ABSTRACT
The tooth, like many other organs, develops from both epithelial and mesenchymal tissues, and has proven to be a valuable tool with which to investigate organ formation and peripheral innervation. Tooth formation is regulated by local epithelial-mesenchymal tissue interactions, and is closely integrated with stereotypic dental nerve navigation and patterning. Recent analyses of the function and regulation of semaphorin 3A (SEMA3A) have shed light on the regulatory mechanisms that coordinate organogenesis and innervation at the tissue and molecular levels. In the tooth, SEM3A acts as a developmentally regulated secretory chemo-repellent, that controls tooth innervation during embryonic and postnatal development. The tooth germ governs its own innervation by a combination of local tissue interactions and SEMA3A expression. SEMA3A signaling, in turn, is controlled by a number of conserved signaling effectors, including TGF-β superfamily members, FGF, and WNT; all function in embryo and organ development, and are essential for tooth histo-morphogenesis. Thus, SEMA3A driven axon guidance is integrated into key odontogenic signaling networks, establishing this protein as a critical molecular tether between 2 distinct developmental processes (morphogenesis and sensory innervation), both of which are required to obtain a functional tooth.
Introduction
The developing tooth germ has turned out to be a useful model system with which to decipher the regulatory mechanisms that underpin organ development and stem cell biology.Citation1-4 Moreover, the tooth is a well-defined target organ for peripheral innervation.Citation5-9 Previous investigations have demonstrated how 2 apparently distinct processes, tooth organ formation and innervation, take place in a highly coordinated fashion. This integration is achieved through local, organ specific tissue interactions, that are mediated by distinct molecular signaling pathways.
The adult tooth nerve supply and its functions
The teeth are unique, greatly specialized organs, which in mammals develop in the 1st branchial arch and are exclusively found in the oral cavity. In addition to 1st branchial arch, some mammalian teeth develop from the frontonasal process. The principal function of teeth in man is mastication, while also making a substantial contribution to articulation and appearance. Teeth receive protective sensory trigeminal innervation from the trigeminal ganglion,Citation10 with an abundant number of sensory nerve endings located in the soft tissue pulp of the crown. The crown itself is the visible part of the tooth within the oral cavity, and is responsible for its masticatory functions. The highest concentration of plentiful number of intricately arranged, sensory nerves, in the crown pulp is distributed in the pulp-dentin border- area of the crown pulp, toward the occlusal surface containing the subodontoblastic region, odontoblast layer, and dentin tubules. These sensory nerves mediate the sensation of pain.Citation10-12 The periodontal space between the root of the tooth and the adjacent alveolar bone, is the second major target area for sensory nerves, with these mediating pain, as well as pressure and touch.Citation10-12 Nerves emanating from the sympathetic superior cervical ganglion are found in association with dental blood vessels in both the dental pulp, and periodontal space, and are involved in vasoregulatory functions.Citation10-13
Peripheral nerves engage in additional, non-neuronal functions, in the developing and adult tooth. Dental nerves are involved in inflammatory responses of the adult tooth, with more extensive pulp necrosis evident in injured denervated teeth than innervated.Citation12-14 Trigeminal nerves have also been shown to be indispensable for the continuous generation of teeth in fish.Citation15 Innervation of the periodontium is required to inhibit the pathological fusion of teeth roots to the surrounding alveolar bone (dentoalveolar ankylosis), and root resorption.Citation16 Recently, the neurovascular bundle was identified as a mesenchymal stem cell niche in the mouse incisor.Citation17 Similarly, the glial cells that envelope neurons, are a source of mesenchymal stem cells that can contribute to the repair of injured pulp.Citation18
Tooth histomorphogenesis and innervation are tightly spatio-temporally integrated
During embryogenesis, the development of tissues, organs, and the nervous system, takes place contemporaneously. Given the specific localization of sensory nerve endings in key areas of the mature tooth, and their important functions, it is plausible to assume that the development of tooth-supporting innervation, together with the tooth germ itself, does not occur haphazardly, but is instead strictly choreographed. Indeed, earlier developmental biology studies on mammalian teeth established that the development of the tooth's shape and dental cells, takes place in a strictly regulated manner.Citation19 This process is characterized by complex epithelial and mesenchymal tissue histomorphogenesis, including a condensation of the mesenchyme, coordinated cell proliferation, folding of the dental epithelium, and a gradual determination and differentiation of tooth-specific cells that includes the enamel and dentin producing amelo- and odontoblasts in the crown.Citation2,3,20
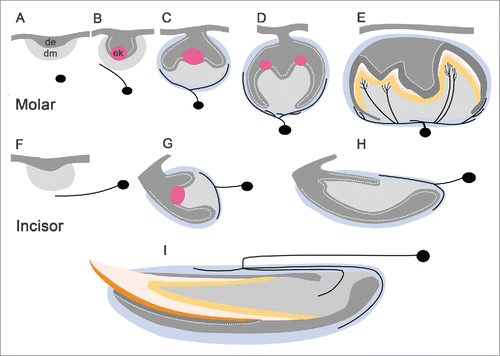
Tooth innervation is tightly linked to advancing tooth development, and occurs stereotypically in a developmentally regulated manner across different species.Citation10 Recent investigations used the mouse mandibular first molar tooth germ as a model system to show that dental sensory axons must grow in precisely defined mesenchymal pathways in order to properly reach the mesenchymal tooth-target areas ().Citation5 Histologically, the pioneer dental axons have been shown to emerge from the deep mandibular inferior alveolar nerve, where they grow toward the first molar tooth germ at embryonic (E) day 12.5.Citation21-24 Approximately half a day later, the first dental axons reach the target area of the bud-stage tooth germ. During the tooth-specific morphogenetic cap and bell stages, nerves innervate the tooth germ, to surround the mesenchymal dental follicle target area, which ultimately forms the periodontium (during root formation and tooth eruption) that attaches roots to alveolar bone.Citation10,13,23,25,26 Of note, although the nerves already surround the developing tooth organ during embryonic stages, axons will only grow into the mesenchymal dental pulp post-natally. Typically this occurs after a delay of about 10 days (from the first encounter of axons with the tooth target), on postnatal day 3–4. This timing is immediately after the onset of enamel formation of the tooth crown.Citation13 It is also intriguing to note that the ingrowth of axons into the pulp does not take place randomly, but instead axons navigate the mandibular molar tooth pulp solely through defined mesial and distal areas, around which the roots of the 2-rooted molar tooth are formed by odontoblasts.Citation7,13 The roots commence to form after the floor of the pulp has been laid down, and at this stage ingrowth of the sympathetic nerve fibers occurs into the dental pulp for the first time.Citation13 The periodontal space receives its final nerve supply after tooth eruption and termination of root growth during later postnatal development. Mouse tooth innervation is developmentally regulated in the embryo and newborn pup, and is not complete until late postnatal stages. Importantly, the timing of axon navigation, the encounter of axons with the tooth germ, as well as patterning of the axons within the mesenchymal tissue compartment of the tooth germ, take place concomitantly with tooth morphogenesis and cell differentiation. These events are therefore closely integrated with the key developmental steps of odontogenesis.
Figure 1. A comparison of nerve localization during embryonic and early postnatal crown morphogenesis of the mouse mandibular incisor, and 2-rooted first molar. Tooth innervation takes place in a stereotypic manner in both tooth types, and is linked to the advancing tooth histomorphogenesis and dental cell differentiation. See main text for a description of the figure. (A, F) initiation stage; (B), bud stage; (C, G) cap stage; (D, E, H, I) bell stage. Abbreviations: cm, condensed dental mesenchyme; de, dental epithelium; dp, dental pulp; p, dental papilla; ek, enamel knot; pm, presumptive dental mesenchyme. Nerve fibers are indicated in black. This schematic is based on data from previous reports.Citation32,23,13,25,26
The tooth germ controls it own innervation, using local signals
Earlier neurobiological investigations have provided fundamental data on the mechanisms of tooth innervation. It has been demonstrated that experimentally separated tooth germs, and adult denervated teeth, undergo re-innervation.Citation27-29 In line with this, explants of dental mesenchyme can influence axonal growth in a developmental manner.Citation30 The expression of neuro-regulatory molecules appears to be independent of nerves in culture, with innervation of the adult tooth regenerating after trauma.Citation12,31 Moreover, rudimentary tooth germs, which degenerate in the mouse diastema, never become innervated.Citation32 To sum up, these data provide evidence that the developing tooth regulates the establishment of its own nerve supply, in a similar fashion to its control of morphogenesis,Citation3 and that the genetic control of these events is mediated by locally expressed effectors.Citation5
The regulation of tooth innervation appears to involve signaling derived from both secretory and membrane-bound neuro-regulatory families; both are critical for the development of the central and peripheral nervous system.Citation5,6 Many neuro-regulatory molecules exhibit spatio-temporal changes in their cellular expression in the tooth germ, with these changes correlating with the growth of axons. Besides the neurotrophin and glial cell line-derived neurotrophic factor (GDNF) protein families (which serve critical roles in tooth innervation), other neuro-regulatory families are also expressed in the developing tooth germ, and are implicated in the development of the tooth's nerve supply.Citation5,6 These include the cell membrane-bound ephrin ligands, their Eph receptors, netrins, laminins, and cell-adhesion molecules (CAMs). Members of these families are likely to control various aspects of tooth innervation such as axon navigation, target field recognition and innervation, as well as nerve survival, and maturation.Citation5,6 Although the exact functions of the majority of neuro-regulatory molecules in tooth innervation are yet to be discovered, there is evidence that the prototypic member of the neurotrophin family, NGF (nerve growth factor),Citation33 serves a key role in the development of the nerve supply. For example, dynamic expression patterns of NGF mRNA during the embryonic and postnatal development of the incisor and molar teeth, correlate with pivotal phases of tooth innervation. These data suggest that NGF may control dental axon guidance, and target field innervation,Citation31,34,25 in addition to its functions in neuronal survival.Citation35,36,35,37
Locally expressed SEMA3A regulates tooth innervation
In addition to being a significant regulator of nervous system development, axonal guidance, and fasciculation, SEMA3A signaling is involved in the development and physiology of many non-neuronal tissues.Citation38,39,40,41,42,43 mRNAs for SEMA3A, which repels both sensory and sympathetic nerves,Citation41 displays developmentally regulated and distinct cellular expression patterns in both epithelial and mesenchymal tissue during mouse molar and incisor development.Citation23,26,44,45 Sema3A is specifically expressed in sites that are devoid of navigating axons, suggesting functions in tooth innervation. During the onset of tooth formation and innervation, Sema3A is expressed in the dental and jaw mesenchyme areas, adjacent to the pioneer nerve branch (the trigeminal ‘molar nerve’) growing toward the early tooth germ. Later, during the bud, cap and bell stages, Sema3A is seen around the tooth germ, flanking the mesenchymal dental follicle target area, where the number of nerves gradually increases in both embryonic and postnatal molar and incisor tooth germs.Citation7,23,26,45 In the dental pulp, although Sema3A is located in the middle part of the base of the 2-rooted mandibular molar tooth, transcripts are specifically absent from the future sites of the mesial and distal roots where nerve fibers grow into the dental pulp. Similarly, in the single-rooted incisor tooth germ, Sema3 transcripts are lacking in the pulp/root areas through which nerves navigate the pulp.Citation7,23,26,45
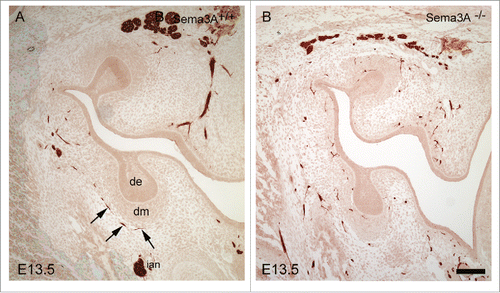
Analyses of Sema3A-deficient transgenic mice confirmed that SEMA3A is a critical regulator of tooth innervation, that is serially exploited at critical embryonic and postnatal stages of tooth innervation.Citation23,25,26 In both Sema3A-deficient incisor and molar tooth germs, pioneer axons reach the tooth germ prematurely and show apparent disturbances in nerve patterning and fasciculation. Although dental mesenchymal target areas become innervated, nerve fibers frequently overshoot their targets, to enter abnormal ectopic locations within mesenchymal exclusion areas, which would suggest a failure of the surrounding repulsion mechanisms ().Citation23,25,26 The dental pulp of Sema3A−/− molars are also prematurely innervated, with axons exhibiting abnormal patterning and fasciculation, in particular within the enlarged and defective subodontoblastic nerve plexus.Citation25 In contrast, the innervation of the dental pulp in Sema3A−/− incisors appears normal, reflecting the finding that Sema3A is largely nonexistent in the postnatal incisor pulp.Citation26,45 However, an abnormal, elevated number of axons and their arborization, is observed in the incisor periodontium, particularly at the labial side.Citation26
Figure 2. Immunohistochemical localization of nerve fibers in Sema3A+/+ (A) and Sema3A−/− (B) bud stage molar tooth germs at E13.5. Nerves in the Sema3A−/− molars and mandible mesenchyme exhibit apparent defasciculation as well as abnormal patterning and tooth target innervation (e.g. ectopic expression in the condensed dental mesenchyme and next to the dental epithelium). In contrast, in the Sema3A+/+ tooth, germ dental nerves show organized, appropriate target innervation (arrows). Abbreviations: cm, condensed dental mesenchyme; de, dental epithelium. Scale bar: 100 µm.
The finding that some degree of correction occurred in the dental nerve patterning of Sema3A−/− molarsCitation25 suggests that other neuro-regulatory molecules may compensate for the lack of SEMA3A signaling. For example, the expression of NGF and GDNF (that exert positive influences on axon growth and tooth target innervation), and their receptors, are unaltered in the trigeminal ganglion during various stages of tooth innervation in Sema3A-mutant teeth.Citation23,25,26 Similarly, mRNA expression of LANR, NCAM, and NET3, all implicated in tooth innervation, appears to be independent of SEMA3A signaling. This supports a model in which SEMA3A's control of dental axon growth, navigation, patterning, and fasciculation, is independent on many tooth-expressed neuro-regulatory molecules, and that tooth innervation involves redundant and independent signaling from neuro-regulatory proteins of different families.Citation5,6,8,9
Collectively, these results establish that SEMA3A mediates dental nerve-tooth target interactions, and is an essential signal needed for the timing and patterning of embryonic and postnatal tooth innervation, as well as dental nerve fasciculation and sprouting.Citation23,25,26 Moreover, these results provide significant evidence for a model in which the tooth germ itself controls its own innervation by local concerted, and apparently redundant signals. Several expression domains of Sema3A, such as the epithelial cervical loops, which contribute to root formation, and preodontoblasts, which later differentiate into dentin producing odontoblasts, imply non-neuronal functions not yet revealed by genetic analyses.Citation23,25,26 Recently, however, SEMA3A was shown to be able to induce mesenchymal-stem-like properties in human periodontal ligament cells in culture conditions.Citation46 Thus, further studies regarding putative non-neuronal functions of SEMA3A signaling in tooth-formation are now warranted.
The regulation of dental Sema3A expression and tooth nerve supply by local tissue interactions
Classic developmental biology studies have demonstrated that tooth formation is regulated by sequential and reciprocal interactions between epithelial and mesenchymal cells. This chain of inductive interaction is defined as secondary induction and regulates all aspects of tooth development.Citation19,7 Recent molecular and genetic studies, particularly with mice, have elaborated on the molecular signatures that define relevant interactions, together with signaling pathways, and networks that control odontogenesis.Citation3
Spatio-temporal changes in the Sema3A expression domain in the incisor and molar tooth germs, especially in the early dental mesenchymal compartments, correlated with inductive signaling between the dental epithelium and mesenchyme.Citation23,45 Analyses of Sema3A regulation using organotypic cultures and tissue separation, and recombination experiments, demonstrated that rather than being controlled by, or being dependent on, peripheral nerves, mesenchymal Sema3A expression was instead regulated by the dental epithelium during early tooth development.Citation23 Thus, these results demonstrated, for the very first time, that local tissue interactions regulate mesenchymal Sema3A expression, and thereby the development of the sensory nerve supply in the tooth germ. Consequently, local inductive signaling within the developing tooth germ provides an explanation as to how the tooth is able to control and orchestrate its own histomorphogenesis and innervation.Citation23 Interestingly, the early dental epithelium has been shown to possess the odontogenic information needed to determine the requisite number of teeth, their size, and shape.Citation21,47,48 Data regarding the regulation of Sema3A suggests that the tooth germ proper possesses the instructions needed to guide innervation and the establishment of a tooth-specific sensory nerve supply, until finalized late after birth.Citation23,25,26
SEMA3A as a link integrating organogenesis and innervation
Tooth formation is dependent on, and controlled by, the activity of a limited number of conserved secreted signaling proteins (and their downstream pathways), including the TGF-β superfamily, FGFs, Hedgehog, and WNT,Citation49,3 all of which are commonly exploited in developmental processes during embryogenesis.Citation50 Certain family members have been shown to mediate reciprocal tissue interactions, and to control the expression of various signals and transcription factors essential for tooth formation. These effectors are collectively integrated into a complex network whose fine-tuning is suggested to underlie the creation of teeth with various morphologies and evolutionary transitions.Citation20,3
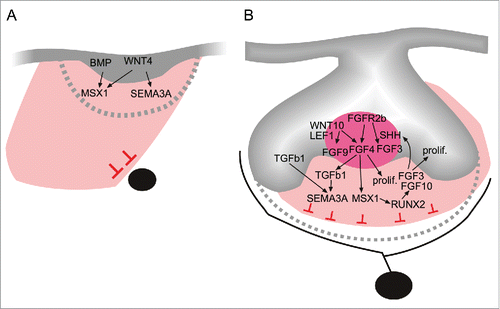
Studies of Sema3A regulation during early tooth development revealed its integration into key odontogenic pathways. WNT and TGF-β signals were found to regulate Sema3A expression in the dental mesenchyme during early stages of pioneer dental axon navigation.Citation5 Many WNT signaling components are present in developing tooth tissues, with WNT signaling acting at multiple stages, including initiation, morphogenesis, and episodes of hard-tissue formation.Citation51-54 Analyses of Sema3A regulation also revealed that WNT4 expressed on early dental epithelium induces not only Sema3A, but also the Msx1 transcription factor. MSX1 is essential for tooth formation in mouse and man, acting within the presumptive dental mesenchyme prior to the arrival of the first dental nerve fibers.Citation23 Moreover, TGF-beta1 stimulates Sema3A expression in the dental mesenchyme at the time of the initial nerve encounter with the tooth target field. TGF-beta1 expression arises at the onset of tooth morphogenesis,Citation55 and during dentinogenesis,Citation57 and it regulate Ngf and Nt3 mRNA expression in maxillary process cells in culture.Citation56 GF signaling regulates embryonic development and organogenesis.Citation58 In the developing tooth, epithelial FGFR2b mediates the signaling of the mesenchymal FGFs requisite for tooth formation.Citation24,59 FGFR2b is essential for tooth morphogenesis from an early stage, as shown by an arrested development at the bud stage when this growth factor receptor is inactivated.Citation24 In FGFR2b−/− molars, Sema3A shows defective expression domains, and is down-regulated from the bud and cap stage in Fgfr2b−/− dental mesenchymes.Citation24 Notably, dental axons show defective patterning as demonstrated by the finding that the trigeminal molar nerve failed to establish its lingual branch at the bud stage.Citation59 Furthermore, it was found that the enamel knot signaling center expressed Fgf4,Citation60-62 which was able to indirectly regulate Sema3A by controlling Tgfß1.Citation24 These results indicate that FGF signaling is essential for tooth morphogenesis, and, by regulating Sema3A, also controls tooth innervation. Collectively, these data show that SEMA3A is regulated by, and integrated into, the TGF-β, FGF, and WNT signaling pathways, as well as more extensive odontogenic signaling networks that control tooth formation ().Citation24,63-65
Figure 3. A model showing select signaling pathways and networks involved in the coordination of tooth morphogenesis and innervation during initiation (A), and the early morphogenetic cap stage (B). Tooth formation is crucially dependent on epithelial-mesenchymal interactions, which also regulate mesenchymal Sema3A, and the subsequent timing and patterning of tooth target innervation. Members of the conserved FGF (FGF4), Wnt (WNT4), and TGF-β superfamily (TGFß1) regulate SEMA3A expression. These signaling pathways are part of a larger odontogenic signaling network involving genes that are absolutely necessary for tooth formation in man and mouse, such as the MSX1 transcription factor.Citation23,24,66-68,69,70,71
Summary
The developing tooth, like many organs, arises from both epithelial and mesenchymal tissues, and is a valuable model organ with which to investigate the regulation of organ histomorphogenesis at the tissue, genetic, and molecular levels.Citation64,65 Studies of the functions and regulation of SEMA3A have unraveled novel developmental regulatory mechanisms regarding tooth innervation. The developing tooth germ controls its own innervation by local epithelial-mesenchymal tissue interactions, with the regulation of SEMA3A achieved via different conserved signaling effector families. These studies have established SEMA3A as a key regulator of innervation for the tooth. In particular, SEMA3A, as part of an integrated molecular network, is employed at consecutive stages of embryonic and postnatal innervation to control tooth development. Importantly, SEMA3A is proposed to serve as a molecular link between the development of the tooth and its specific nerve supply. Further detailed studies regarding the functions of SEMA3A signaling in tooth innervation, and how these signals are integrated within larger regulatory networks, are now warranted.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Kjellfrid Haukanes is acknowledged for skillful technical assistance. We thank the staff of the animal facility for careful mouse husbandry.
Funding
This study was supported by the University of Bergen.
References
- Leso H, Brook AH. Epithelial histogenesis during tooth development. Arch Oral Biol 2009; 54 (Suppl 1):S25-33; PMID:18656852; http://dx.doi.org/10.1016/j.archoralbio.2008.05.019
- Cobourne MT, Sharpe PT. Making up the numbers: The molecular control of mammalian dental formula. Semin Cell Dev Biol 2010; 21:314-24; PMID:20080198; http://dx.doi.org/10.1016/j.semcdb.2010.01.007
- Thesleff I. Current understanding of the process of tooth formation: transfer from the laboratory to the clinic. Australian Dental J 2014; 59 (Suppl 1):48-54; http://dx.doi.org/10.1111/adj.12102
- Mitsiadis TA, Harada H. Regenerated teeth: the future of tooth replacement. An update. Regenerative Med 2015; 10:5-8; http://dx.doi.org/10.2217/rme.14.78
- Luukko K, Kvinnsland IH, Kettunen P. Tissue interactions in the regulation of axon pathfinding during tooth morphogenesis. Dev Dyn 2005; 234:482-8; PMID:16217735; http://dx.doi.org/10.1002/dvdy.20586
- Fried K, Nosrat C, Lillesaar C, Hildebrand C. Molecular signaling and pulpal nerve development. Crit Rev Oral Biol Med 2000; 11:318-32; PMID:11021633; http://dx.doi.org/10.1177/10454411000110030301
- Luukko K, Moe K, Sijaona A, Furmanek T, Hals Kvinnsland I, Midtbo M, Kettunen P. Secondary induction and the development of tooth nerve supply. Ann Anat 2008; 190:178-87; PMID:18413271; http://dx.doi.org/10.1016/j.aanat.2007.10.003
- Fried K, Lillesaar C, Sime W, Kaukua N, Patarroyo M. Target finding of pain nerve fibers: neural growth mechanisms in the tooth pulp. Physiol Behav 2007; 92:40-5; PMID:17585959; http://dx.doi.org/10.1016/j.physbeh.2007.05.032
- Luukko K, Kettunen P. Coordination of tooth morphogenesis and neuronal development through tissue interactions: Lessons from mouse models. Exp Cell Res 2014; PMID:24631295
- Hildebrand C, Fried K, Tuisku F, Johansson CS. Teeth and tooth nerves. Prog Neurobiol 1995; 45:165-222; PMID:7777672; http://dx.doi.org/10.1016/0301-0082(94)00045-J
- Byers MR, Narhi MV. Dental injury models: experimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions. Crit Rev Oral Biol Med 1999; 10:4-39; PMID:10759425; http://dx.doi.org/10.1177/10454411990100010101
- Byers MR, Suzuki H, Maeda T. Dental neuroplasticity, neuro-pulpal interactions, and nerve regeneration. Microsc Res Tech 2003; 60:503-15; PMID:12619126; http://dx.doi.org/10.1002/jemt.10291
- Moe K, Kettunen P, Kvinnsland IH, Luukko K. Development of the pioneer sympathetic innervation into the dental pulp of the mouse mandibular first molar. Arch Oral Biol 2008; 53:865-73; PMID:18436190; http://dx.doi.org/10.1016/j.archoralbio.2008.03.004
- Haug SR, Heyeraas KJ. Modulation of dental inflammation by the sympathetic nervous system. J Dent Res 2006; 85:488-95; PMID:16723642; http://dx.doi.org/10.1177/154405910608500602
- Tuisku F, Hildebrand C. Evidence for a neural influence on tooth germ generation in a polyphyodont species. Dev Biol 1994; 165:1-9; PMID:8088427; http://dx.doi.org/10.1006/dbio.1994.1228
- Fujiyama K, Yamashiro T, Fukunaga T, Balam TA, Zheng L, Takano-Yamamoto T. Denervation resulting in dento-alveolar ankylosis associated with decreased Malassez epithelium. J Dent Res 2004; 83:625-9; PMID:15271971; http://dx.doi.org/10.1177/154405910408300808
- Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P, Chai Y. Secretion of Shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell 2014; 14:160-73; PMID:24506883; http://dx.doi.org/10.1016/j.stem.2013.12.013
- Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L, Hultman I, Ahrlund-Richter L, et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature 2014; 513:551-4; PMID:25079316; http://dx.doi.org/10.1038/nature13536
- Thesleff I, Nieminen P. Tooth induction. Encyclopedia Life Sci 2005; 1-9; http://dx.doi.org/10.1038/npg.els.0004183
- Jernvall J, Thesleff I. Tooth shape formation and tooth renewal: evolving with the same signals. Development 2012; 139:3487-97; PMID:22949612; http://dx.doi.org/10.1242/dev.085084
- Lumsden AG. Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development 1988; 103 (Suppl):155-69; PMID:3250849
- Obara N, Takeda M. Innervation of mouse molars during the early states of tooth germ development. Higashi Nippon Shigaku Zasshi 1989; 8:115-24; PMID:2486712
- Kettunen P, Loes S, Furmanek T, Fjeld K, Kvinnsland IH, Behar O, Yagi T, Fujisawa H, Vainio S, Taniguchi M, et al. Coordination of trigeminal axon navigation and patterning with tooth organ formation: epithelial-mesenchymal interactions, and epithelial Wnt4 and Tgfbeta1 regulate semaphorin 3a expression in the dental mesenchyme. Development 2005; 132:323-34; PMID:15604101; http://dx.doi.org/10.1242/dev.01541
- Kettunen P, Spencer-Dene B, Furmanek T, Kvinnsland IH, Dickson C, Thesleff I, Luukko K. Fgfr2b mediated epithelial-mesenchymal interactions coordinate tooth morphogenesis and dental trigeminal axon patterning. Mech Dev 2007; 124:868-83; PMID:17951031; http://dx.doi.org/10.1016/j.mod.2007.09.003
- Moe K, Sijaona A, Shrestha A, Kettunen P, Taniguchi M, Luukko K. Semaphorin 3A controls timing and patterning of the dental pulp innervation. Differentiation 2012; 84:371-9; PMID:23142733; http://dx.doi.org/10.1016/j.diff.2012.09.003
- Shrestha A, Moe K, Luukko K, Taniguchi M, Kettunen P. Sema3A chemorepellant regulates the timing and patterning of dental nerves during development of incisor tooth germ. Cell Tissue Res 2014; 357:15-29; PMID:24752460
- Erdelyi G, Fried K, Hildebrand C. Nerve growth to tooth buds after homotopic or heterotopic autotransplantation. Brain Res 1987; 430:39-47; PMID:3594271; http://dx.doi.org/10.1016/0165-3806(87)90174-X
- Fried K, Erdelyi G. Inferior alveolar nerve regeneration and incisor pulpal reinnervation following intramandibular neurotomy in the cat. Brain Res 1982; 244:259-68; PMID:7116174; http://dx.doi.org/10.1016/0006-8993(82)90084-1
- Holland GR, Robinson PP. Pulp re-innervation in re-implanted canine teeth of the cat. Arch Oral Biol 1987; 32:593-7; PMID:3479101; http://dx.doi.org/10.1016/0003-9969(87)90069-0
- Lillesaar C, Fried K. Neurites from trigeminal ganglion explants grown in vitro are repelled or attracted by tooth-related tissues depending on developmental stage. Neuroscience 2004; 125:149-61; PMID:15051154; http://dx.doi.org/10.1016/j.neuroscience.2004.01.008
- Luukko K, Arumae U, Karavanov A, Moshnyakov M, Sainio K, Sariola H, Saarma M, Thesleff I. Neurotrophin mRNA expression in the developing tooth suggests multiple roles in innervation and organogenesis. Dev Dyn 1997; 210:117-29; PMID:9337133; http://dx.doi.org/10.1002/(SICI)1097-0177(199710)210:2%3c117::AID-AJA5%3e3.0.CO;2-J
- Loes S, Kettunen P, Kvinnsland H, Luukko K. Mouse rudimentary diastema tooth primordia are devoid of peripheral nerve fibers. Anat Embryol 2002; 205:187-91; PMID:12107488; http://dx.doi.org/10.1007/s00429-002-0247-8
- Thoenen H, Sendtner M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat Neurosci 2002; 5 (Suppl):1046-50; PMID:12403983; http://dx.doi.org/10.1038/nn938
- Nosrat CA, Fried K, Lindskog S, Olson L. Cellular expression of neurotrophin mRNAs during tooth development. Cell Tissue Res 1997; 290:569-80; PMID:9369532; http://dx.doi.org/10.1007/s004410050962
- Naftel JP, Qian XB, Bernanke JM. Effects of postnatal anti-nerve growth factor serum exposure on development of apical nerves of the rat molar. Brain Res Dev Brain Res 1994; 80:54-62; PMID:7955360; http://dx.doi.org/10.1016/0165-3806(94)90089-2
- Matsuo S, Ichikawa H, Henderson TA, Silos-Santiago I, Barbacid M, Arends JJ, Jacquin MF. trkA modulation of developing somatosensory neurons in oro-facial tissues: tooth pulp fibers are absent in trkA knockout mice. Neuroscience 2001; 105:747-60; PMID:11516838; http://dx.doi.org/10.1016/S0306-4522(01)00223-8
- Qian XB, Naftel JP. Effects of neonatal exposure to anti-nerve growth factor on the number and size distribution of trigeminal neurones projecting to the molar dental pulp in rats. Arch Oral Biol 1996; 41:359-67; PMID:8771327; http://dx.doi.org/10.1016/0003-9969(95)00128-X
- Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, Yagi T, Fujisawa H. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron 1997; 19:995-1005; PMID:9390514; http://dx.doi.org/10.1016/S0896-6273(00)80392-X
- Taniguchi M, Yuasa S, Fujisawa H, Naruse I, Saga S, Mishina M, Yagi T. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron 1997; 19:519-30; PMID:9331345; http://dx.doi.org/10.1016/S0896-6273(00)80368-2
- Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol 2007; 23:263-92; PMID:17539753; http://dx.doi.org/10.1146/annurev.cellbio.22.010605.093554
- Fujisawa H. Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development. J Neurobiol 2004; 59:24-33; PMID:15007824; http://dx.doi.org/10.1002/neu.10337
- Yazdani U, Terman JR. The semaphorins. Genome Biol 2006; 7:211; PMID:16584533; http://dx.doi.org/10.1186/gb-2006-7-3-211
- Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3A. Nature 2012; 485:69-74; PMID:22522930; http://dx.doi.org/10.1038/nature11000
- Loes S, Kettunen P, Kvinnsland IH, Taniguchi M, Fujisawa H, Luukko K. Expression of class 3 semaphorins and neuropilin receptors in the developing mouse tooth. Mech Dev 2001; 101:191-4; PMID:11231073; http://dx.doi.org/10.1016/S0925-4773(00)00545-1
- Moe K, Shrestha A, Kvinnsland IH, Luukko K, Kettunen P. Developmentally regulated expression of Sema3A chemorepellant in the developing mouse incisor. Acta Odontol Scand 2012; 70:184-9; PMID:21793640; http://dx.doi.org/10.3109/00016357.2011.600717
- Wada N, Maeda H, Hasegawa D, Gronthos S, Bartold PM, Menicanin D, Fujii S, Yoshida S, Tomokiyo A, Monnouchi S, et al. Semaphorin 3A induces mesenchymal-stem-like properties in human periodontal ligament cells. Stem Cell Dev 2014; 23:2225-36; PMID:24380401; http://dx.doi.org/10.1089/scd.2013.0405
- Mina M, Kollar EJ. The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch Oral Biol 1987; 32:123-7; PMID:3478009; http://dx.doi.org/10.1016/0003-9969(87)90055-0
- Brook AH, Jernvall J, Smith RN, Hughes TE, Townsend GC. The dentition: the outcomes of morphogenesis leading to variations of tooth number, size and shape. Aust Den J 2014; 59 (Suppl 1):131-42; PMID:24646162; http://dx.doi.org/10.1111/adj.12160
- Cobourne MT, Sharpe PT. Diseases of the tooth: the genetic and molecular basis of inherited anomalies affecting the dentition. Wiley Interdiscip Rev Dev Biol 2013; 2:183-212; PMID:24009033; http://dx.doi.org/10.1002/wdev.66
- Weiss A, Attisano L. The TGFbeta superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol 2013; 2:47-63; PMID:23799630; http://dx.doi.org/10.1002/wdev.86
- Fjeld K, Kettunen P, Furmanek T, Kvinnsland IH, Luukko K. Dynamic expression of Wnt signaling-related Dickkopf1, −2, and −3 mRNAs in the developing mouse tooth. Dev Dyn 2005; 233:161-6; PMID:15759274; http://dx.doi.org/10.1002/dvdy.20285
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, et al. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol 2008; 313:210-24; PMID:18022614; http://dx.doi.org/10.1016/j.ydbio.2007.10.016
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev 1994; 8:2691-703; PMID:7958926; http://dx.doi.org/10.1101/gad.8.22.2691
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell 2002; 2:643-53; PMID:12015971; http://dx.doi.org/10.1016/S1534-5807(02)00167-3
- Vaahtokari A, Vainio S, Thesleff I. Associations between transforming growth factor beta 1 RNA expression and epithelial-mesenchymal interactions during tooth morphogenesis. Development 1991; 113:985-94; PMID:1726565
- Buchman VL, Sporn M, Davies AM. Role of transforming growth factor-beta isoforms in regulating the expression of nerve growth factor and neurotrophin-3 mRNA levels in embryonic cutaneous cells at different stages of development. Development 1994; 120:1621-9; PMID:8050368
- Martin A, Unda FJ, Begue-Kirn C, Ruch JV, Arechaga J. Effects of aFGF, bFGF, TGFbeta1 and IGF-I on odontoblast differentiation in vitro. Eur J Oral Sci 1998; 106 (Suppl 1):117-21; PMID:9541212; http://dx.doi.org/10.1111/j.1600-0722.1998.tb02162.x
- Ornitz DM, Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol 2015; 4:215-66; PMID:25772309; http://dx.doi.org/10.1002/wdev.176
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun 2000; 277:643-9; PMID:11062007; http://dx.doi.org/10.1006/bbrc.2000.3721
- Jernvall J, Kettunen P, Karavanova I, Martin LB, Thesleff I. Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int J Dev Biol 1994; 38:463-9; PMID:7848830
- Thesleff I, Jernvall J. The enamel knot: a putative signaling center regulating tooth development. Cold Spring Harb Symp Quant Biol 1997; 62:257-67; PMID:9598359; http://dx.doi.org/10.1101/SQB.1997.062.01.032
- Luukko K, Loes S, Furmanek T, Fjeld K, Kvinnsland IH, Kettunen P. Identification of a novel putative signaling center, the tertiary enamel knot in the postnatal mouse molar tooth. Mech Dev 2003; 120:270-6; PMID:12591596; http://dx.doi.org/10.1016/S0925-4773(02)00458-6
- Salazar-Ciudad I, Jernvall J. A gene network model accounting for development and evolution of mammalian teeth. Proc Natl Acad Sci U S A 2002; 99:8116-20; PMID:12048258; http://dx.doi.org/10.1073/pnas.132069499
- Jussila M, Thesleff I. Signaling networks regulating tooth organogenesis and regeneration, and the specification of dental mesenchymal and epithelial cell lineages. Cold Spring Harb Perspect Biol 2012; 4:a008425; PMID:22415375
- Balic A, Thesleff I. Chapter Seven - Tissue Interactions Regulating Tooth Development and Renewal. In: Yang C, ed. Current topics in developmental biology. Academic Press, 2015:157-86.
- Vainio S, Karavanova I, Jowett A, Thesleff I. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 1993; 75:45-58; PMID:8104708; http://dx.doi.org/10.1016/S0092-8674(05)80083-2
- Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R. FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1(−/−) mice. Genes Dev 2002; 16:3173-85; PMID:12502739; http://dx.doi.org/10.1101/gad.1035602
- Klein OD, Minowada G, Peterkova R, Kangas A, Yu BD, Lesot H, Peterka M, Jernvall J, Martin GR. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev Cell 2006; 11:181-90; PMID:16890158; http://dx.doi.org/10.1016/j.devcel.2006.05.014
- Thesleff I, Sharpe P. Signalling networks regulating dental development. Mech Dev 1997; 67:111-23; PMID:9392510; http://dx.doi.org/10.1016/S0925-4773(97)001-15-9
- Bei M. Molecular genetics of tooth development. Cur Opin Genet Dev 2009; 19:504-10; PMID:19875280; http://dx.doi.org/10.1016/j.gde.2009.09.002
- Tummers M, Thesleff I. The importance of signal pathway modulation in all aspects of tooth development. J Exp Zool B Mol Dev Evol 2009; 312B:309-19; PMID:19156667; http://dx.doi.org/10.1002/jez.b.21280
