ABSTRACT
The low-density lipoprotein receptor-related protein-1 (LRP-1) is a member of Low Density Lipoprotein Receptor (LDLR) family, which is ubiquitously expressed and which is described as a multifunctional endocytic receptor which mediates the clearance of various extracellular matrix molecules including serine proteinases, proteinase-inhibitor complexes, and matricellular proteins. Several studies showed that high LRP-1 expression promotes breast cancer cell invasiveness, and LRP-1 invalidation leads to cell motility abrogation in both tumor and non-tumor cells. Furthermore, our group has reported that LRP-1 silencing prevents the invasion of a follicular thyroid carcinoma despite increased pericellular proteolytic activities from MMP2 and uPA using a 2D-cell culture model. As the use of 3D culture systems is becoming more and more popular due to their promise as enhanced models of tissue physiology, the aim of the present work is to characterize for the first time how the 3D collagen type I matrix may impact the ability of LRP-1 to regulate the migratory properties of thyroid carcinoma using as a model FTC-133 cells. Our results show that inhibition of LRP-1 activity or expression leads to morphological changes affecting cell-matrix interactions, reorganizations of the actin-cytoskeleton especially by inhibiting FAK activation and increasing RhoA activity and MLC-2 phosphorylation, thus preventing cell migration. Taken together, our results suggest that LRP-1 silencing leads to a decrease of cell migratory capacity in a 3D configuration.
Introduction
The low-density lipoprotein receptor-related protein-1 (LRP-1) is a member of low density lipoprotein receptor (LDLR) family, which is ubiquitously expressed in a variety of organs including adipose tissue, liver and brain.Citation8 It is composed of a 515 kDa extracellular ligand-binding subunit (α-chain) that contains 4 clusters of ligand binding domains linked non-covalently to a 85 kDa transmembrane subunit encompassing a short cytoplasmic tail (β-chain).Citation14,26 Initially, LRP-1 was identified as a receptor playing an active role in the lipid metabolism, and then as a receptor of activated α2-macroglobulin.Citation53 Subsequent work has revealed that this receptor is a large endocytic receptor mediating the clearance of various extracellular matrix molecules including serine proteinases, proteinase-inhibitor complexes, and matricellular proteins such as thrombospondins.Citation21,26,57 Firstly considered to act only as a scavenger receptor, numerous evidence indicate that LRP-1 may mediate intracellular signalingCitation4,28,54 and modulate the activity of other transmembrane receptors such as tyrosine kinase receptors and integrins.Citation5 Although several works have described its involvement in tumorigenesis, the precise role of LRP-1 remains controversial and the potential underlying mechanisms need to be clarified. Several studies have reported that low expression of LRP-1 is closely related to aggressiveness of tumor cells derived from various tissues such as human endometrial carcinoma,Citation16 thyroid cancer,Citation51 breast and prostate cancer.Citation31 However, other studies showed that high LRP-1 expression promotes breast cancer cell invasiveness, and LRP-1 neutralization leads to cell motility abrogation in both tumor and non-tumor cells.Citation52 Furthermore, our group has reported that LRP-1 silencing prevents the invasion of a follicular thyroid carcinoma despite increased pericellular proteolytic activities from MMP2 and uPA.Citation12 In this previous work, we established that LRP-1 may coordinate the adhesion and de-adhesion balance of malignant cells to support tumor progression by regulating the amount and distribution of focal contact proteins, such as FAK, paxillin and talin, and thus by contributing to the control of integrin activation and adhesion complex turnover.Citation11 LRP-1 has also been shown to regulate the internalization of CD44 in follicular thyroid carcinoma, a receptor known to control the adhesion dynamics of cancer cells.Citation45 Moreover, in schwann cells, LRP-1 expression has been shown to regulate their migration by inhibiting RhoA and activating Rac1.Citation38 Taken together, these results demonstrate that the role of LRP-1 in tumor cell migration and invasion is highly complex and likely depends on the tumor cell type and the composition and arrangement of the microenvironment. Previous studies indeed demonstrated that the extracellular matrix composition and organization are crucial to determine the endocytic and signaling functions of LRP-1.Citation55,58
One of the limitations of in vitro studies is that they were performed on conventional tissue culture substrate, a situation that does not take into account the tumor cell microenvironment in which tumor cells exert in vivo complex molecular and cellular interactions especially through the extracellular matrix (ECM). Tumor cells exist within a dynamic tridimensional (3D) matrix which is not completely reproduced by standard in vitro cell culture techniques. Several recent works showed clearly that cells can sense chemical composition of the ECM and its physical properties such as dimensionality, stiffness, and architecture.Citation13 Bidimensional (2D) plastic or ECM coating and 3D matrices, as scaffolds for cell growth, supply the cell with very different biochemical and mechanical environments. In 2D conditions cell surface interact with a solid substrate or ECM coating via their basal surface. However, cells growing in 3D matrix exhibit adapted cell-surface mechano-transducers and ECM adhesion proteins.Citation10,25 Setting in culture of cells in 2D versus 3D platforms results in significant modifications in cell growth,Citation6,22 migration,Citation3,39,46 morphologyCitation2,62 and gene expression.Citation22,36 The use of in vitro 3D collagen matrix to mimic in vivo cellular environment becomes increasingly popular and improves our understanding of cell growth, survival, migration, and cell-ECM interactions that may occur in vivo under physiological and pathological conditions.Citation13
Several studies have thus reported a positive contribution of LRP-1 to migration and invasion events in various cell types,Citation7,8,37 including malignant tumor cells.Citation12,15 In the present study, we characterized for the first time how the 3D collagen type I matrix may impact the ability of LRP-1 to regulate the migratory properties of thyroid carcinoma.
Results
LRP-1 supports thyroid carcinoma cell migration in 3D collagen matrix
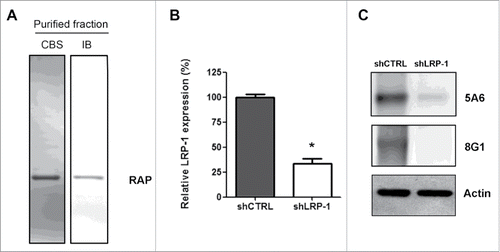
To demonstrate the involvement of LRP-1 in tumor cell migration in a 3D environment, 2 different strategies were used: treatment with RAP (Receptor Associated Protein) to inhibit LRP-1 activityCitation60 and LRP-1 silencing. The recombinant protein RAP was purified on a nickel-agarose column and controlled after SDS-PAGE by Coomassie blue staining and immunoblotting (). LRP-1 silencing was conducted by using previously validated short interfering sequence.Citation12 A clonal cell line that stably overexpresses a specific short hairpin RNA for LRP-1 (shLRP-1) was selected, and a control cell line was established after transfection with pSuppressorNeo carrying a non-silencing sequence (shCTRL). The expression of LRP-1 was analyzed by both real-time PCR () and western-blotting (). Transfection with shCTRL had no effect on the LRP-1 expression level compared to wild type cells (data not shown).Citation12 At the opposite, stable transfection of FTC-133 cells by the LRP-1-specific shRNA plasmid resulted in a 70 % down regulation of the expression of LRP-1 at the mRNA and the protein levels ().
Figure 1. Validation of LRP-1-silencing efficiency and purification of human recombinant RAP. (A) Purified recombinant RAP was assessed by SDS-PAGE followed by CBS and immunoblotting using anti-RAP antibody (IB). (B) Total RNAs were purified from FTC-133 cells transfected with non-silencing shRNA (shCTRL) or shRNA targeting LRP-1 (shLRP-1). The transcriptional level of LRP-1 was assessed by reverse transcription followed by a real-time PCR. β-actin primers were used as a normalization control. Graph represents the relative LRP-1 expression in percent. *, P< 0.05 (C) Whole-cell extracts from each clonal cell were subjected to immunoblot analysis under non-reducing conditions with anti-LRP-1 α-chain (8G1) and β-chain (5A6) antibodies. β-actin antibody was used for normalization.
We first investigated whether shRNA-mediated inhibition of LRP-1 expression altered migration of FTC-133 cells into 3-D collagen gels. The migration speed of individual cells cultured within collagen type I matrix has been determined by quantifying their migration speed and 3D trajectories using computer-assisted videomicroscopy. To this end, FTC-133 cells were seeded into a type-I collagen matrix and individual cells were examined for their migrating potential during the first 6 h () or after 18h (). From one hand, migration of shLRP-1 overexpressing cells was diminished 2.8 fold after 18h whereas there is no modification of the migration rate during the six first hours of culture. On the other hand, RAP treatment decreased cell migration speed of FTC-133 cells by 2 fold regardless of the observed migration times. Indeed, the length of trajectory of FTC-133 cells is more than twice as high as the length of trajectory of RAP-treated cells during the six first hours of culture and after 18h (). Furthermore, the distance traveled by LRP-1-silenced cells is 2.9 times lower than for control cells between 18 h and 24 h.
Figure 2. Thyroid carcinoma cell migration on 3D collagen matrix is drastically altered by LRP-1 blockade or silencing. FTC-133 cells were cultured for 24 h within 3D collagen I matrix, and then were tracked by time-lapse videomicroscopy. (A) Cell migration speed (µm/h) was assessed during the first 6 h and the last 6 h of culture and data represent the average of 3 independent experiments (at least 25 cells were tracked per experiment). Error bars represent SEM. ***, P < 0.001 significant difference. (B) Distance of migration covered by cells during the first 6 h and the last 6 h. Data represent the average of 3 independent experiments (at least 25 cells tracked per experiment). (C) Quantitative analysis of 3D FTC-133 cell trajectories during the last 6 h of culture. Each color corresponds to a distinct cell.
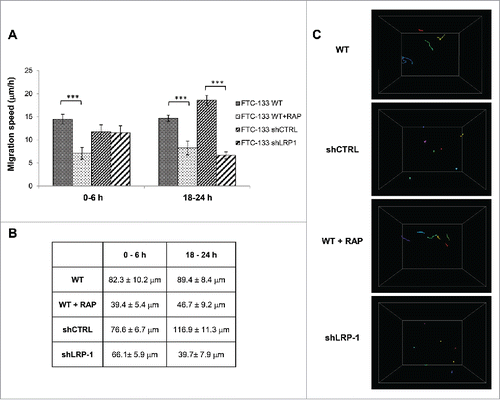
Using live-cell microscopy, we measured the individual cell trajectory length inside collagen matrix. Migration of the cells embedded in the collagen was evaluated by tracing cell movements projected into the x–y-z plane. Trajectories of cells expressing or not LRP-1 revealed significant distinct patterns (). Wild-type FTC-133 actively migrated through the collagen matrix whereas LRP-1-silenced cells exhibited circular trajectories around their starting point. Similar data were obtained under RAP treatment and are together in accordance with the inhibition of cell migration speed and observed traveled distance in .
LRP-1 promotes cell elongation in 3D collagen matrix
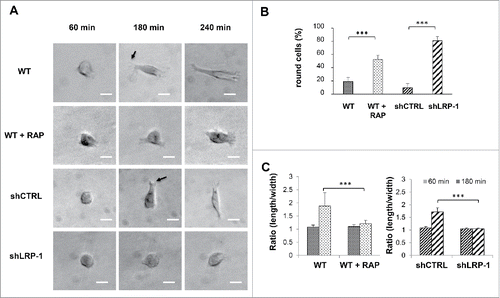
To determine whether cell spreading could be LRP-1 dependent, wild-type FTC-133 cells treated or not with RAP, and both control and LRP-1-silenced carcinoma cells were seeded inside collagen matrix for different time periods before analysis of spreading (). After 60 min, wild-type and control cells were spread and begun to exhibit thin projections as shown by arrows in . Several distinct protrusions and filopodiae consistent with migratory activity were detected after 240 min in control cells. In striking contrast, LRP-1-silenced cells displayed overspread morphology with membrane ruffles and failed to extend filopodiae. To support this observation, the number of cells exhibiting a rounded form was quantified. As expected, the number of rounded cells was increased by 2.7 and 8.1-fold under RAP treatment and LRP-1 silencing, respectively (). Additionally, quantitative analysis by measurement of length-width ratio confirmed that cell elongation was reduced by about 40% under RAP treatment or LRP-1-silencing, as shown in . Altogether, these results demonstrate that unlike fast-invading carcinoma control cells, inhibition of LRP-1 activity or expression leads to major morphological changes affecting cell-matrix interactions.
Figure 3. LRP-1 blockade or silencing impacts the FTC-133 carcinoma cell morphology on 3D collagen matrix. (A) Cells were seeded within 3D type I collagen matrix. Typical morphology of cells cultured within 3D matrix was obtained by phase contrast microscopy. Arrows show examples of nascent thin projections. Bar: 100 μm. (B) Percentage of round cell was calculated by using 30 isolated cells (180 min after seeding) for each condition in 3 independent experiments. ***, P < 0.001. (C) Ratio (length/width) was calculated using Image J software by using 20 isolated cells (60 min and 180 min after seeding) for each condition in 3 independent experiments. ***, P < 0.001.
LRP-1 induces FAK activation and MLC-2 inhibition in 3D collagen matrix
Focal adhesion kinase (FAK) is a cytoplasmic tyrosine kinase which is widely expressed and located in integrin-mediated focal adhesions that regulates integrin signaling. FAK can be rapidly activated by a variety of mechanical stimuli and plays an important role in control of cell adhesion and migration making it a major mechanosensitive kinase.Citation41,44 Previous results obtained by Dedieu et al. (2008) established that LRP-1 is required for FAK association with focal contacts and reported that FAK activation is reduced in cells lacking LRP-1.Citation12 To determine whether FAK activation could be differentially regulated by LRP-1 in 3D collagen environment, we analyzed FAK phosphorylation on tyrosine 397 (p397-FAK) in control cells, RAP-treated cells or LRP-1 silenced cells seeded inside collagen matrix (). Western-blotting of cell lysates demonstrated 36% and 84% decrease in p397-FAK expression when cells were treated with RAP for 3 h and 24 h, respectively. FAK phosphorylation was similarly decreased in LRP-1-deficient cells by 28% and 40% after 3 h and 24 h after seeding, respectively.
Figure 4. FAK and MLC-2 Phosphorylation and Rho activation are LRP-1 dependent in 3D matrix. Wild-type FTC-133 (treated with or without RAP) and clonal cells (shCTRL and shLRP-1) were seeded in 3D collagen matrix for 3 h and 24 h. Whole-cell extracts were subjected to Western-blot analysis to evaluate the level of FAK activation (A) and the phosphorylation of MLC-2 (B). The respective phosphorylation rates of FAK (A) and MLC-2 (B) were determined as intensity ratios of phospho-protein to corresponding pan-protein and expressed in relative units +/− SD, with a value of 100% ascribed to wild-type cells (right panels). GAPDH antibody was used as a loading control. (C) Cell lysates were incubated with GST-RBD beads. The bound RhoA was detected with a monoclonal anti-RhoA antibody (Top). The relative amount of total RhoA in the cell lysates were assessed by using a monoclonal antibody against RhoA (Bottom). Quantitative analysis of the GTP-RhoA associated with RBD beads was obtained by densitometry. The amount of RhoA bound to RBD was normalized to the RhoA content of cell extracts. *, P<0.05; ***, P < 0.001.
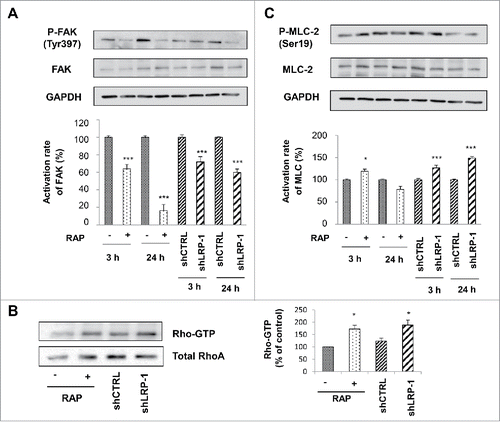
Mesenchymal-type movement in 3D matrix is known to be driven by GTPase Rac pathway which activates WASP-family verprolin-homologous protein 2 (WAVE2) to shift cell movement from amoeboid to mesenchymal form through decreasing actinomyosin contractility and inhibiting the RhoA GTPase.Citation49 To address this point, we assessed the impact of silencing LRP-1 expression or RAP treatment in FTC-133 cells on RhoA activation in cells seeded in 3D collagen matrix (). Interestingly, both RAP treatment and down-regulation of LRP-1 expression induce an increase of the formation of active GTP-bound RhoA by 72% and 88%, respectively and as compared to corresponding controls. One of the major downstream effector of RhoA is the serine/threonine RhoA-associated kinase ROCK which phosphorylates MLC-2 at serine 19.Citation23 To confirm whether RhoA is inhibited by LRP-1 in our experimental model, we analyzed phosphorylated MLC-2 level in control cells, RAP-treated cells or LRP-1 silenced cells seeded in 3D collagen matrix. Whereas RAP treatment appears to moderately and transiently influence the phosphorylation of MLC-2, LRP-1 silencing leads to an increase in the amount of phosphorylated-MLC-2 by 27% and 49% after 3 h and 24 h incubation respectively ().
Altogether, our results clearly indicate that LRP-1 silencing or inhibition of LRP-1 mediated-endocytosis induce reorganizations of the actin-cytoskeleton especially by inhibiting FAK activation and increasing MLC-2 phosphorylation, thus preventing cell migration in a 3D environment.
Discussion
Several works have shown recently that cells can sense chemical composition of the extracellular matrix and its physical properties such as dimensionality, stiffness, and architecture. The use of in vitro 3D collagen matrix to mimic in vivo cellular environments becomes increasingly popular and improves our understanding of cell growth, survival, migration, and cell-ECM interactions that occur in vivo under physiologically normal and diseased conditions.Citation9 For this reason, we proposed to study the impact of extracellular matrix on the role of LRP-1 in FTC-133 cells by using a complex cellular system such as 3D culture systems. Our data show the pro-migratory effect of LRP-1 receptor in 3D culture. In fact, inhibition of the LRP-1 function or its silencing induce a decrease in cell migration speed by suppressing cell protrusion and by regulating FAK and MLC-2 phosphorylation. The discrepancies in results observed with RAP treatment and shLRP-1 transfected cells could be explained by the 2 methods used to invalidate LRP-1. Indeed, a treatment by RAP does not modify LRP-1 expression in cell membrane, endocytosis kinetic and intracellular signaling pathways associated with this last mechanism, whereas downregulation of LRP-1 by shLRP-1 induces a strong decrease of LRP-1 expression at the cell membrane and then subsequent modifications of the membrane proteome (including sometimes middle or long-term changes), concomitantly with drastic modifications of intracellular signaling associated with LRP-1 co-receptors). To our knowledge, this is the first study to show that LRP-1 silencing leads to a decrease of cell migratory capacity in a 3D configuration.
Cell migration is dependent of physical and extracellular modulators which include: confinement, determined by pore-size through which the cell migrates; geometry defined by ECM alignment and dimensionality (2D vs. 3D); and stiffness depending on the composition, flexibility, density and cross-link status of ECM components.Citation32,33,61 To mimic in vivo microenvironment, we proposed to use a representative model which is 3D gel of type I collagen. Type I collagen is the most abundant collagen of extracellular matrix in the human body and represents a pre-intravasation microenvironment.Citation50 Previous data from our groupCitation17,40 have shown that the 3D collagen gel prepared here at physiological concentrationCitation47 is close to the 3D fibrous nature of a mesenchymal stroma because of a highly fibrillar organization. Furthermore, our native fibrillar collagen is prepared without pepsinization and presents intact telopeptides, which correspond to the flanking regions of the molecules and permit to form intra- and intermolecular cross-links that promote the staggering and the resilience of fibrillar collagen.Citation29
Morphology of FTC-133 carcinomas cultured in 3D matrix appears different from that of cells cultured on rigid 2D substrate. Indeed, FTC-133 cells on plastic or substrate-coated support were broader and more flattened and exhibit most of the time prominent lamellae and retraction fibers at the rear of the cell.Citation45 In collagen 3D matrix, wild-type FTC-133 cells displayed a spindle-shaped, elongated, or stellate phenotype with thinner and longer protrusions and dendritic extensions at the migration front, which is rather in favor of a mesenchymal migration mode.Citation56 LRP-1 silencing or RAP treatment induce morphological changes, especially by maintaining carcinoma cells in a rounded morphology. The cells are then characterized by an increase in circularity, a decrease in elongation and a higher fraction of rounded cells. Interestingly, a similar phenotype was obtained in 2D culture as LRP-1-silenced cells displayed overspread morphology and failed to extend membrane extension onto gelatin.Citation12 Thus, LRP-1 expression in tumor cells may be, at least in part, responsible for mesenchymal phenotype acquisition.
This change in shape could arise from the decrease of cell/matrix adhesion and could suggest that along with loss of traction the amoeboid-like cells may have greater difficulty to migrate in absence of LRP-1 and thus results in a decrease in the cell migration speed. Indeed, previous results obtained in 2D have shown that LRP-1 may coordinate the adhesion-deadhesion balance in malignant cells to support tumor progression.Citation11 We have previously provided molecular knowledge on LRP-1 roles in multiple cancer-related events. Indeed, LRP-1 silencing prevents thyroid carcinoma cell invasiveness despite a major increase in pericellular proteolytic activities. This suggests that LRP-1 promotes cell invasion by subtly controlling the adhesive properties and the actin network structure. In addition, LRP-1 promotes cell invasion through ERK and JNK MAPK pathways to regulate focal adhesion disassembly to support invasion.Citation11,34,45 By controlling focal adhesions components and the turn-over of the cytoskeleton organization proteins and integrins, Dedieu and collaborators described LRP-1 as a crucial actor of the epithelial-mesenchymal transition.Citation12 In addition, signaling induced by urokinase-type plasminogen activator (uPA)-urokinase receptor (uPAR) is another migration- and invasion-related pathway regulated by LRP-1 that can enhance cell invasion and migration.Citation1,19,59 Furthermore, Hu et al. have described in a previous work that LRP-1 is involved in the β1-integrin recruitment and the subsequent activation of the β1-integrin-linked kinase (ILK).Citation27 The focal adhesion protein kinase, ILK, is known to consolidate the integrin/cytoskeleton connections., LRP-1 could play a key role in regulating the actin polymerization and cytoskeleton dynamics during retraction of migrating cells by controlling ILK activity.Citation63 Furthermore, in rounded amoeboid movement, cells move with high levels of actomyosin contractility driven by Rho-Rho kinase (ROCK) signaling.Citation48,49 ROCK decreases myosin phosphatase activity, increasing phosphorylation of MLC-2 and activity of myosin II,Citation30 as suggested by our data.
Although most studies have suggested that rounded-amoeboid cancer cells do not require MMPs to invade, a recent work demonstrated that rounded amoeboid melanoma cells secrete higher levels of several MMPs, including collagenase MMP-13 and gelatinase MMP-9 to invade through type I collagen.Citation42 Moreover, Orgaz and collaborators showed that MMP-9 promotes rounded-amoeboid 3D migration using a non-catalytic mechanism through regulation of actomyosin contractility via CD44 receptor Citation42 LRP-1 was previously reported to bind with high affinity to MMP-9 and to mediate its cellular catabolism.Citation24 Besides, both LRP-1 silencing and RAP treatment led to accumulation of CD44 at the tumor cell surface in our thyroid carcinoma environment.Citation45 We therefore believe that the residual migratory activity observed in our study under LRP-1 silencing or blocking, could be attributed to amoeboid movement.
Materials and methods
Cell line
The human FTC-133 cell line was derived from a lymph node metastasis of a follicular thyroid carcinoma.Citation20 This cell line was obtained from the European Collection of Cell Cultures, ECACC. Cells were cultured in Dulbecco's Modified Eagle Medium-Ham's F12 (DMEM:F12, Invitrogen, Cergy Pontoise, France) supplemented with 10% fetal bovine serum (FBS) and was maintained at 37˚C in a humidified 5% CO2 atmosphere. Generation of 2 clonal cell lines that stably overexpress a non-silencing sequence (shCTRL) or a specific short hairpin RNA (shRNA) for LRP-1 (shLRP-1) was described previously.Citation11 Cells were routinely passaged at preconfluency until 15 subcultures using 0.05% trypsin, 0.53 mM EDTA (Invitrogen) and screened for the presence of mycoplasma using PCR methods.
Purification of recombinant RAP
Histidine-tagged RAP was expressed in Escherichia coli BL21 pLysS (Promega, Charbonnières-les-Bains, France) using the pT7H6FX-RAP construct kindly provided by M. S. Nielsen (Department of Medical Biochemistry, University of Aarhus, Denmark). Purification of recombinant RAP was performed by gravity-flow chromatography using a nickel-charged resin (Ni-nitrilotriacetic acid [NTA]- agarose from Qiagen, Courtaboeuf, France) and then controlled after SDS-PAGE by Coomassie blue staining (CBS) and immunoblotting. The LRP-1 binding capacity was confirmed using a BIAcore X system (BIAcore AB, Uppsala, Sweden), as previously reported (Selvais et al., 2009)
3D culture
Acid-extracted, non-pepsinized collagen type I from rat tail tendons was prepared as described by Garnotel et al.Citation18 Lyophilized collagen was dissolved in a sterile 18 mM acetic acid solution at a concentration of 3 mg/ml.
For 3D cell culture, 5 × 104 FTC-133 cells were resuspended in 100 μl fetal bovine serum and mixed with a solution containing 100 μl DMEM:F12 10X, 100 μl NaHCO3 0.26 M, 100 μl H2O, 90 μl NaOH 0.1 M, 10 μl glutamine 200 mM and 500 μl collagen 3 mg/ml. This solution was deposited in 24-well plates (1 ml/well). After polymerization at 37°C during 10 min, gels were recovered by 1 ml DMEM:F12 10% foetal bovine serum (FBS) and 3D cultures were maintained at 37˚C in a humidified 5% CO2 atmosphere. For RAP treatment, RAP was added at 500 nM both in the culture medium and in the collagen gel
3D cell migration quantification and cell morphology
3D cultures, prepared as described above, were seeded in 24-well plates (1 ml/well). Cell motility was analyzed by time-lapse videomicroscopy using an inverted microscope Axiovert 200 M (Zeiss, Le Pecq, France) equipped with a small transparent environmental chamber Climabox (Zeiss) with 5% (v/v) CO2 in air at 37°C. The microscope was driven by the Metamorph software (Roper Scientific, Evry, France) and images were recorded with a charge-coupled device camera CoolsnapHQ (Roper scientific). Cell speed was quantified (μm/h) for each individual non-mitotic cell using an interactive tracking method as previously described.Citation40 The length/width ratio was calculated using ImageJ, as follows: length was scored as the longest distance between any 2 points of the cells; width was scored as the secondary axis of the best fit ellipse of the circled cells. This factor estimates the degree of variance from a circle. A value of 1.00 is a perfect circle.Citation43
RNA isolation, reverse transcription, and real-time PCR
Total RNAs were isolated and purified with an Extract-All kit (Eurobio Laboratories, Courtaboeuf, France). Reverse transcription (RT) and real-time PCR were performed with Verso SYBR 2-Step QRT Rox kit (AB-4113/A) and Absolute QPCR SYBR green Rox (AB-1162/B), respectively (Thermo Fisher Scientific). Quantitative PCR was carried out on a Chromo4 real-time detector (Bio-Rad Laboratories, Marne-la-Vallée, France), and β-actin was used for normalization. Primers for LRP-1Citation12 and β-actinCitation34 were previously described. All primers were synthesized by Eurogentec France (Angers, France). Results shown are representative of 3 independent experiments.
Expression and purification of recombinant proteins in bacteria
GST-tagged fusion proteins of the Rho-binding domain (RBD) of Rhotekin were expressed using the bacterial expression vector pGEX4T1 in the BL21DE3 strain of Escherichia coli and purified. We thank Dr. Dario Diviani (Department of Pharmacology and Toxicology, Lausanne, Switzerland) for the gift of the bacterial expression vector pGEX4T1. To induce Expression of fusion proteins was induced with 1 mM IPTG in exponentially growing bacterial cultures incubated 24 h at 16°C. After centrifugation. bacteria were lysed in buffer A (20 mM Tris, pH 7.4/100 mM NaCl/5 mM MgCl2/1% (wt/vol) Triton X-100/1 mM benzamidine/2 mg/ml leupeptin/2 mg/ml pepstatin), sonicated, and centrifuged at 38,000 ´ g for 30 min at 4°C. Supernatants were then incubated with glutathione Sepharose beads (Pharmacia) for 2 h at 4°C, the resin was washed 5 times with 10 bed volumes of buffer A and used immediately for the Rhotekin RBD pulldown assay.
Rhotekin Rho-binding domain (RBD) pulldown assay
FTC-133 cells were grown for 3 h in 3D culture. Gels were then digested by collagenase P at 1 mg/ml. After centrifugation, cells were lysed in Rho-binding domain (RBD) lysis buffer (50 mM Tris pH 7.2, 150 mM NaCl, 1% (w/v) Triton X-100, 30 mM MgCl2, 1 mM DTT, 10% glycerol, 1 mM benzamidine, 10 μg/ml leupeptin, 10 μg/ml aprotinin and 1 mM PMSF). After centrifugation at 38 000 g for 10 min at 4°C, lysates were incubated with 30 μg of RDB beads for 1 h at 4°C. Beads were then washed 3 times with RBD buffer without sodium deoxycholate, resuspended in SDS sample buffer and analyzed by SDS–PAGE.
Western-blotting
FTC-133 cells were grown for 3 h or 24 h in 3D culture. Gels were digested by collagenase P at 1 mg/ml. After centrifugation, cells were harvested in lysis buffer (50 mM Tris pH 7.2, 150 mM NaCl, 1% (w/v) Triton X-100, 30 mM MgCl2, 1 mM DTT, 10% glycerol, 1 mM PMSF supplemented with proteinase inhibitor cocktail from Sigma-Aldrich). Cell lysates were cleared by centrifugation at 21,000 x g for 10 min at 4 C. Proteins in total lysates were assayed before SDS-PAGE using the the Bradford method (Bio-Rad Laboratories, Marnes-la-Coquette, France). Protein aliquots were applied to a 10% SDS-PAGE. After transfer onto nitrocellulose membrane (Whatman GmbH, Dassel, Germany), blots were blocked overnight with 5% non-fat dry milk in Tris-buffered saline, 0.1% Tween 20 and then incubated overnight at 4°C with primary antibody: mouse monoclonal anti-LRP-1 α-chain (clone 8G1, 1:1000, Merck Biosciences), mouse monoclonal anti-LRP-1 β-chain (clone 5A6, 1:1000, Merck Biosciences), mouse monoclonal anti-RAP (clone 7F1, Merck), rabbit polyclonal anti-MLC-2 (Myosin Light Chain 2, 1:1000, Cell Signaling), rabbit polyclonal anti-(S19) phosphorylated-MLC-2 (1:1000, Cell signaling), mouse monoclonal antibodies raised against total FAK (1:5000, Millipore) or (Y397) phosphorylated-FAK (1:1000, Millipore), mouse monoclonal anti-RhoA (1:250, Santa Cruz), rabbit polyclonal anti-GAPDH (1:1000, Cell signaling). The membranes were then washed and incubated with secondary antibody for 1 h: Horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibodies (1:10000, Cell Signaling Technology). Chemiluminescent detection was conducted using an ECL plus chemiluminescence kit from Amersham Biosciences. β-actin antibodies or GAPDH antibodies were used to ensure equal loading of the protein samples and for normalization. Immunoblots presented are representative of at least 3 separate experiments.
Statistical analysis
Results are expressed as means ± SD. The significance of differences between 2 groups was determined using Student's t-test. Statistical significance was set at p < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors acknowledge the technical assistance of Cathy Hachet and Laurence Van Gulick.
Funding
This work was supported by funding from the CNRS and by Université de Reims Champagne Ardenne.
References
- Amos S, Mut M, diPierro CG, Carpenter JE, Xiao A, Kohutek ZA, Redpath GT, Zhao Y, Wang J, Shaffrey ME, Hussaini IM. Protein kinase C-α-mediated regulation of low-density lipoprotein receptor related protein and urokinase increases astrocytoma invasion. Cancer Res 2007; 67:10241-51; PMID:17974965; https://doi.org/10.1158/0008-5472.CAN-07-0030
- Baker EL, Bonnecaze RT, Zaman MH. Extracellular matrix stiffness and architecture govern intracellular rheology in cancer. Biophys J 2009; 97:1013-21; PMID:19686648; https://doi.org/10.1016/j.bpj.2009.05.054
- Baker EL, Srivastava J, Yu D, Bonnecaze RT, Zaman MH. Cancer cell migration: integrated roles of matrix mechanics and transforming potential. PLoS One 2011; 6:e20355; PMID:21647371; https://doi.org/10.1371/journal.pone.0020355
- Barnes H, Ackermann EJ, van der Geer P. v-Src induces Shc binding to tyrosine 63 in the cytoplasmic domain of the LDL receptor-related protein 1. Oncogene 2003; 22:3589-97; PMID:12789267; https://doi.org/10.1038/sj.onc.1206504
- Barnes H, Larsen B, Tyers M, van Der Geer P. Tyrosine-phosphorylated low density lipoprotein receptor-related protein 1 (Lrp1) associates with the adaptor protein SHC in SRC-transformed cells. J Biol Chem 2001; 276:19119-25; PMID:11259429; https://doi.org/10.1074/jbc.M101216200
- Barralet JE, Wang L, Lawson M, Triffitt JT, Cooper PR, Shelton RM. Comparison of bone marrow cell growth on 2D and 3D alginate hydrogels. J Mater Sci Mater Med 2005; 16:515-9; PMID:15928866; https://doi.org/10.1007/s10856-005-0526-z
- Cao C, Lawrence DA, Li Y, Von Arnim CA, Herz J, Su EJ, Makarova A, Hyman BT, Strickland DK, Zhang L. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J 2006; 25:1860-70; PMID:16601674; https://doi.org/10.1038/sj.emboj.7601082
- Cheng CF, Fan J, Fedesco M, Guan S, Li Y, Bandyopadhyay B, Bright AM, Yerushalmi D, Liang M, Chen M, et al. Transforming growth factor α (TGFalpha)-stimulated secretion of HSP90alpha: using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Mol Cell Biol 2008; 28:3344-58; PMID:18332123; https://doi.org/10.1128/MCB.01287-07
- Cukierman E, Bassi DE. Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. Semin Cancer Biol 2010; 20:139-45; PMID:20452434; https://doi.org/10.1016/j.semcancer.2010.04.004
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science 2001; 294:1708-12; PMID:11721053; https://doi.org/10.1126/science.1064829
- Dedieu S, Langlois B. LRP-1: a new modulator of cytoskeleton dynamics and adhesive complex turnover in cancer cells. Cell Adh Migr 2008; 2:77-80; PMID:19271352; https://doi.org/10.4161/cam.2.2.6374
- Dedieu S, Langlois B, Devy J, Sid B, Henriet P, Sartelet H, Bellon G, Emonard H, Martiny L. LRP-1 silencing prevents malignant cell invasion despite increased pericellular proteolytic activities. Mol Cell Biol 2008; 28:2980-95; PMID:18316405; https://doi.org/10.1128/MCB.02238-07
- Doyle AD, Yamada KM. Mechanosensing via cell-matrix adhesions in 3D microenvironments. Exp Cell Res 2015; 343(1):60-6; PMID:26524505; https://doi.org/10.1016/j.yexcr.2015.10.033
- Emonard H, Theret L, Bennasroune AH, Dedieu S. Regulation of LRP-1 expression: make the point. Pathol Biol (Paris) 2014; 62:84-90; PMID:24661974; https://doi.org/10.1016/j.patbio.2014.02.002
- Fayard B, Bianchi F, Dey J, Moreno E, Djaffer S, Hynes NE, Monard D. The serine protease inhibitor protease nexin-1 controls mammary cancer metastasis through LRP-1-mediated MMP-9 expression. Cancer Res 2009; 69:5690-8; PMID:19584287; https://doi.org/10.1158/0008-5472.CAN-08-4573
- Foca C, Moses EK, Quinn MA, Rice GE. Differential expression of the α(2)-macroglobulin receptor and the receptor associated protein in normal human endometrium and endometrial carcinoma. Mol Hum Reprod 2000; 6:921-7; PMID:11006321; https://doi.org/10.1093/molehr/6.10.921
- Fourre N, Millot JM, Garnotel R, Jeannesson P. In situ analysis of doxorubicin uptake and cytotoxicity in a 3D culture model of human HT-1080 fibrosarcoma cells. Anticancer Res 2006; 26:4623-6; PMID:17201187
- Garnotel R, Rittie L, Poitevin S, Monboisse JC, Nguyen P, Potron G, Maquart FX, Randoux A, Gillery P. Human blood monocytes interact with type I collagen through α x β 2 integrin (CD11c-CD18, gp150–95). J Immunol 2000; 164:5928-34; PMID:10820275; https://doi.org/10.4049/jimmunol.164.11.5928
- Gonias SL, Gaultier A, Jo M. Regulation of the urokinase receptor (uPAR) by LDL receptor-related protein-1 (LRP1). Curr Pharm Des 2011; 17:1962-9; PMID:21711236; https://doi.org/10.2174/138161211796718224
- Goretzki PE, Frilling A, Simon D, Roeher HD. Growth regulation of normal thyroids and thyroid tumors in man. Recent Results Cancer Res 1990; 118:48-63; PMID:2173080; https://doi.org/10.1007/978-3-642-83816-3_6
- Greenaway J, Lawler J, Moorehead R, Bornstein P, Lamarre J, Petrik J. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1). J Cell Physiol 2007; 210:807-18; PMID:17154366; https://doi.org/10.1002/jcp.20904
- Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol 2003; 13:264-9; PMID:12742170; https://doi.org/10.1016/S0962-8924(03)00057-6
- Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol 2011; 21:718-26; PMID:21924908; https://doi.org/10.1016/j.tcb.2011.08.002
- Hahn-Dantona E, Ruiz JF, Bornstein P, Strickland DK. The low density lipoprotein receptor-related protein modulates levels of matrix metalloproteinase 9 (MMP-9) by mediating its cellular catabolism. J Biol Chem 2001; 276:15498-503; PMID:11279011; https://doi.org/10.1074/jbc.M100121200
- Hakkinen KM, Harunaga JS, Doyle AD, Yamada KM. Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue Eng Part A 2011; 17:713-24; PMID:20929283; https://doi.org/10.1089/ten.tea.2010.0273
- Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest 2001; 108:779-84; PMID:11560943; https://doi.org/10.1172/JCI200113992
- Hu K, Wu C, Mars WM, Liu Y. Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J Clin Invest 2007; 117:3821-32; PMID:18037995; https://doi.org/10.1172/JCI32405
- Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem 2006; 281:2120-7; PMID:16303771; https://doi.org/10.1074/jbc.M504988200
- Imai K, Sato T, Senoo H. Adhesion between cells and extracellular matrix with special reference to hepatic stellate cell adhesion to three-dimensional collagen fibers. Cell Struct Funct 2000; 25:329-36; PMID:11280703
- Ito M, Nakano T, Erdodi F, Hartshorne DJ. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem 2004; 259:197-209; PMID:15124925; https://doi.org/10.1023/B:MCBI.0000021373.14288.00
- Kancha RK, Stearns ME, Hussain MM. Decreased expression of the low density lipoprotein receptor-related protein/α 2-macroglobulin receptor in invasive cell clones derived from human prostate and breast tumor cells. Oncol Res 1994; 6:365-72; PMID:7534510
- Karsdal MA, Nielsen MJ, Sand JM, Henriksen K, Genovese F, Bay-Jensen AC, Smith V, Adamkewicz JI, Christiansen C, Leeming DJ. Extracellular matrix remodeling: the common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev Technol 2013; 11:70-92; PMID:23046407; https://doi.org/10.1089/adt.2012.474
- Kraning-Rush CM, Reinhart-King CA. Controlling matrix stiffness and topography for the study of tumor cell migration. Cell Adh Migr 2012; 6:274-9; PMID:22863740; https://doi.org/10.4161/cam.21076
- Langlois B, Perrot G, Schneider C, Henriet P, Emonard H, Martiny L, Dedieu S. LRP-1 promotes cancer cell invasion by supporting ERK and inhibiting JNK signaling pathways. PLoS One 2010; 5:e11584; PMID:20644732; https://doi.org/10.1371/journal.pone.0011584
- Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev 2008; 88:887-918; PMID:18626063; https://doi.org/10.1152/physrev.00033.2007
- Luca AC, Mersch S, Deenen R, Schmidt S, Messner I, Schafer KL, Baldus SE, Huckenbeck W, Piekorz RP, Knoefel WT, et al. Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PLoS One 2013; 8:e59689; PMID:23555746; https://doi.org/10.1371/journal.pone.0059689
- Mantuano E, Inoue G, Li X, Takahashi K, Gaultier A, Gonias SL, Campana WM. The hemopexin domain of matrix metalloproteinase-9 activates cell signaling and promotes migration of schwann cells by binding to low-density lipoprotein receptor-related protein. J Neurosci 2008; 28:11571-82; PMID:18987193; https://doi.org/10.1523/JNEUROSCI.3053-08.2008
- Mantuano E, Jo M, Gonias SL, Campana WM. Low density lipoprotein receptor-related protein (LRP1) regulates Rac1 and RhoA reciprocally to control Schwann cell adhesion and migration. J Biol Chem 2010; 285:14259-66; PMID:20197276; https://doi.org/10.1074/jbc.M109.085126
- Meyer AS, Hughes-Alford SK, Kay JE, Castillo A, Wells A, Gertler FB, Lauffenburger DA. 2D protrusion but not motility predicts growth factor-induced cancer cell migration in 3D collagen. J Cell Biol 2012; 197:721-9; PMID:22665521; https://doi.org/10.1083/jcb.201201003
- Millerot-Serrurot E, Guilbert M, Fourre N, Witkowski W, Said G, Van Gulick L, Terryn C, Zahm JM, Garnotel R, Jeannesson P. 3D collagen type I matrix inhibits the antimigratory effect of doxorubicin. Cancer Cell Int 2010; 10:26; PMID:20707917; https://doi.org/10.1186/1475-2867-10-26
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 2005; 6:56-68; PMID:15688067; https://doi.org/10.1038/nrm1549
- Orgaz JL, Pandya P, Dalmeida R, Karagiannis P, Sanchez-Laorden B, Viros A, Albrengues, J, Nestle FO, Ridley AJ, Gaggioli C, et al. Diverse matrix metalloproteinase functions regulate cancer amoeboid migration. Nat Commun 2014; 5:4255; PMID:24963846; https://doi.org/10.1038/ncomms5255
- Pankova D, Jobe N, Kratochvilova M, Buccione R, Brabek J, Rosel D. NG2-mediated Rho activation promotes amoeboid invasiveness of cancer cells. Eur J Cell Biol 2012; 91:969-77; PMID:22699001; https://doi.org/10.1016/j.ejcb.2012.05.001
- Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene 2000; 19:5606-13; PMID:11114741; https://doi.org/10.1038/sj.onc.1203877
- Perrot G, Langlois B, Devy J, Jeanne A, Verzeaux L, Almagro S, Sartelet H, Hachet C, Schneider C, Sick E, et al. LRP-1–CD44, a new cell surface complex regulating tumor cell adhesion. Mol Cell Biol 2012; 32:3293-307; PMID:22711991; https://doi.org/10.1128/MCB.00228-12
- Petrie RJ, Doyle AD, Yamada KM. Random vs. directionally persistent cell migration. Nat Rev Mol Cell Biol 2009; 10:538-49; PMID:19603038; https://doi.org/10.1038/nrm2729
- Ramanujan S, Pluen A, McKee TD, Brown EB, Boucher Y, Jain RK. Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys J 2002; 83:1650-60; PMID:12202388; https://doi.org/10.1016/S0006-3495(02)73933-7
- Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol 2003; 5:711-9; PMID:12844144; https://doi.org/10.1038/ncb1019
- Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell 2008; 135:510-23; PMID:18984162; https://doi.org/10.1016/j.cell.2008.09.043
- Serebriiskii I, Castello-Cros R, Lamb A, Golemis EA, Cukierman E. Fibroblast-derived 3D matrix differentially regulates the growth and drug-responsiveness of human cancer cells. Matrix Biol 2008; 27:573-85; PMID:18411046; https://doi.org/10.1016/j.matbio.2008.02.008
- Sid B, Dedieu S, Delorme N, Sartelet H, Rath GM, Bellon G, Martiny L. Human thyroid carcinoma cell invasion is controlled by the low density lipoprotein receptor-related protein-mediated clearance of urokinase plasminogen activator. Int J Biochem Cell Biol 2006; 38:1729-40; PMID:16807059; https://doi.org/10.1016/j.biocel.2006.04.005
- Song H, Li Y, Lee J, Schwartz AL, Bu G. Low-density lipoprotein receptor-related protein 1 promotes cancer cell migration and invasion by inducing the expression of matrix metalloproteinases 2 and 9. Cancer Res 2009; 69:879-86; PMID:19176371; https://doi.org/10.1158/0008-5472.CAN-08-3379
- Strickland DK, Ashcom JD, Williams S, Burgess WH, Migliorini M, Argraves WS. Sequence identity between the α 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem 1990; 265:17401-4; PMID:1698775
- Strickland DK, Ranganathan S. Diverse role of LDL receptor-related protein in the clearance of proteases and in signaling. J Thromb Haemost 2003; 1:1663-70; PMID:12871303; https://doi.org/10.1046/j.1538-7836.2003.00330.x
- Troeberg L, Lazenbatt C, Anower-E-Khuda MF, Freeman C, Federov O, Habuchi H, Habuchi O, Kimata K, Nagase H. Sulfated glycosaminoglycans control the extracellular trafficking and the activity of the metalloprotease inhibitor TIMP-3. Chem Biol 2014; 21:1300-9; PMID:25176127; https://doi.org/10.1016/j.chembiol.2014.07.014
- Van Goethem E, Poincloux R, Gauffre F, Maridonneau-Parini I, Le Cabec V. Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J Immunol 2010; 184:1049-61; PMID:20018633; https://doi.org/10.4049/jimmunol.0902223
- Van Gool B, Dedieu S, Emonard H, Roebroek AJ. The matricellular receptor LRP1 forms an interface for signaling and endocytosis in modulation of the extracellular tumor environment. Front Pharmacol 2015; 6:271; PMID:26617523; https://doi.org/10.3389/fphar.2015.00271
- Wang S, Herndon ME, Ranganathan S, Godyna S, Lawler J, Argraves WS, Liau G. Internalization but not binding of thrombospondin-1 to low density lipoprotein receptor-related protein-1 requires heparan sulfate proteoglycans. J Cell Biochem 2004; 91:766-76; PMID:14991768; https://doi.org/10.1002/jcb.10781
- Webb DJ, Nguyen DH, Gonias SL. Extracellular signal-regulated kinase functions in the urokinase receptor-dependent pathway by which neutralization of low density lipoprotein receptor-related protein promotes fibrosarcoma cell migration and matrigel invasion. J Cell Sci 2000; 113(Pt 1):123-34; PMID:10591631
- Webb DJ, Nguyen DH, Sankovic M, Gonias SL. The very low density lipoprotein receptor regulates urokinase receptor catabolism and breast cancer cell motility in vitro. J Biol Chem 1999; 274:7412-20; PMID:10066806; https://doi.org/10.1074/jbc.274.11.7412
- Wolf K, Friedl P. Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol 2011; 21:736-44; PMID:22036198; https://doi.org/10.1016/j.tcb.2011.09.006
- Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007; 130:601-10; PMID:17719539; https://doi.org/10.1016/j.cell.2007.08.006
- Zhang W, Wu Y, Wu C, Gunst SJ. Integrin-linked kinase regulates N-WASp-mediated actin polymerization and tension development in tracheal smooth muscle. J Biol Chem 2007; 282:34568-80; PMID:17897939; https://doi.org/10.1074/jbc.M704966200
