ABSTRACT
Integrins, following binding to proteins of the extracellular matrix (ECM) including collagen, laminin and fibronectin (FN), are able to transduce molecular signals inside the cells and to regulate several biological functions such as migration, proliferation and differentiation. Besides activation of adaptor molecules and kinases, integrins transactivate Receptor Tyrosine Kinases (RTK). In particular, adhesion to the ECM may promote RTK activation in the absence of growth factors. The Colony-Stimulating Factor-1 Receptor (CSF-1R) is a RTK that supports the survival, proliferation, and motility of monocytes/macrophages, which are essential components of innate immunity and cancer development. Macrophage interaction with FN is recognized as an important aspect of host defense and wound repair. The aim of the present study was to investigate on a possible cross-talk between FN-elicited signals and CSF-1R in macrophages. FN induced migration in BAC1.2F5 and J774 murine macrophage cell lines and in human primary macrophages. Adhesion to FN determined phosphorylation of the Focal Adhesion Kinase (FAK) and Src Family Kinases (SFK) and activation of the SFK/FAK complex, as witnessed by paxillin phosphorylation. SFK activity was necessary for FAK activation and macrophage migration. Moreover, FN-induced migration was dependent on FAK in either murine macrophage cell lines or human primary macrophages. FN also induced FAK-dependent/ligand-independent CSF-1R phosphorylation, as well as the interaction between CSF-1R and β1. CSF-1R activity was necessary for FN-induced macrophage migration. Indeed, genetic or pharmacological inhibition of CSF-1R prevented FN-induced macrophage migration. Our results identified a new SFK-FAK/CSF-1R signaling pathway that mediates FN-induced migration of macrophages.
Introduction
Cell adhesion to the extracellular matrix (ECM) plays an important role in the regulation of different cellular functions such as survival, proliferation, and migration. The latter phenomenon is mediated by different cell surface receptors, including integrins.Citation1,2 Integrins are heterodimers, composed of one α and one β subunit, endowed with a large extracellular domain that binds to ECM components.Citation3-5 Integrins couple the ECM to the actin cytoskeleton, by recruiting proteins such as paxillin and vinculin, and are involved in focal adhesion formation.Citation1,4 Different members of the integrin family include the receptors for fibronectin (FN), collagen and laminin.Citation2 In particular, FN has an important role in inducing cell attachment and migration and is involved in the pathogenesis of several diseases including cancer.Citation6-8 Moreover, interaction of the cell with the ECM regulates differentiation and motility, that are often deregulated during cancer onset and progression.Citation4
FN elicits intracellular signaling by inducing integrin clustering, that results in the recruitment and activation of tyrosine kinases including focal adhesion kinase (FAK), Src family kinases (SFK) and their substrates.Citation1 FAK is a non-receptor tyrosine kinase that is auto-phosphorylated at tyrosine 397 (Y397) following integrin clustering. Phosphorylated Y397 is a binding site for SFK, that phosphorylate FAK further, leading to the formation of an active SFK/FAK complex.Citation1,9 Integrins may also induce ligand-independent tyrosine phosphorylation and activation of Receptor Tyrosine Kinases (RTK).Citation10,11
The Colony-Stimulating Factor-1 (CSF-1, also referred to as Macrophage Colony-Stimulating Factor), by binding to its receptor CSF-1R, sustains the survival, proliferation, differentiation and motility of monocytes/macrophages.Citation12 CSF-1R and the αvβ3 integrin collaborate during differentiation of osteoclasts, that are CSF-1R expressing cells.Citation13 Because macrophage interaction with FN is important in inflammation and host defense as well as cancer progression,Citation14 we undertook the present study to investigate on a possible cross-talk between FN-induced signaling and CSF-1R in macrophages.
Results
FN induces migration and FAK activation in macrophages
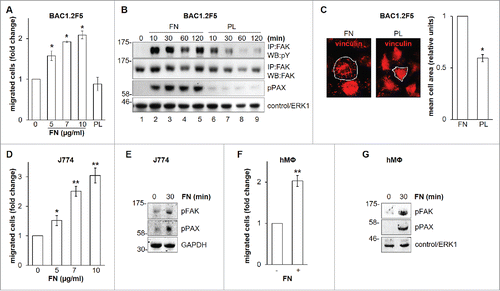
Early studies indicated that FN may reduce Hartley guinea pig macrophage migration in fibrin gel matrices in vitro.Citation15 By contrast, it has been recently reported that FN may be a good substrate for random migration of primary murine macrophages.Citation16 We further deepened the relevance of FN with respect to macrophage migration () and found that BAC1.2F5 murine macrophages migrate toward FN in a concentration-dependent manner (). This effect was marked, and comparable to that determined by CSF-1,Citation17 the master regulator of macrophage functions, including migration.Citation18 Polylysine (PL), a non-specific cell attachment factor that does not induce integrin clustering, did not affect macrophage migration (). It is well known that FN induces FAK phosphorylation in several cell types.Citation4,6 We were among the first to identify the important role of FAK in macrophage biology.Citation19 Moreover, later evidences definitively linked FAK to macrophage migration.Citation20,21 In particular, experiments carried out using murine FAK−/− cells showed that FAK mediates haptotaxis elicited by the activation of α5β1 integrin, that binds a number of substrates including FN.Citation20 We therefore characterized the effects of FN on FAK activation in macrophages. No previous data were indeed available on a possible link between FN-elicited signaling and FAK phosphorylation in macrophages. Adhesion on FN induced FAK activation, as indicated by the robust increase of total tyrosine phosphorylation of FAK and paxillin, a well-known substrate of FAK ().Citation9 In contrast, PL failed to induce marked and prolonged phosphorylation of FAK and paxillin (). Accordingly, cell spreading on FN was much more marked than that on PL (), as indicated by the significantly higher mean cell surface area following 30-minute adhesion on FN than on PL. The effect of FN on macrophage migration and FAK activation, as witnessed by FAK phosphorylation on Y397, was also confirmed in the murine macrophage cell line J774 () and in primary human macrophages ().
Figure 1. Effects of FN on migration and FAK in macrophages. Dose-response effect of FN on the migration of murine BAC1.2F5 macrophages. (A) Cells were cultured in the absence of CSF-1 for 18 hours and then subjected to migration assay toward DMEM containing or not FN at the indicated concentrations or PL (10 μg/ml). Migrated cells were counted. Histograms represent means ± SEM of data from 4 independent experiments each performed in triplicate. Student's t test: *, p< 0.05 versus untreated. Kinetics of FAK activation induced by FN in BAC1.2F5 cells. (B) Cells were cultured in the absence of CSF-1 for 18 hours, kept in suspension for 45 minutes and then plated on FN- or PL-coated dishes (10 μg/ml) for the indicated times. Protein lysates were then subjected to immunoprecipitation (IP) and/or immunoblotting (WB) with the indicated antibodies. Representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. FN-induced macrophage adhesion. (C) Cells were cultured in the absence of CSF-1 for 18 hours and then let adhere on FN- or PL-coated coverglass (10 μg/ml) for the indicated times. Cells were then stained with anti-vinculin antibodies and analyzed by confocal microscopy. Cell area was then measured. Histograms represent means ± SEM of data from 2 independent experiments. Student's t test: *, p < 0.05 vs. FN. Examples of selected areas are indicated. FN induces macrophage migration and FAK activation in J774 murine macrophages as well as human primary macrophages. (D, F) Migration assay was performed as described in (A). Histograms represent means ± SEM of data from 2 independent experiments each performed in triplicate. Student's t test: *, p < 0.05 versus untreated; **, p < 0.01 vs. untreated. (E, G) Total cell lysates were subjected to immunoblotting with the indicated antibodies. Representative images of WB from one out of 3 (E) or 2 (G) independent experiments are shown. Molecular weight markers are reported on the left of gels.
FAK mediates FN-induced macrophage migration
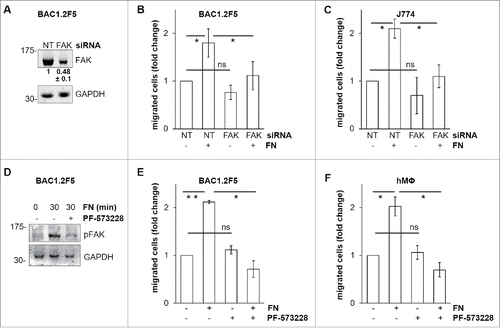
FAK is a master regulator of focal adhesion remodeling during cell migrationCitation22 and is involved in macrophage migration toward CSF-1 as well as in haptotaxis elicited by FN-induced α5β1 integrin activation in primary murine macrophages.Citation20,21 To evaluate whether FAK is involved in macrophage migration toward FN, we silenced FAK using specific siRNA (). FAK silencing prevented FN-induced migration of BAC1.2F5 () and J774 () cells. Furthermore, pharmacological inhibition of FAK, using the FAK inhibitor PF-573228 that prevented FN-induced FAK activation (), determined comparable results in either BAC1.2F5 () or primary human macrophages (). These findings demonstrated that FAK mediates FN-induced macrophage migration.
Figure 2. Effects of genetic or pharmacological inhibition of FAK on FN-induced macrophage migration. Genetic inhibition of FAK prevents FN-induced macrophage migration. (A-C) Cells were transfected with non-targeting control (NT) or FAK-specific (FAK) siRNA, incubated in complete medium for 48 hours and then cultured in the absence of CSF-1 (BAC1.2F5) or FBS (J774) for 18 or 24 hours, respectively. (A) Cells were then lysed and subjected to western blotting with the indicated antibodies. Numerical values represent the densitometric ratio FAK/NT (+/− SEM) relative to the FAK band (normalized for the densitometric value of the respective GAPDH band within the same gel) and are the average of data from 3 independent experiments. Likewise, representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. (B, C) Cells were subjected to migration assay toward DMEM containing or not 10 μg/ml FN. Histograms represent means ± SEM of data from 3 independent experiments performed in triplicate. Student's t test for paired samples; *, p ≤ 0.05; ns, not significant. Pharmacological inhibition of FAK prevents FN-induced macrophage migration. (D) Cells were cultured in the absence of CSF-1 for 18 hours and then kept in suspension for 45 minutes and lysed (0) or plated on FN-coated dishes (10 μg/ml) for 30 minutes in the presence or the absence of PF-573228 (1 μM). Cells were then lysed and proteins subjected to immunoblotting with the indicated antibodies. Representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. (E, F) Cells were cultured in the absence of CSF-1 for 18 hours and then treated or not with PF-573228 (1 μM) for 30 minutes before being subjected to migration assay toward DMEM containing or not FN at the indicated concentrations. Migrated cells were counted. Histograms represent means ± SEM of data from 3 (E) or 2 (F) independent experiments performed in triplicate. Student's t test: *, p < 0.05; **, p < 0.01; ns, not significant.
FN-induced FAK activation requires a functional cytoskeleton and is mediated by SFK
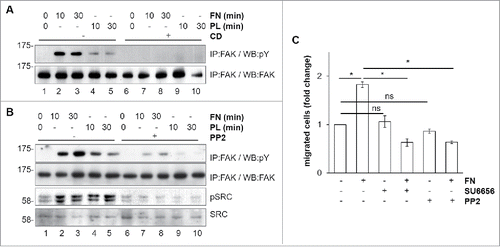
FN-induced FAK phosphorylation was abolished by pretreatment with cytochalasin D (CD), an inhibitor of actin polymerization (; compare lanes 7 and 8 to 2 and 3, respectively). These results confirmed that also in macrophages actin polymerization is important for FAK activation.Citation23 Macrophages and BAC1.2F5 cells are known to express several SFKCitation24 that bind to FAK following its auto-phosphorylation on Y397.Citation9 Adhesion on FN induced SFK activation, as determined by evaluating SFK phosphorylation using an antibody raised against the Src-activating residue Y416 (; compare lanes 2 and 3 to 1). It should be noted that adhesion on PL (lanes 4 and 5) induced SFK phosphorylation at levels comparable to those induced by adhesion on FN. Nevertheless, following adhesion on PL, the SFK/FAK complex is likely to be inactive, as witnessed by the lack of paxillin phosphorylation (). However, we cannot exclude that this lack is a consequence of the action of phosphatases. Pretreatment of cells with the SFK inhibitor PP2 prevented FN-induced FAK tyrosine phosphorylation, indicating that FN-induced FAK activation involves SFK (, compare lanes 7 and 8 to 2 and 3, respectively). In keeping with the fact that SFK-dependent FAK activity is necessary for macrophage migration, FN-induced macrophage migration was completely suppressed by the treatment with either SU6656 or PP2, two SFK chemical inhibitors (). In the experiments of , we were unable to discriminate which SFK was involved in FAK activation. It should also be noted that SU6656 or PP2 () and PF-573228 () not only suppressed the increase of BAC1.2F5 cell migration in the presence of FN, but even reduced migration below that of untreated cells. This effect was not due to a decrease in cell adhesiveness. Indeed, PF-573228, PP2, SU6656 (and even GW2580), used at the same concentrations, had no effect on the ability of BAC1.2F5 cells to adhere onto cell culture plastics (as a surrogate for polycarbonate - i.e. plastic - nucleopore filters) with respect to vehicle-treated cells (not shown).
Figure 3. Mechanism of FN-induced FAK activation in BAC1.2F5 cells. Actin polymerization is necessary for FN-induced FAK phosphorylation. (A) Cells were cultured in the absence of CSF-1 for 18 hours and then kept in suspension in the presence or not of cytochalasin D (CD, 10 μM) before being plated on FN- or PL-coated (10 μg/ml) dishes for the indicated times. Protein lysates were subjected to immunoprecipitation (IP) and immunoblotting with the indicated antibodies. Representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. SFK mediate FN-induced FAK phosphorylation. (B) Cells were cultured in the absence of CSF-1 for 18 hours and then kept in suspension for 45 minutes in the presence or the absence of the SFK inhibitor PP2 (10 μM) before being plated on FN- or PL-coated (10 μg/ml) dishes for the indicated times. Protein lysates were subjected to immunoprecipitation (IP) and/or immunoblotting with the indicated antibodies. Representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. SFK mediate FN-induced macrophage migration. (C) Cells were cultured in the absence of CSF-1 for 18 hours and then treated or not with the SFK inhibitors PP2 or SU6656 (10 μM) for 30 minutes before being subjected to migration assay toward DMEM containing or not FN (10 μg/ml). Migrated cells were counted. Histograms represent means ± SEM of data from 2 independent experiments performed in triplicate. Student's t test: *, p < 0.05; ns, not significant.
FN induces FAK-dependent CSF-1R phosphorylation that is required for macrophage migration
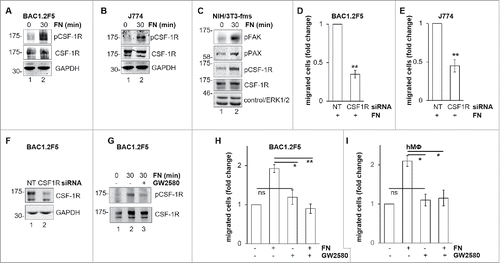
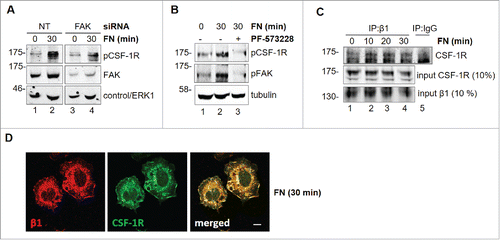
The existence of a cross-talk between integrins and RTK has been previously reported.Citation25 In order to identify a possible cross-talk between FN-elicited signals and CSF-1R, we determined whether adhesion on FN was able to induce CSF-1R phosphorylation (). We found that FN induced CSF-1R phosphorylation, as determined by evaluating CSF-1R phosphorylation on Y723, in BAC1.2F5 () and J774 () macrophages, but also in NIH/3T3 fibroblasts expressing ectopic CSF-1R (). In the latter cells, as expected, FN also induced FAK and paxillin phosphorylation. FN-induced CSF-1R phosphorylation was already marked after 10 minutes of stimulation (not shown). We then tested the impact of CSF-1R inhibition on FN-induced macrophage migration. Genetic inhibition of CSF-1R by specific siRNA () markedly reduced this migration in BAC1.2F5 () and J774 cells (). Furthermore, pharmacological inhibition of CSF-1R by GW2580 () prevented FN-induced migration in BAC1.2F5 cells () and human primary macrophages (). These results demonstrated that FN-induced macrophage migration requires CSF-1R activity.
Figure 4. Involvement of CSF-1R in FN-induced macrophage migration. FN induces CSF-1R phosphorylation. (A-C) Cells were cultured in the absence of CSF-1 for 18 hours (A) or in the absence of FBS for 24 hours (B, C) and kept in suspension for 45 minutes and then lysed or plated on FN-coated (10 μg/ml) dishes for 30 minutes before lysis. Protein lysates were subjected to immunoblotting with the indicated antibodies. Molecular weight markers are reported on the left of gels. Representative images of WB from one out of 3 independent experiments are shown. Genetic inhibition of CSF-1R prevents FN-induced macrophage migration. (D-F) Cells were transfected with non-targeting control (NT) or CSF-1R-specific (CSF1R) siRNA for 48 hours and then cultured in the absence of CSF-1 for 18 hours (D, F) or in the absence of FBS for 24 hours (E) and subjected to migration assay toward FN (D, E) or lysed and subjected to immunoblotting with the indicated antibodies (F). (D, E) Histograms represent means ± SEM of data from 3 independent experiments performed in triplicate. Student's t test: **, p< 0.01. (F) Representative images of WB from one out of 4 independent experiments are shown. Molecular weight markers are reported on the left of gels. Pharmacological inhibition of CSF-1R prevents FN-induced macrophage migration. (G) Cells were cultured in the absence of CSF-1 for 18 hours and then kept in suspension for 45 minutes and lysed or plated on FN-coated dishes (10 μg/ml) for 30 minutes in the presence or the absence of GW2580 (1μM). Cells were then lysed and proteins were subjected to immunoblotting with the indicated antibodies. Representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. (H, I) Cells were cultured in the absence of CSF-1 for 18 hours and then were treated or not with GW2580 (1μM) for 30 minutes before being subjected to migration assay toward DMEM containing or not 10 μg/ml FN. Migrated cells were then counted. Histograms represent means ± SEM of data from 3 (H) or 2 (I) independent experiments performed in triplicates. Student's t test: *, p < 0.05; **, p < 0.01; ns, not significant.
FN induces FAK-dependent CSF-1R activation
In order to characterize the mechanisms of the cross-talk between FN-elicited signals and CSF-1R, we tested whether FAK is involved. Genetic or pharmacological inhibition of FAK markedly reduced CSF-1R phosphorylation (), indicating that FAK mediates FN-induced CSF-1R phosphorylation. Previous reports indicated that β1 integrin, which is important for macrophage migration, is involved in integrin-RTK cross-talk.Citation26,27 Co-immunoprecipitation experiments () showed that the association of CSF-1R with β1 integrin is increased after 10 minutes of stimulation with FN. This interaction is maintained after 20 minutes and starts to decrease after 30 minutes. This association was confirmed by the co-localization of CSF-1R together with B1 at the level of cell edges following adhesion onto FN (). CSF-1R and β1 integrin appear to co-localize in structures that look more like ruffles than extending lamellipodia. This is possibly due to the fact that in the time-frame of the experiments of cells are adhering rather than moving. These results indicated that the formation of molecular complexes is the basis of the cross-talk between FN-elicited signals and CSF-1R.
Figure 5. Involvement of FAK in CSF-1R activation induced by FN in BAC1.2F5 cells. Genetic inhibition of FAK decreases FN-induced CSF-1R phosphorylation. (A) Cells were transfected with non-targeting control (NT) or FAK-specific (FAK) siRNA for 48 hours, kept in suspension for 45 minutes and then lysed or plated on FN-coated (10 μg/ml) dishes for 30 minutes. Protein lysates were subjected to immunoblotting with the indicated antibodies. Representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. Pharmacological inhibition of FAK prevents FN-induced CSF-1R phosphorylation. (B) Cells were cultured in the absence of CSF-1 for 18 hours and then kept in suspension for 45 minutes and lysed (0) or plated on FN-coated dishes (10 μg/ml) for 30 minutes in the presence or the absence of PF-573228 (1 μM) before lysis. Proteins were subjected to immunoblotting with the indicated antibodies. Representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. CSF-1R interacts with integrin β1 following adhesion on FN. (C) Cells were cultured in the absence of CSF-1 for 18 hours and then kept in suspension for 45 minutes and lysed (0) or plated on (10 μg/ml) FN-coated dishes for the indicated times (minutes) before lysis. Protein lysates were then subjected to co-immunoprecipitation (IP) and immunoblotting with the indicated antibodies. Representative images of WB from one out of 2 independent experiments are shown. CSF-1R co-localizes with B1 following adhesion onto FN. (D) Cells were cultured in the absence of CSF-1 for 18 hours, kept in suspension for 45 minutes and then plated on FN-coated coverglass (10 μg/ml) for 30 minutes. Cells were then stained with the indicated antibodies and analyzed by confocal microscopy. Representative images are from one out of 3 independent experiments. Scale bar: 10 μm.
Discussion
An altered expression of FN is observed in several pathological contexts, including inflammation and cancer.Citation6,28 Moreover, increased macrophage numbers may, with few exceptions, exacerbate inflammatory diseases and enhance cancer progression.Citation29 In this work, we identified a new pathway, SFK-FAK>CSF-1R, relevant for FN-induced macrophage migration. To strengthen our results, we used two different murine cell lines, one dependent on and one independent of CSF-1, as well as primary human macrophages.
We found that FN stimulates the migration of murine or human macrophages and the activation of SFK/FAK complex, as witnessed by the increased phosphorylation of activating residues in either kinase, as well as of paxillin.Citation9 We also observed that macrophage migration depends on FAK activity. A second member of the FAK family, Pyk2 (Proline-rich tyrosine kinase), is known to be central to macrophage migration.Citation30,31 Nevertheless, the specific involvement of FAK rather than of PYK2 was revealed using FAK-specific siRNA, as well as the chemical compound PF-573228, a selective inhibitor of FAK (IC50=4 nM) over Pyk2 (IC50>1μM). Our results are at variance with those showing that FAK phosphorylation in primary murine macrophages results in the increase of adhesion rather than chemotaxis.Citation32 These differences may be due to the different models used. Importantly, the fact that FAK inhibition markedly impaired macrophage migration is in keeping with a previous report indicating that the motility defects exhibited by FAK−/− macrophages are not compensated by endogenous Pyk2.Citation20
We also found that FAK activation and macrophage migration depend on SFK, as reported in other cellular contexts.Citation9,19,33 Moreover, FN-induced FAK activation was found to be dependent on cytoskeleton integrity, in keeping with the fact that, in response to migration stimuli, macrophages are known to polarize and extend lamellipodia and filopodia in the direction of the increasing chemotactic gradient.Citation34 Indeed, the formation of these protrusive structures is controlled by the dynamic reorganization of actin cytoskeleton.Citation35
The fact that FN induced a ligand-independent CSF-1R phosphorylation is in keeping with what already reported for other RTK following cross-talk with integrins.Citation10,11 Moreover, FN-induced CSF-1R phosphorylation was a rapid event, in keeping with previous studies relative to other RTK such as EGFR.Citation36 We also demonstrated that the trans-activation of CSF-1R by adhesion stimuli is relevant to macrophage migration. Indeed, inhibition of CSF-1R impaired FN-induced migration. This is consistent with the notion that CSF-1R activation induces morphological changes in response to CSF-1, including lamellipodia formation, dorsal ruffling, polarization, and CSF-1-directed chemotaxis.Citation18,37 Moreover, activated CSF-1R triggers the small GTPases Rac1 and Cdc42, which contribute to membrane ruffling and cell polarization.Citation37 An intriguing result was the fact that FN-induced CSF-1R phosphorylation occurred in fibroblasts expressing ectopic CSF-1R. This result points to a possibly wider role of this phenomenon in other CSF-1R-expressing cells including dendritic or tumor cells.Citation29,38
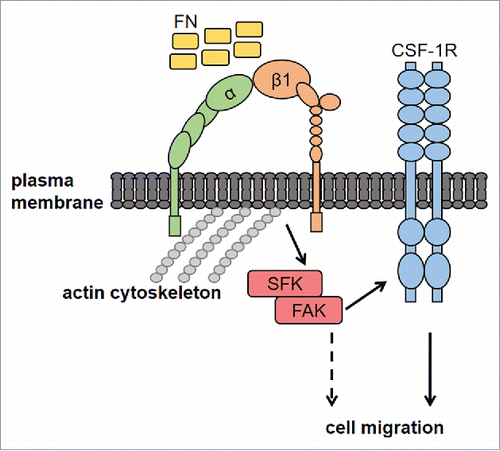
Different mechanisms are involved in the transactivation of RTK by integrin ligands.Citation11 In particular, kinases and adaptor molecules may be involved in the cross-talk between integrins and RTK. The mechanism we envision for the cross-talk between FN and CSF-1R in macrophages is summarised in . Our results indicated that the SFK/FAK complex is necessary for FN-induced transactivation of CSF-1R. Moreover, we found that CSF-1R and β1 integrin co-immunoprecipitate following stimulation with FN. The fact that CSF-1R and β1 integrin co-localize at the level of cell edges further supports their association. Nevertheless, we could not exclude an effect on CSF-1R stabilization following interaction with integrins.Citation11 We recently reported that CSF-1R may be trans-activated by the G-protein-coupled receptor for prostaglandin E2 and that CSF-1R is involved in macrophage migration induced by prostaglandin E2.Citation17 Therefore, CSF-1R appears to be an important junction for the regulation of macrophage migration in response to stimuli surrounding macrophages within physiologic as well as pathological microenvironments.
Figure 6. Proposed mechanisms of FN-induced macrophage migration through the SFK-FAK/CSF-1R pathway. Macrophage adhesion onto FN, through β1 integrin, activates the SFK/FAK complex, thereby leading to CSF-1R phosphorylation and migration. CSF-1R-independent migration induced by FN (dashed line) cannot be excluded, although not observed in our experiments. FN, fibronectin; CSF-1R, Colony-Stimulating Factor-1 Receptor; SFK, Src Family Kinases; FAK, Focal Adhesion Kinase; α and β1, integrin subunits.
Materials and methods
Cells and cell culture
Murine macrophages of the BAC1.2F5 cell line, which depends on CSF-1 for survival and proliferation were cultured in DMEM supplemented with 4 mM glutamine, 10% foetal bovine serum (FBS) and 10% L929 cell-conditioned medium as a source of CSF-1 (“complete medium”) as previously described.Citation17 NIH-3T3 murine fibroblasts expressing ectopic CSF-1R (NIH/3T3-Fms; kind gift of M.F. Roussel, St. Jude Children's Research Hospital, Memphis, TN, USA) and J774 murine macrophage cell line, derived from a reticular cell sarcoma, were cultured in DMEM supplemented with 4 mM glutamine and 10 % FBS.Citation39 Primary human macrophages were obtained following differentiation of monocytes from peripheral blood of healthy donor after informed consent (and after approval of the project BIO 14.015 by the ethical committee of Azienda Ospedaliero-Universitaria di Careggi, Firenze, Italy; prot. no 2014/00 26551). Mononucleated cells were obtained by density gradient centrifugation with Lympholyte (CEDARLANE) from buffy-coat preparations following the manufacturer's instructions. Monocytes were then separated from lymphocytes by adherence to tissue culture plastics. Cells were incubated for 2 hours at 37 °C with 5% CO2. Non -adherent cells were then removed and adherent cells were washed 2 or 3 times with PBS (37 °C; 10 mM Na2HPO4, 150 mM NaCl, pH 7.4) and then incubated for 7 d in RPMI culture medium supplemented with 10% FBS and 50 ng/ml human recombinant CSF-1 (ImmunoTools, Friesoythe, Germany). All the cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Cell lysis and western blotting
BAC1.2F5 cells and primary human macrophages were incubated in the absence of CSF-1 for 18 hours, whereas J774 and NIH/3T3-Fms cells in the absence of FBS for 24 hours, before being stimulated or not with the appropriate stimuli. Noteworthy, CSF-1 was never added to the cells as a stimulus in the experiments reported in the present manuscript. In experiments where adhesion on FN (cat no F1141; Sigma) or poly-L-lysine (PL; cat no P6407; Sigma) was allowed, cells were kept in suspension for 45 minutes in order to lower adhesion-dependent stimuli. Cell culture Petri dishes coating was performed by incubating overnight at room temperature with different concentration of FN or PL dissolved in PBS. Dishes were then washed twice with PBS, treated with 2% heat-inactivated bovine serum albumin (cat no A3311; Sigma) for 2 h at room temperature, and washed twice with PBS prior to use.
Total cell lysates and western blotting were performed as previously described.Citation40 To immunoprecipitate FAK, adherent cells were lysed in RIPA buffer (20 mM Tris-HCl, pH 7.05, 5 mM EDTA, 50 mM NaF, 30 mM Na4P2O7, 1 mM Na3VO4, 1% (v/v) triton, 1 mM PMSF, 0.2 U/ml Aprotinin, 1 mM TPKC). Samples were then incubated with anti-FAK (C-20; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) primary antibodies for 2 hours at 4°C, followed by incubation with protein A-Sepharose beads overnight at 4°C. After several washes with RIPA buffer, bound proteins were eluted in Laemmli buffer (62.5 mM Tris-HCl, pH 6.8, 10% glycerol, 0.005% blue bromophenol, 2% SDS) and analyzed by SDS-PAGE. Primary antibodies used for immunoblotting were: rabbit monoclonal anti-phospho-Y723-M-CSFReceptor/CSF-1R (corresponding to Y721 of murine CSF-1R, cat no 3155), rabbit polyclonal anti-phospho-Y118 Pax (cat no 2541), rabbit polyclonal anti-phospho-ERK1 (T202/Y204) and ERK2 (T185/Y187) (cat no 9101), rabbit polyclonal anti-phospho-Y416-Src (cat no 2101), from Cell Signaling Technology, Inc. (Danvers, MA, USA); rabbit polyclonal anti-phospho-Y397 FAK (cat no sc-11765), goat polyclonal anti-β1 (cat no sc-6622), rabbit polyclonal anti-FAK (sc-558), rabbit polyclonal anti-ERK1 (cat no sc-93), goat polyclonal anti-GAPDH (cat no sc-20357), rabbit polyclonal anti-CSF-1R (cat no sc-692), rabbit polyclonal anti-c-Src (cat no sc-19) from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). In general, immunoblotting was performed after striping on the same membrane or, more rarely, on parallel gels performed with the sample of the same experiment.
Co-immunoprecipitation
For co-immunoprecipitation (Co-IP), adherent cells were lysed in Co-IP buffer (20 mM Tris-HCl, pH 7.6, 5 mM EDTA, 1% (v/v) NP-40, with 0.1 mM Na3VO4, 30 mM Na4P2O7, 50 mM NaF, 1 mM PMSF, 0.2 U/ml Aprotinin, 1 mM TPKC). Samples were then incubated with primary antibodies for 2 hours at 4°C and then with protein A-Sepharose beads overnight at 4°C. After several washes with Co-IP buffer, bound proteins were eluted in Laemmli buffer and analyzed by SDS-PAGE.
FAK and CSF1R silencing with small interfering RNA (siRNA)
Cells were seeded in 60mm-diameter dishes in complete medium and incubated for 24 hours (50% confluence). FAK or CSF1R gene silencing was performed with 40 nM SMARTpool siRNA, targeting FAK (cat no M-041099-00, Dharmacon Inc., Lafayette, CO, USA), targeting CSF-1R (cat no M-044650-01, Dharmacon Inc., Lafayette, CO, USA) or 40 nM siCONTROL non-targeting (siNT, cat no D-001206-13, Dharmacon Inc.) pool, as previously described.Citation39 It should be noted that smart-pool siRNA from Dharmacon are designed in order to lower off-side effects. The manufacturer's protocol shows in particular that, while individual duplexes delivered at 40 nM can modulate a varying numbers of off-targeted genes, transfection of the corresponding SMARTpool siRNA (40 nM total concentration) alters only a fraction of the total off-target profile. On the other hand, the SMARTpool non-targeting siRNA we used as control should result in similar off-targets effects determined by the SMARTpool siRNA targeting FAK or SMARTpool siRNA targeting CSF1R. Treatment with lipofectamine alone did not determine significant effects in migration assays or Western blotting.
Migration assay
Migration assay was performed as previously described.Citation17 Briefly, macrophages (4 × 104 or 7 × 104/well for macrophage cell lines or primary human macrophages, respectively) were seeded in DMEM, in the presence or the absence of inhibitory treatment, onto the top chamber of 48-well trans-well plates equipped with 8 μm polycarbonate nucleopore filters (Neuro Probe, Gaithersburg, MD, USA). The bottom chamber was supplemented with treatment-containing or control medium. After incubation (1 or 2 hours for macrophage cell lines or primary macrophages, respectively) cells that had not migrated were removed with a cotton swab from the upper surface of filters, and cells that had migrated to the lower surface of the membrane were subjected to Diff-Quick staining (Medion Diagnostics AG, Dudingen, Switzerland) and observed with a light microscope. The number of cells per well was evaluated by counting cells in 5 randomly-chosen microscope fields (20x magnification).
Immunofluorescence
Cells were plated on glass coverslips that had been previously coated with 10 μg/ml PL or FN. Immunofluorescence was performed as previously described.Citation41 Briefly, after fixation with 4% paraformaldehyde and permeabilization cells were incubated (overnight, 4°C) in PBS-1% BSA supplemented with a 1:500 dilution of a mouse anti-Vinculin antibody (cat no V9131, Sigma-Aldrich, St. Louis, MO, USA), goat anti-β1 (cat no sc-6622) or rabbit polyclonal anti-CSF-1R (cat no sc-692) and then with Cy3- or Cy2-labeled secondary antibodies (Chemicon, Temecula, CA, USA). After extensive washing, the coverslips were mounted with propyl-thiogallate and then observed with an inverted confocal Nikon Eclipse TE2000 microscope. The C1 software was used for image acquisition. Cell contours have been drawn by following focal contacts at cellular edges using ImageJ Software. The ImageJ Software for also used for cell area calculation (around 50 cells/condition were counted). Incubation with the secondary Ab alone did not produce any significant fluorescence.
Reagents
The inhibitors used (substrate; time of pretreatment; manufacturer) were: PP2 and SU6656Citation42,43 (SFK; 30 minutes; Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), GW2580Citation44 (CSF-1R; 30 minutes; BioVision, Milpitas, CA, USA), PF-573228Citation45 (FAK, 30 minutes; MedChem Express). Kinase inhibitors were used at the concentrations reported in literature.Citation17,45 Treatment with dimethyl sulfoxide (DMSO), used as vehicle for all inhibitors, did not determine any effect in migration assays or Western blotting. Protein A Sepharose, FN and cytochalasin D (CD, cat no C8273) were from Sigma-Aldrich (St. Louis, MO, USA).
Abbreviations
| CSF-1 | = | Colony-Stimulating Factor-1 |
| CSF-1R | = | CSF-1 Receptor |
| RTK | = | Receptor Tyrosine Kinase |
| FN | = | fibronectin |
| PL | = | polylysine |
| FBS | = | foetal bovine serum |
| DMEM | = | Dulbecco's modified eagle medium |
| RPMI | = | Roswell Park Memorial Institute |
| ERK | = | Extracellular signal-Regulated Kinase |
| M-CSF | = | Macrophage Colony-Stimulating Factor |
| GAPDH | = | glyceraldehyde-3-phosphate dehydrogenase |
| SFK | = | Src Family Kinases |
| MEK | = | Mitogen-activated ERK Kinase |
| siRNA | = | small interfering RNA |
| DMSO | = | dimethylsulfoxide |
| CD | = | cytochalasin D |
| FAK | = | Focal Adhesion Kinase |
| SEM | = | standard error of the mean |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
GD, MGC, ER, MB and IT performed experiments. ER and GD designed experiments. GD and ER analyzed results and wrote the paper together with PDS.
Funding
This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC, #IG13466 to PDS, #IG15282 to ER), Istituto Toscano Tumori (PDS), Ministero della Salute (Ricerca Finalizzata, Grant #RF-TOS-2008-1163728 to PDS), Regione Toscana (Programma per la Ricerca in Materia di Salute to PDS), Fondazione Cassa di Risparmio di Volterra (PDS) and Università degli Studi di Firenze (Fondo di Ateneo ex-60 %; ER).
References
- Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci 2009; 122:159-63; PMID:19118207; https://doi.org/10.1242/jcs.018093
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110(6):673-87; PMID:12297042; https://doi.org/10.1016/S0092-8674(02)00971-6
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol 2001; 2(11):793-805; PMID:11715046; https://doi.org/10.1038/35099066
- Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol 2011; 209(2):139-51; PMID:21307119; https://doi.org/10.1530/JOE-10-0377
- Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb Perspect Biol 2011; 3(9):a005074; PMID:21885598; https://doi.org/; https://doi.org/10.1101/cshperspect.a005074
- Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res 1999; 76:1-20; PMID:10218097; https://doi.org/10.1016/S0065-230X(08)60772-1
- Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci 2010; 123:4195-200; PMID:21123617; https://doi.org/10.1242/jcs.023820
- Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol 2010; 341(1):126-40; PMID:19854168; https://doi.org/10.1016/j.ydbio.2009.10.026
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 2006; 18(5):516-23; PMID:16919435; https://doi.org/10.1016/j.ceb.2006.08.011
- Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol 2003; 19:173-206; PMID:14570568; https://doi.org/10.1146/annurev.cellbio.19.031103.133334
- Alam N, Goel HL, Zarif MJ, Butterfield JE, Perkins HM, Sansoucy BG, Sawyer TK, Languino LR. The integrin-growth factor receptor duet. J Cell Physiol 2007; 213(3):649-53; PMID:17886260; https://doi.org/10.1002/jcp.21278
- Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol 2014; 6(6):a021857; PMID:24890514; https://doi.org/10.1101/cshperspect.a021857
- Faccio R, Takeshita S, Zallone A, Ross FP, Teitelbaum SL. c-Fms and the alphavbeta3 integrin collaborate during osteoclast differentiation. J Clin Invest 2003; 111(5):749-58; PMID:12618529; https://doi.org/10.1172/JCI200316924
- White ES, Livant DL, Markwart S, Arenberg DA. Monocyte-fibronectin interactions, via α(5)β(1) integrin, induce expression of CXC chemokine-dependent angiogenic activity. J Immunol 2001; 167(9):5362-66; PMID:11673553; https://doi.org/10.4049/jimmunol.167.9.5362
- Lanir N, Ciano PS, Van de Water L, McDonagh J, Dvorak AM, Dvorak HF. Macrophage migration in fibrin gel matrices. II. Effects of clotting factor XIII, fibronectin, and glycosaminoglycan content on cell migration. J Immunol 1988; 140(7):2340-9; PMID:3351302
- Murray MY, Birkland TP, Howe JD, Rowan AD, Fidock M, Parks WC, Gavrilovic J. Macrophage migration and invasion is regulated by MMP10 expression. PLoS One 2013; 8(5):e63555; PMID:23691065; https://doi.org/10.1371/journal.pone.0063555
- Digiacomo G, Ziche M, Dello Sbarba P, Donnini S, Rovida E. Prostaglandin E2 transactivates the colony-stimulating factor-1 receptor and synergizes with colony-stimulating factor-1 in the induction of macrophage migration via the mitogen-activated protein kinase ERK1/2. FASEB J 2015; 29(6):2545-54; PMID:25757564; https://doi.org/10.1096/fj.14-258939
- Boocock CA, Jones GE, Stanley ER, Pollard JW. Colony-stimulating factor-1 induces rapid behavioural responses in the mouse macrophage cell line, BAC1.2F5. J Cell Sci 1989; 93(Pt 3):447-56; PMID:2532650
- Rovida E, Lugli B, Barbetti V, Giuntoli S, Olivotto M, Dello Sbarba P. Focal adhesion kinase is redistributed to focal complexes and mediates cell spreading in macrophages in response to M-CSF. Biol Chem 2005; 386(9):919-29; PMID:16164417; https://doi.org/10.1515/BC.2005.107
- Abshire MY, Thomas KS, Owen KA, Bouton AH. Macrophage motility requires distinct α5β1/FAK and α4β1/paxillin signaling events. J Leukoc Biol 2011; 89(2):251-7; PMID:21084629; https://doi.org/10.1189/jlb.0710395
- Owen KA, Pixley FJ, Thomas KS, Vicente-Manzanares M, Ray BJ, Horwitz AF, Parsons JT, Beggs HE, Stanley ER, Bouton AH. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J Cell Biol 2007; 179(6):1275-87; PMID:18070912; https://doi.org/10.1083/jcb.200708093
- Hauck CR, Hsia DA, Schlaepfer DD. The focal adhesion kinase–a regulator of cell migration and invasion. IUBMB Life 2002; 53(2):115-9; PMID:12049193; https://doi.org/10.1080/15216540211470
- Lipfert L, Haimovich B, Schaller MD, Cobb BS, Parsons JT, Brugge JS. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol 1992; 119(4):905-12; PMID:1385445; https://doi.org/10.1083/jcb.119.4.905
- Abram CL, Lowell CA. The diverse functions of Src family kinases in macrophages. Front Biosci 2008; 13:4426-50; PMID:18508521; https://doi.org/10.2741/3015
- Sundberg C, Rubin K. Stimulation of beta1 integrins on fibroblasts induces PDGF independent tyrosine phosphorylation of PDGF β-receptors. J Cell Biol 1996; 132(4):741-52; PMID:8647902; https://doi.org/10.1083/jcb.132.4.741
- Yu X, Miyamoto S, Mekada E. Integrin α 2 β 1-dependent EGF receptor activation at cell-cell contact sites. J Cell Sci 2000; 113(Pt 12):2139-47; PMID:10825287
- Veevers-Lowe J, Ball SG, Shuttleworth A, Kielty CM. Mesenchymal stem cell migration is regulated by fibronectin through α5β1-integrin-mediated activation of PDGFR-β and potentiation of growth factor signals. J Cell Sci 2011; 124(Pt 8):1288-300; PMID:21429937; https://doi.org/10.1242/jcs.076935
- To WS, Midwood KS. Plasma and cellular fibronectin: distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair 2011; 4:21; PMID:21923916; https://doi.org/10.1186/1755-1536-4-21
- Rovida E, Dello Sbarba P. Colony-stimulating factor-1 receptor in the polarization of macrophages: A target for turning bad to good ones? J Clin Cell Immunol 2015; 6:6; https://doi.org/10.4172/2155-9899.1000379
- Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, Schlessinger J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci U S A 2003 Sep 16; 100(19):10740-5; PMID:12960403; https://doi.org/10.1073/pnas.1834348100
- Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol 1999; 71(3–4):435-78. Review; PMID:10354709; https://doi.org/10.1016/S0079-6107(98)00052-2
- Osma-Garcia IC, Punzón C, Fresno M, Díaz-Muñoz MD. Dose-dependent effects of prostaglandin E2 in macrophage adhesion and migration. Eur J Immunol 2016; 46(3):677-88; PMID:26631603; https://doi.org/10.1002/eji.201545629
- Dwyer AR, Mouchemore KA, Steer JH, Sunderland AJ, Sampaio NG, Greenland EL, Joyce DA, Pixley FJ. Src family kinase expression and subcellular localization in macrophages: implications for their role in CSF-1-induced macrophage migration. J Leukoc Biol 2016; 100(1):163-75, pii: jlb.2A0815-344RR. [Epub ahead of print]; PMID:26747837; https://doi.org/10.1189/jlb.2A0815-344RR
- Calle Y, Burns S, Thrasher AJ, Jones GE. The leukocyte podosome. Eur J Cell Biol 2006; 85(3–4):151-7; PMID:16546557; https://doi.org/10.1016/j.ejcb.2005.09.003
- Jones GE. Cellular signaling in macrophage migration and chemotaxis. J Leukoc Biol 2000; 68(5):593-602; PMID:11073096
- Moro L, Dolce L, Cabodi S, Bergatto E, Boeri Erba E, Smeriglio M, Turco E, Retta SF, Giuffrida MG, Venturino M, et al. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J Biol Chem 2002; 277(11):9405-14; PMID:11756413; https://doi.org/10.1074/jbc.M109101200
- Allen WE, Jones GE, Pollard JW, Ridley AJ. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci 1997; 110(Pt6):707-20; PMID:9099945
- MacDonald KP, Rowe V, Bofinger HM, Thomas R, Sasmono T, Hume DA, Hill GR. The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J Immunol 2005; 175(3):1399-405; PMID:16034075; https://doi.org/10.4049/jimmunol.175.3.1399
- Rovida E, Spinelli E, Sdelci S, Barbetti V, Morandi A, Giuntoli S, Dello Sbarba P. ERK5/BMK1 is indispensable for optimal colony-stimulating factor 1 (CSF-1)-induced proliferation in macrophages in a Src-dependent fashion. J Immunol 2008; 180(6):4166-72; PMID:18322228; https://doi.org/10.4049/jimmunol.180.6.4166
- Barbetti V, Tusa I, Cipolleschi MG, Rovida E, Dello Sbarba P. AML1/ETO sensitizes via TRAIL acute myeloid leukemia cells to the pro-apoptotic effects of hypoxia. Cell Death Dis 2013; 4:e536; PMID:23492767; https://doi.org/10.1038/cddis.2013.49
- Barbetti V, Morandi A, Tusa I, Digiacomo G, Riverso M, Marzi I, Cipolleschi MG, Bessi S, Giannini A, Di Leo A, Dello Sbarba P, Rovida E. Chromatin-associated CSF-1R binds to the promoter of proliferation-related genes in breast cancer cells. Oncogene 2014; 33(34):4359-64; PMID:24362524; https://doi.org/10.1038/onc.2013.542
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem 1996; 271(2):695-701; PMID:8557675; https://doi.org/10.1074/jbc.271.2.695
- Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol 2000; 20(23):9018-27; PMID:11074000; https://doi.org/10.1128/MCB.20.23.9018-9027.2000
- Conway JG, McDonald B, Parham J, Keith B, Rusnak DW, Shaw E, Jansen M, Lin P, Payne A, Crosby RM, et al. Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. Proc Natl Acad Sci U S A 2005; 102(44):16078-83; PMID:16249345; https://doi.org/10.1073/pnas.0502000102
- Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, Luzzio MJ, Cooper B, Kath JC, Roberts WG, et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem 2007; 282(20):14845-52; PMID:17395594; https://doi.org/10.1074/jbc.M606695200
