,80 For example, growth cone collapse was observed after a short-term exposure to Sema3A, while multiple branch formation after a long-term exposure to Sema3A occurred in Xenopus retinal growth cones.Citation99 Indeed, retrograde signaling from distal axons to the nucleus contributes to axonal extension. Downstream of neurotrophin receptors, including TrkA, several pathways signal to transcription factors, including CREB, that contribute not only to survival but also to axon growth and target innervation.Citation15 Thus the crosstalk of Sema3A with neurotrophin signaling may underlie both its local and remote effects.
Although the essential role of TrkA in Sema3A signaling indicates that Sema3A and NGF share a common mechanism for retrograde signaling, some differences exist between the roles of Sema3A and NGF. TrkA may play a role in mediating axonal transport but not growth cone collapse in response to Sema3A. Either siRNA knockdown of TrkA or treatment with K252a suppressed the Sema3A-induced axonal transport, but neither affected Sema3A-induced growth cone collapse (unpublished observation). Furthermore, Sema3A induced retrograde transport of both PlexA4 and TrkA, whereas NGF induced the transport of TrkA without modifying that of PlexA4.Citation86 Moreover, unlike Sema3A, NGF did not induce colocalization of PlexA4 and TrkA. These findings indicate that despite a shared interaction with TrkA, Sema3A and NGF play different biological roles through distinct signaling pathways in developing neurons.
Future implications
The following questions remain to be answered. Are CRMPs, and other signaling molecules involved in the ion channel-coupled retrograde Sema3A signaling? If so, how do they mediate Sema3A signaling in axons, cell bodies or dendrites? How does the retrograde Sema3A signaling regulate interactions between PlexAs and glutamate receptors thereby promoting dendritic arborization and synapse maturation? To answer these questions, the effects of local inactivation of CRMPs and other signaling molecules in a certain subcellular region such as growth cone, dendrite or cell body must be examined.Citation100 Estimation of membrane potential and local Ca2+ imaging will be required to elucidate the mechanism by which ion channel–coupled transport is elicited in response to Sema3A.
Overall, the signaling mechanisms for semaphorins including Sema3A have been investigated in reconstituted in vitro systems and confirmed in in vivo systems. Multiple signaling mechanisms for semaphorins may exist in the heterogenous neuron populations of the central and peripheral nervous system. Therefore single-cell analysis with highly sensitive detection and an understanding of the complex interplay between heterogenous cell populations will be required for determining the in vivo relevance of the wide variety of signaling mechanisms of semaphorins.
Conclusion
At least 3 models of retrograde signaling have been proposed: signaling endosome model, retrograde waves of Trk receptor activation and retrograde calcium waves emanating from activated Trk receptors.Citation9,11,12,15 The crosstalk between TrkA and PlexAs may provide a fourth model of retrograde signaling in the developing nervous system.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are particularly grateful for the assistance given by Ms Okada, Ms Chen and Ms Ogawara.
Funding
This work was funded by Core Research for Evolutional Science and Technology of the Japan Science and Technology, Grants-in-Aid for Scientific Research in a Priority Area from the Ministry of Education, Science, Sports and Culture (No. 17082006), Targeted Proteins Research Program (No. 0761890004), Global COE Program and Innovative Integration between Medicine and Engineering Based on Information Communication Technology (No. 1542140002).
References
- Shen K, Cowan CW. Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol 2010; 2:a001842; PMID:20452946; http://dx.doi.org/10.1101/2shperspect.a001842
- Colon-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science 2007; 318:103-6; PMID:17916735; http://dx.doi.org/10.1126/2cience.1143762
- Santiago C, Bashaw GJ. Transcription factors and effectors that regulate neuronal morphology. Development 2014; 141:4667-80; PMID:25468936; http://dx.doi.org/10.1242/2ev.110817
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science 1996; 274:1123-33; PMID:8895455; http://dx.doi.org/10.1126/2cience.274.5290.1123
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci 2004; 7:244-53; PMID:14770185; http://dx.doi.org/10.1038/2n1189
- Kacharmina JE, Job C, Crino P, Eberwine J. Stimulation of glutamate receptor protein synthesis and membrane insertion within isolated neuronal dendrites. Proc Natl Acad Sci U S A 2000; 97:11545-50; PMID:11027353; http://dx.doi.org/10.1073/2nas.97.21.11545/
- Xu NJ, Henkemeyer M. Ephrin reverse signaling in axon guidance and synaptogenesis. Semin Cell Dev Biol 2012; 23:58-64; PMID:22044884; http://dx.doi.org/10.1016/2.semcdb.2011.10.024
- Cosker KE, Courchesne SL, Segal RA. Action in the axon: generation and transport of signaling endosomes. Curr Opin Neurobiol 2008; 18:270-5; PMID:18778772; http://dx.doi.org/10.1016/2.conb.2008.08.005
- Ginty DD, Segal RA. Retrograde neurotrophin signaling: Trk-ing along the axon. Curr Opin Neurobiol 2002; 12:268-74; PMID:12049932; http://dx.doi.org/10.1016/20959-4388(02)00326-4
- Harrington AW, Ginty DD. Long-distance retrograde neurotrophic factor signalling in neurons. Nat Rev Neurosci 2013; 14:177-87; PMID:23422909; http://dx.doi.org/10.1038/2rn3253
- Howe CL, Mobley WC. Signaling endosome hypothesis: A cellular mechanism for long distance communication. J Neurobiol 2004; 58:207-16; PMID:14704953; http://dx.doi.org/10.1002/2eu.10323
- Howe CL, Mobley WC. Long-distance retrograde neurotrophic signaling. Curr Opin Neurobiol 2005; 15:40-8; PMID:15721743; http://dx.doi.org/10.1016/2.conb.2005.01.010
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 2003; 72:609-42; PMID:12676795; http://dx.doi.org/10.1146/2nnurev.biochem.72.121801.161629
- Wu C, Cui B, He L, Chen L, Mobley WC. The coming of age of axonal neurotrophin signaling endosomes. J Proteomics 2009; 72:46-55; PMID:19028611; http://dx.doi.org/10.1016/2.jprot.2008.10.007
- Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci 2005; 6:615-25; PMID:16062170; http://dx.doi.org/10.1038/2rn1727
- Unified nomenclature for the semaphorins/2ollapsins. Semaphorin Nomenclature Committee. Cell 1999; 97:551-2; PMID:10367884; http://dx.doi.org/10.1016/20092-8674(00)80766-7
- Raper JA. Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol 2000; 10:88-94; PMID:10679438; http://dx.doi.org/10.1016/20959-4388(99)00057-4
- Kolodkin AL, Ginty DD. Steering clear of semaphorins: neuropilins sound the retreat. Neuron 1997; 19:1159-62; PMID:9427240; http://dx.doi.org/10.1016/20896-6273(00)80408-0
- Tamagnone L, Comoglio PM. Signalling by semaphorin receptors: cell guidance and beyond. Trends in cell biology 2000; 10:377-83; PMID:10932095; http://dx.doi.org/10.1016/20962-8924(00)01816-X
- Sharma A, Verhaagen J, Harvey AR. Receptor complexes for each of the Class 3 Semaphorins. Front Cell Neurosci 2012; 6:28; PMID:22783168; http://dx.doi.org/10.3389/2ncel.2012.00028
- Pascual M, Pozas E, Soriano E. Role of class 3 semaphorins in the development and maturation of the septohippocampal pathway. Hippocampus 2005; 15:184-202; PMID:15386596; http://dx.doi.org/10.1002/2ipo.20040
- Godenschwege TA, Hu H, Shan-Crofts X, Goodman CS, Murphey RK. Bi-directional signaling by Semaphorin 1a during central synapse formation in Drosophila. Nat Neurosci 2002; 5:1294-301; PMID:12436113; http://dx.doi.org/10.1038/2n976
- Fenstermaker V, Chen Y, Ghosh A, Yuste R. Regulation of dendritic length and branching by semaphorin 3A. J Neurobiol 2004; 58:403-12; PMID:14750152; http://dx.doi.org/10.1002/2eu.10304
- Morita A, Yamashita N, Sasaki Y, Uchida Y, Nakajima O, Nakamura F, Yagi T, Taniguchi M, Usui H, Katoh-Semba R, et al. Regulation of dendritic branching and spine maturation by semaphorin3A-Fyn signaling. J Neurosci 2006; 26:2971-80; PMID:16540575; http://dx.doi.org/10.1523/2NEUROSCI.5453-05.2006
- Polleux F, Giger RJ, Ginty DD, Kolodkin AL, Ghosh A. Patterning of cortical efferent projections by semaphorin-neuropilin interactions. Science 1998; 282:1904-6; PMID:9836643; http://dx.doi.org/10.1126/2cience.282.5395.1904
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature 2000; 404:567-73; PMID:10766232; http://dx.doi.org/10.1038/25007001
- Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, Huganir RL, Ginty DD, Kolodkin AL. Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature 2009; 462:1065-9; PMID:20010807; http://dx.doi.org/10.1038/2ature08628
- Sahay A, Kim CH, Sepkuty JP, Cho E, Huganir RL, Ginty DD, Kolodkin AL. Secreted semaphorins modulate synaptic transmission in the adult hippocampus. J Neurosci 2005; 25:3613-20; PMID:15814792; http://dx.doi.org/10.1523/2NEUROSCI.5255-04.2005
- Bouzioukh F, Daoudal G, Falk J, Debanne D, Rougon G, Castellani V. Semaphorin3A regulates synaptic function of differentiated hippocampal neurons. Eur J Neurosci 2006; 23:2247-54; PMID:16706833; http://dx.doi.org/10.1111/2.1460-9568.2006.04783.x
- Yamashita N, Morita A, Uchida Y, Nakamura F, Usui H, Ohshima T, Taniguchi M, Honnorat J, Thomasset N, Takei K, et al. Regulation of spine development by semaphorin3A through cyclin-dependent kinase 5 phosphorylation of collapsin response mediator protein 1. J Neurosci 2007; 27:12546-54; PMID:18003833; http://dx.doi.org/10.1523/2NEUROSCI.3463-07.2007
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature 2009; 462:920-4; PMID:19946265; http://dx.doi.org/10.1038/2ature08577
- Fujisawa H. Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development. J Neurobiol 2004; 59:24-33; PMID:15007824; http://dx.doi.org/10.1002/2eu.10337
- Hota PK, Buck M. Plexin structures are coming: opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions. Cell Mol Life Sci 2012; 69:3765-805; PMID:22744749; http://dx.doi.org/10.1007/200018-012-1019-0
- Jongbloets BC, Pasterkamp RJ. Semaphorin signalling during development. Development 2014; 141:3292-7; PMID:25139851; http://dx.doi.org/10.1242/2ev.105544
- Kumanogoh A, Kikutani H. Immunological functions of the neuropilins and plexins as receptors for semaphorins. Nat Rev Immunol 2013; 13:802-14; PMID:24319778; http://dx.doi.org/10.1038/2ri3545
- Yamashita N, Goshima Y. Collapsin response mediator proteins regulate neuronal development and plasticity by switching their phosphorylation status. Mol Neurobiol 2012; 45:234-46; PMID:22351471; http://dx.doi.org/10.1007/212035-012-8242-4
- Negishi M, Oinuma I, Katoh H. R-ras as a key player for signaling pathway of plexins. Mol Neurobiol 2005; 32:217-22; PMID:16385138; http://dx.doi.org/10.1385/2N:32:3:217
- Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci 2008; 33:161-70; PMID:18374575; http://dx.doi.org/10.1016/2.tibs.2008.01.006
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci 2001; 4:367-73; PMID:11276226; http://dx.doi.org/10.1038/26011
- Vastrik I, Eickholt BJ, Walsh FS, Ridley A, Doherty P. Sema3A-induced growth-cone collapse is mediated by Rac1 amino acids 17-32. Curr Biol 1999; 9:991-8; PMID:10508610; http://dx.doi.org/10.1016/20960-9822(99)80447-3
- Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science 2004; 305:862-5; PMID:15297673; http://dx.doi.org/10.1126/2cience.1097545
- Puschel AW. GTPases in semaphorin signaling. Adv Exp Med Biol 2007; 600:12-23; PMID:17607943; http://dx.doi.org/10.1007/278-0-387-70956-7_2
- Gu Y, Ihara Y. Evidence that collapsin response mediator protein-2 is involved in the dynamics of microtubules. J Biol Chem 2000; 275:17917-20; PMID:10770920; http://dx.doi.org/10.1074/2bc.C000179200
- Arimura N, Hattori A, Kimura T, Nakamuta S, Funahashi Y, Hirotsune S, Furuta K, Urano T, Toyoshima YY, Kaibuchi K. CRMP-2 directly binds to cytoplasmic dynein and interferes with its activity. J Neurochem 2009; 111:380-90; PMID:19659462; http://dx.doi.org/10.1111/2.1471-4159.2009.06317.x
- Uchida Y, Ohshima T, Sasaki Y, Suzuki H, Yanai S, Yamashita N, Nakamura F, Takei K, Ihara Y, Mikoshiba K, et al. Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3beta phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer's disease. Genes Cells 2005; 10:165-79; PMID:15676027; http://dx.doi.org/10.1111/2.1365-2443.2005.00827.x
- Niisato E, Nagai J, Yamashita N, Abe T, Kiyonari H, Goshima Y, Ohshima T. CRMP4 suppresses apical dendrite bifurcation of CA1 pyramidal neurons in the mouse hippocampus. Dev Neurobiol 2012; 72:1447-57; PMID:22234963; http://dx.doi.org/10.1002/2neu.22007
- Schmidt EF, Shim SO, Strittmatter SM. Release of MICAL autoinhibition by semaphorin-plexin signaling promotes interaction with collapsin response mediator protein. J Neurosci 2008; 28:2287-97; PMID:18305261; http://dx.doi.org/10.1523/2NEUROSCI.5646-07.2008
- Terman JR, Mao T, Pasterkamp RJ, Yu HH, Kolodkin AL. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell 2002; 109:887-900; PMID:12110185; http://dx.doi.org/10.1016/20092-8674(02)00794-8
- Nakamura F, Kumeta K, Hida T, Isono T, Nakayama Y, Kuramata-Matsuoka E, Yamashita N, Uchida Y, Ogura K, Gengyo-Ando K, et al. Amino- and carboxyl-terminal domains of Filamin-A interact with CRMP1 to mediate Sema3A signalling. Nat Commun 2014; 5:5325; PMID:25358863; http://dx.doi.org/10.1038/2comms6325
- Goshima Y, Ito T, Sasaki Y, Nakamura F. Semaphorins as signals for cell repulsion and invasion. J Clin Invest 2002; 109:993-8; PMID:11956234; http://dx.doi.org/10.1172/2CI0215467
- Goshima Y, Kawakami T, Hori H, Sugiyama Y, Takasawa S, Hashimoto Y, Kagoshima-Maezono M, Takenaka T, Misu Y, Strittmatter SM. A novel action of collapsin: collapsin-1 increases antero- and retrograde axoplasmic transport independently of growth cone collapse. J Neurobiol 1997; 33:316-28; PMID:9298768; http://dx.doi.org/10.1002/(SICI)1097-4695(199709)33:3%3c316::AID-NEU9%3e3.0.CO;2-4
- Goshima Y, Hori H, Sasaki Y, Yang T, Kagoshima-Maezono M, Li C, Takenaka T, Nakamura F, Takahashi T, Strittmatter SM, et al. Growth cone neuropilin-1 mediates collapsin-1/2ema III facilitation of antero- and retrograde axoplasmic transport. J Neurobiol 1999; 39:579-89; PMID:10380079; http://dx.doi.org/10.1002/(SICI)1097-4695(19990615)39:4%3c579::AID-NEU11%3e3.0.CO;2-9
- Yamashita N, Usui H, Nakamura F, Chen S, Sasaki Y, Hida T, Suto F, Taniguchi M, Takei K, Goshima Y. Plexin-A4-dependent retrograde semaphorin 3A signalling regulates the dendritic localization of GluA2-containing AMPA receptors. Nat Commun 2014; 5:3424; PMID:24599038
- Yamane M, Yamashita N, Yamamoto H, Iizuka A, Shouji M, Usui H, Goshima Y. Semaphorin3A facilitates axonal transport through a local calcium signaling and tetrodotoxin-sensitive voltage-gated sodium channels. Biochem Biophys Res Commun 2012; 422:333-8; PMID:22575508; http://dx.doi.org/10.1016/2.bbrc.2012.05.003
- Yamashita N, Aoki R, Chen S, Jitsuki-Takahashi A, Ohura S, Kamiya H, Goshima Y. Voltage-gated calcium and sodium channels mediate Sema3A retrograde signaling that regulates dendritic development. Brain Res 2015; 1631:127-36; PMID:26638837; http://dx.doi.org/10.1016/2.brainres.2015.11.034
- Togashi K, von Schimmelmann MJ, Nishiyama M, Lim CS, Yoshida N, Yun B, Molday RS, Goshima Y, Hong K. Cyclic GMP-gated CNG channels function in Sema3A-induced growth cone repulsion. Neuron 2008; 58:694-707; PMID:18549782; http://dx.doi.org/10.1016/2.neuron.2008.03.017
- Li-Smerin Y, Swartz KJ. Gating modifier toxins reveal a conserved structural motif in voltage-gated Ca2+ and K+ channels. Proc Natl Acad Sci U S A 1998; 95:8585-9; PMID:9671721; http://dx.doi.org/10.1073/2nas.95.15.8585
- Henley J, Poo MM. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol 2004; 14:320-30; PMID:15183189; http://dx.doi.org/10.1016/2.tcb.2004.04.006
- Nishiyama M, von Schimmelmann MJ, Togashi K, Findley WM, Hong K. Membrane potential shifts caused by diffusible guidance signals direct growth-cone turning. Nat Neurosci 2008; 11:762-71; PMID:18536712; http://dx.doi.org/10.1038/2n.2130
- Tojima T, Itofusa R, Kamiguchi H. Asymmetric clathrin-mediated endocytosis drives repulsive growth cone guidance. Neuron 2010; 66:370-7; PMID:20471350; http://dx.doi.org/10.1016/2.neuron.2010.04.007
- Behar O, Mizuno K, Badminton M, Woolf CJ. Semaphorin 3A growth cone collapse requires a sequence homologous to tarantula hanatoxin. Proc Natl Acad Sci U S A 1999; 96:13501-5; PMID:10557350; http://dx.doi.org/10.1073/2nas.96.23.13501
- Fournier AE, Nakamura F, Kawamoto S, Goshima Y, Kalb RG, Strittmatter SM. Semaphorin3A enhances endocytosis at sites of receptor-F-actin colocalization during growth cone collapse. The Journal of cell biology 2000; 149:411-22; PMID:10769032; http://dx.doi.org/10.1083/2cb.149.2.411
- Hida T, Yamashita N, Usui H, Nakamura F, Sasaki Y, Kikuchi A, Goshima Y. GSK3beta/2xin-1/2eta-catenin complex is involved in semaphorin3A signaling. J Neurosci 2012; 32:11905-18; PMID:22933777; http://dx.doi.org/10.1523/2NEUROSCI.6139-11.2012
- Nishiyama M, Togashi K, von Schimmelmann MJ, Lim CS, Maeda S, Yamashita N, Goshima Y, Ishii S, Hong K. Semaphorin 3A induces CaV2.3 channel-dependent conversion of axons to dendrites. Nat Cell Biol 2011; 13:676-85; PMID:21602796; http://dx.doi.org/10.1038/2cb2255
- Treinys R, Kaselis A, Jover E, Bagnard D, Satkauskas S. R-type calcium channels are crucial for semaphorin 3A-induced DRG axon growth cone collapse. PLoS One 2014; 9:e102357; PMID:25032951; http://dx.doi.org/10.1371/2ournal.pone.0102357
- Naidoo V, Dai X, Galligan JJ. R-type Ca(2+) channels contribute to fast synaptic excitation and action potentials in subsets of myenteric neurons in the guinea pig intestine. Neurogastroenterol Motil 2010; 22:e353-63; PMID:20879993; http://dx.doi.org/10.1111/2.1365-2982.2010.01596.x
- Brittain JM, Wang Y, Eruvwetere O, Khanna R. Cdk5-mediated phosphorylation of CRMP-2 enhances its interaction with CaV2.2. FEBS Lett 2012; 586:3813-8; PMID:23022559; http://dx.doi.org/10.1016/2.febslet.2012.09.022
- Brittain JM, Piekarz AD, Wang Y, Kondo T, Cummins TR, Khanna R. An atypical role for collapsin response mediator protein 2 (CRMP-2) in neurotransmitter release via interaction with presynaptic voltage-gated calcium channels. J Biol Chem 2009; 284:31375-90; PMID:19755421; http://dx.doi.org/10.1074/2bc.M109.009951
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, et al. Nomenclature of voltage-gated calcium channels. Neuron 2000; 25:533-5; PMID:10774722; http://dx.doi.org/10.1016/20896-6273(00)81057-0
- Sasaki Y, Cheng C, Uchida Y, Nakajima O, Ohshima T, Yagi T, Taniguchi M, Nakayama T, Kishida R, Kudo Y, et al. Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron 2002; 35:907-20; PMID:12372285; http://dx.doi.org/10.1016/20896-6273(02)00857-7
- Granger AJ, Gray JA, Lu W, Nicoll RA. Genetic analysis of neuronal ionotropic glutamate receptor subunits. J Physiol 2011; 589:4095-101; PMID:21768264; http://dx.doi.org/10.1113/2physiol.2011.213033
- Lujan R, Shigemoto R, Lopez-Bendito G. Glutamate and GABA receptor signalling in the developing brain. Neuroscience 2005; 130:567-80; PMID:15590141; http://dx.doi.org/10.1016/2.neuroscience.2004.09.042
- Prithviraj R, Kelly KM, Espinoza-Lewis R, Hexom T, Clark AB, Inglis FM. Differential regulation of dendrite complexity by AMPA receptor subunits GluR1 and GluR2 in motor neurons. Dev Neurobiol 2008; 68:247-64; PMID:18000827; http://dx.doi.org/10.1002/2neu.20590
- Kita EM, Bertolesi GE, Hehr CL, Johnston J, McFarlane S. Neuropilin-1 biases dendrite polarization in the retina. Development 2013; 140:2933-41; PMID:23739132; http://dx.doi.org/10.1242/2ev.088286
- Zhuang B, Su YS, Sockanathan S. FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane Semaphorin6A and PlexinA4 signaling. Neuron 2009; 61:359-72; PMID:19217374; http://dx.doi.org/10.1016/2.neuron.2008.12.022
- Grooms SY, Noh KM, Regis R, Bassell GJ, Bryan MK, Carroll RC, Zukin RS. Activity bidirectionally regulates AMPA receptor mRNA abundance in dendrites of hippocampal neurons. J Neurosci 2006; 26:8339-51; PMID:16899729; http://dx.doi.org/10.1523/2NEUROSCI.0472-06.2006
- Li C, Sasaki Y, Takei K, Yamamoto H, Shouji M, Sugiyama Y, Kawakami T, Nakamura F, Yagi T, Ohshima T, et al. Correlation between semaphorin3A-induced facilitation of axonal transport and local activation of a translation initiation factor eukaryotic translation initiation factor 4E. J Neurosci 2004; 24:6161-70; PMID:15240808; http://dx.doi.org/10.1523/2NEUROSCI.1476-04.2004
- Setou M, Hayasaka T, Yao I. Axonal transport versus dendritic transport. J Neurobiol 2004; 58:201-6; PMID:14704952; http://dx.doi.org/10.1002/2eu.10324
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature 2002; 417:83-7; PMID:11986669; http://dx.doi.org/10.1038/2ature743
- Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron 2003; 39:69-84; PMID:12848933; http://dx.doi.org/10.1016/20896-6273(03)00397-0
- Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods 2006; 3:995-1000; PMID:17072308; http://dx.doi.org/10.1038/2meth947
- Hamad MI, Ma-Hogemeier ZL, Riedel C, Conrads C, Veitinger T, Habijan T, Schulz JN, Krause M, Wirth MJ, Hollmann M, et al. Cell class-specific regulation of neocortical dendrite and spine growth by AMPA receptor splice and editing variants. Development 2011; 138:4301-13; PMID:21865324; http://dx.doi.org/10.1242/2ev.071076
- Saglietti L, Dequidt C, Kamieniarz K, Rousset MC, Valnegri P, Thoumine O, Beretta F, Fagni L, Choquet D, Sala C, et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron 2007; 54:461-77; PMID:17481398; http://dx.doi.org/10.1016/2.neuron.2007.04.012
- Nakamura F, Ugajin K, Yamashita N, Okada T, Uchida Y, Taniguchi M, Ohshima T, Goshima Y. Increased proximal bifurcation of CA1 pyramidal apical dendrites in sema3A mutant mice. J Comp Neurol 2009; 516:360-75; PMID:19655386; http://dx.doi.org/10.1002/2ne.22125
- Heerssen HM, Pazyra MF, Segal RA. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nat Neurosci 2004; 7:596-604; PMID:15122257; http://dx.doi.org/10.1038/2n1242
- Yamashita N, Yamane M, Suto F, Goshima Y. TrkA mediates retrograde semaphorin3A signaling through PlexinA4 to regulate dendritic branching. J Cell Sci 2016; PMID:26945060
- Guan F, Villegas G, Teichman J, Mundel P, Tufro A. Autocrine class 3 semaphorin system regulates slit diaphragm proteins and podocyte survival. Kidney Int 2006; 69:1564-9; PMID:16541019; http://dx.doi.org/10.1038/2j.ki.5000313
- Ben-Zvi A, Yagil Z, Hagalili Y, Klein H, Lerman O, Behar O. Semaphorin 3A and neurotrophins: a balance between apoptosis and survival signaling in embryonic DRG neurons. J Neurochem 2006; 96:585-97; PMID:16336628; http://dx.doi.org/10.1111/2.1471-4159.2005.03580.x
- Blum R, Kafitz KW, Konnerth A. Neurotrophin-evoked depolarization requires the sodium channel Na(V)1.9. Nature 2002; 419:687-93; PMID:12384689; http://dx.doi.org/10.1038/2ature01085
- Berridge MJ. Neuronal calcium signaling. Neuron 1998; 21:13-26; PMID:9697848; http://dx.doi.org/10.1016/20896-6273(00)80510-3
- Dontchev VD, Letourneau PC. Nerve growth factor and semaphorin 3A signaling pathways interact in regulating sensory neuronal growth cone motility. J Neurosci 2002; 22:6659-69; PMID:12151545
- Ben-Zvi A, Ben-Gigi L, Klein H, Behar O. Modulation of semaphorin3A activity by p75 neurotrophin receptor influences peripheral axon patterning. J Neurosci 2007; 27:13000-11; PMID:18032673; http://dx.doi.org/10.1523/2NEUROSCI.3373-07.2007
- Ben-Zvi A, Ben-Gigi L, Yagil Z, Lerman O, Behar O. Semaphorin3A regulates axon growth independently of growth cone repulsion via modulation of TrkA signaling. Cell Signal 2008; 20:467-79; PMID:18096366; http://dx.doi.org/10.1016/2.cellsig.2007.10.023
- Yoshida H, Watanabe A, Ihara Y. Collapsin response mediator protein-2 is associated with neurofibrillary tangles in Alzheimer's disease. J Biol Chem 1998; 273:9761-8; PMID:9545313; http://dx.doi.org/10.1074/2bc.273.16.9761
- Isono T, Yamashita N, Obara M, Araki T, Nakamura F, Kamiya Y, Alkam T, Nitta A, Nabeshima T, Mikoshiba K, et al. Amyloid-beta 25-35 induces impairment of cognitive function and long-term potentiation through phosphorylation of collapsin response mediator protein 2. Neurosci Res 2013; 77:180-5; PMID:23994236; http://dx.doi.org/10.1016/2.neures.2013.08.005
- Good PF, Alapat D, Hsu A, Chu C, Perl D, Wen X, Burstein DE, Kohtz DS. A role for semaphorin 3A signaling in the degeneration of hippocampal neurons during Alzheimer's disease. J Neurochem 2004; 91:716-36; PMID:15485501; http://dx.doi.org/10.1111/2.1471-4159.2004.02766.x
- Hefti F, Weiner WJ. Nerve growth factor and Alzheimer's disease. Ann Neurol 1986; 20:275-81; PMID:3532929; http://dx.doi.org/10.1002/2na.410200302
- Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL. Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron 1995; 14:949-59; PMID:7748562; http://dx.doi.org/10.1016/2896-6273(95)90333-X
- Campbell DS, Regan AG, Lopez JS, Tannahill D, Harris WA, Holt CE. Semaphorin 3A elicits stage- collapse, turning, and branching in Xenopus retinal growth cones. J Neurosci 2001; 21:8538-47; PMID:11606642
- Higurashi M, Iketani M, Takei K, Yamashita N, Aoki R, Kawahara N, Goshima Y. Localized role of CRMP1 and CRMP2 in neurite outgrowth and growth cone steering. Dev Neurobiol 2012; 72:1528-40; PMID:22378692; http://dx.doi.org/10.1002/2neu.22017
