ABSTRACT
Osteosarcoma patients often exhibit pulmonary metastasis, which results in high patient mortality. Understanding the mechanisms of advanced metastasis in osteosarcoma cell is important for the targeted treatment and drug development. Our present study revealed that transforming growth factor-β (TGF-β) treatment can significantly promote the in vitro migration and invasion of human osteosarcoma MG-63 and HOS cells. The loss of epithelial characteristics E-cadherin (E-Cad) and up regulation of mesenchymal markers Vimentin (Vim) suggested TGF-β induced epithelial-mesenchymal transition (EMT) of osteosarcoma cells. TGF-β treatment obviously increased the expression of Snail, a key EMT-related transcription factor, in both MG-63 and HOS cells. Silencing of Snail markedly attenuated TGF-β induced down regulation of E-cad and up regulation of Vim. TGF-β treatment also significantly increased the expression and nuclear translocation of estrogen-related receptors α (ERRα), while had no obvious effect on the expression of ERα, ERβ, or ERRγ. Knock down of ERRα or its inhibitor XCT-790 significantly attenuated TFG-β induced EMT and transcription of Snail in osteosarcoma cells. Collectively, our present study revealed that TGF-β treatment can trigger the EMT of osteosarcoma cells via ERRα/Snail pathways. Our data suggested that ERRα/Snail pathways might be potential therapeutic targets of metastasis of osteosarcoma cells.
KEYWORDS:
Introduction
Osteosarcoma, which develops from primitive transformed cells of mesenchymal origin, is the most common histological form of primary bone cancer.Citation21 Half of the osteosarcoma patients often exhibit pulmonary metastasis, which results in high patient mortality. Despite that development of combination treatment with radical surgery and chemotherapy has significantly increased the survival rates from 20 to 75%,Citation11 the outcome is still poor and most of them will die due to local relapse or pulmonary metastases.Citation8 The clinical prognosis and outcome for these recurrent or metastatic patients are extremely poor.Citation33 Therefore, understanding the mechanisms of advanced metastasis in osteosarcoma cell is important for the targeted treatment and drug development.
Epithelial-mesenchymal transition (EMT), which is a process through which epithelial cells lose their polarity and are converted into a mesenchymal phenotype, has been considered as the first and critical step for cancer metastasis.Citation26 During EMT, epithelial cells will loss the epithelial characteristics and acquire a mesenchymal phenotype accompanied by increased Vimentin (Vim) expression. Loss or reduction of E-cadherin (E-Cad) is a well-established hallmark of EMT.Citation27 EMT-related transcription factors such as Twist, Snail, Slug, and ZEB can repress E-cad expression by binding to the E-box in the E-cad gene promoter, and consequently promote EMT.Citation3,30 Recent studies indicated that expression of EMT-related transcription factors, such as Twist, Snail, and ZEB are involved in the complex pathogenesis of osteosarcoma.Citation31 Therefore targeting EMT might provide a novel opportunity in osteosarcoma treatment by controlling metastasis.
Estrogen-related receptors α (ERRα) is an orphan nuclear receptor which expressed in tissues with high-energy demand such as heart, kidney, skeletal muscle.Citation18 Due to the structural similarities with estrogen receptor, initial studies mainly focused on the potential cross talk between these 2 receptors. Further studies indicated that ERRα can bind to and activate transcription through ERR-response elements (ERREs), which is different from those mediating the estrogenic response (Estrogen Response Elements, EREs).Citation9 ERRα can directly regulate tumor progression of various cancer cells via modulation of cell motility. Studies indicated that over expression of ERRα in xenografted breast cancer cells increases their metastatic capacities.Citation1,10,24 The expression of ERRα is highly detected in osteoblastic cells.Citation4 Further, it can confer methotrexate resistance via attenuation of reactive oxygen species (ROS) production in OS cells.Citation6 However, the roles of ERRα in OS progression and whether it is related to EMT process are still not studied.
In this study, we demonstrated that transforming growth factor-β (TGF-β) treatment can promote the in vitro cell motility of osteosarcoma cells via induction of EMT. The up regulation of Snail was essential for TGF-β induced EMT. TGF-β can trigger the expression and nuclear translocation of ERRα. While inhibition of ERRα obviously attenuated TGF-β induced EMT and Snail expression in osteosarcoma cells.
Materials and methods
Cell culture and transfection
The human osteosarcoma cell line MG-63 and HOS were purchased from the American Type Cell Culture Collection (Manassas, VA, USA). The cells were maintained in DMEM medium, which was supplemented with 20 mM HEPES, 10% heat-inactivated fetal bovine serum, 2 mM-glutamine, penicillin (100 U/mL), and streptomycin (100 μg/mL), at 37°C with 5% CO2. For cell transfection, cells were seeded into plates in order to reach 30–50% confluence and transfected with siRNA negative control (si-NC: 5′-GGC TAC GTC CAG GAG CGC A-3′), si-Snail (5′-UGC AGU UGA AGA UCU UCC GCG ACU G-3′), or si-ERRα (5′-ATC GAG AGA TAG TGG TCA CCA TCA G −3′) by use of Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instruction.
In vitro would healing assay
Confluent cell monolayers were seeded and scratched by use of a 100 μl tip after cells formed a confluent mono-layer. The closure of scratch was analyzed under the microscope and images were captured after incubation for the indicated times. Average distances between wound edges were calculated by measuring the uncovered wound area and dividing by the width of the field of view. Distance migrated was calculated by subtracting the average distance between wound edges from that at the beginning. For each experiment a total of 12 wounds were measured per group, and each experiment was repeated 3 times.
In vitro invasion assay
Cancer cell invasion was assessed by a chamber-based invasion assay.Citation7 Briefly, the upper surface of a filter (pore size, 8.0 μm; Millipore, Billerica, USA) was coated with basement membrane matrigel (BD Biosciences, Franklin Lakes, USA). The cells were suspended in medium containing 1% FBS. Then the cells in suspension (1.0 × 105) were added to the upper chambers. Simultaneously, 500 μl of DMEM containing 10% FBS was placed in the lower chambers. Then cells were allowed to migrate at 37 C for the indicated times. Then membranes were fixed in 70% methanol at −20°C and the migrated cells were stained for nuclei with Hoechst 33342 dye (1 μg/mL) (blue fluorescent) and evaluated by counting cell nuclei in 10 randomly chosen fields under fluorescence microscopy. Each invasion assay was repeated in 3 independent experiments.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from cells using an RNeasy Plus Mini Kit (Qiagen) according to the manufacturer's instructions. The first strand of cDNA was synthesized using Superscript II Reverse transcriptase (Invitrogen Ltd., Paisley, Scotland, UK) and random hexamer primers. Quantitative real time PCR (qRT-PCR) was carried out as previously described.Citation28 Expression values were measured in triplicate on a Roche LightCycler 480 and normalized to GAPDH expression. Results are computed as fold induction relative to controls. E-cad, 5′-GGT TAT TCC TCC CAT CAG CT-3′ (forward) and 5′-CTT GGC TGA GGA TGG TGT A-3′ (reverse); zonula occludens-1 (ZO-1), 5′-CTG AAG AGG ATG AAG AGT ATT ACC-3′ (forward) and 5′-TGA GAA TGG ACT GGC TTG G-3′ (reverse); firbonectin (FN), 5′-GGA CT GCA TTG CCT ACT CG-3′ (forward) and 5′-GAA TCC TGG CAT TGG TCG AC-3′ (reverse); Vim, 5′-GAG TCC ACT GAG TAC CGG AG-3′ (forward) and 5′-ACG AGC CT TTC CTC CTT CA-3′ (reverse); Snail, 5′-GAC CAC TAT GCC GCG CTC TT−3′ (forward) and 5′-TCG CTG TAG TTA GGC TTC CGA TT−3′ (reverse); Slug, 5′-AGC AGT TGC ACT GTG ATG CC-3′ (forward) and 5′-ACA CAG CAG CCA GAT TCC TC-3′ (reverse); Twist, 5′-CGG ACA AGC TGA GCA AGA TT-3′ (forward) and 5′-CCT TCT CTG GAA ACA ATG AC-3′ (reverse); ZEB1, 5′-GCA CCT GAA GAG GAC CAG AG-3′ (forward) and 5′-TGC ATC TGG TGT TCC ATT TT-3′ (reverse); GAPDH, 5′-GAC TCA TGA CCA CAG TCC ATG C-3′ (forward) and 5′-AGA GGC AGG GAT GAT GTT CTG-3′ (reverse). CT values were reported relative to GAPDH RNA. The results were expressed by the comparative CT method (2−ΔΔCT). All experiments were performed 3 times independently and the average was used for comparison.
Western blot analysis
Cells at the logarithmic phase were lysed in lysis buffer. The total proteins were extracted by 12% SDS-PAGE, transferred onto PVDF membranes (Pierce, Rockford, IL, USA) and then incubated overnight with specific antibodies followed by incubation with HRP-conjugated secondary antibodies (Abcam). GAPDH (Santa Cruz) was used as loading control. Protein expression was detected by a chemiluminescence kit (Amersham Biosciences).
Immunofluorescence
Cells were treated with or without TGF-β (20 ng/ml) for the indicated times and then fixed in 4% paraformaldehyde for 10 minutes and blocked with goat serum over night at 4°C, then incubated with antibody at 1:100 for 1 h at 37 °C. Cells were washed 3 times with PBS and then incubated with a secondary anti-mouse antibody conjugated to FITC at 1:1000 for 1 h at 37 °C. Finally, cells were washed, incubated with DAPI (10 μg/ml) for 10 min to visualize cell nuclei, and examined with Confocal Laser Scanning Microscopy (Zeiss, Germany) to analyze nuclear translocation of ERRα.
Statistical analysis
Data are presented as mean ± standard deviation. Statistical comparison was performed using the Student's t test in Microsoft Excel. In all cases, P < 0.05 was considered significant.
Results
TGF-β triggers the in vitro motility of osteosarcoma cells
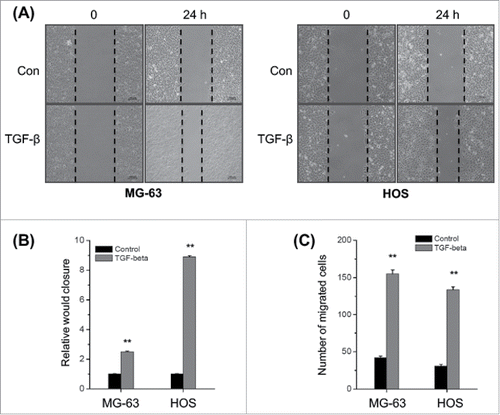
Previous studies suggested that TGF-β can promote the cell motility and EMT of various cancer cells.Citation22 However, the data about effects of TGF-β on the metastasis of osteosarcoma cells are very limited. We then evaluated the effects of TGF-β on the in vitro migration and invasion of osteosarcoma cells by use of would healing and transwell assays. Our results revealed that TGF-β (20 ng/ml) treatment can significantly (p < 0.05) increase the wound closure of both MG-63 and HOS cells (). Furthermore, TGF-β (20 ng/ml) treatment also significantly (p < 0.05) increased the in vitro invasion of MG-63 and HOS cells (). These results confirmed that TGF-β can trigger the in vitro motility of osteosarcoma cells.
Figure 1. TGF-β triggers the in vitro motility of osteosarcoma cells. (A) MG-63 and HOS cells were treated with or without TGF-β (20 ng/ml) and then scraped by a pipette tip to generate wounds for 48 h, representative images of wounds were observed; (B) The statistic results of wound healing assays; (C) MG-63 and HOS cells treated with or without TGF-β (20 ng/ml) were allowed to invade transwell chambers for 48 h, then the invaded cells were fixed, stained, and counted. Data are presented as means ± SD of 3 independent experiments. **p < 0.01 compared with control.
TGF-β triggers the EMT of osteosarcoma cells
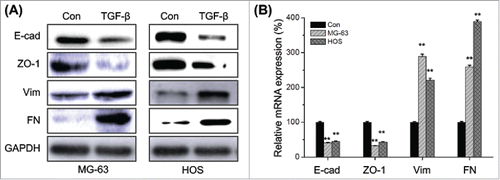
We therefore investigated the effects of TGF-β on the EMT of osteosarcoma cells. The EMT related markers in TGF-β treated MG-63 and HOS cells were measured by Western blot analysis. Our results showed that TGF-β treatment can obviously down regulate the expression of E-Cad and ZO-1, while increase the expression of Vim and FN in both MG-63 and HOS cells (). Furthermore, qRT-PCR results also confirmed that TGF-β treatment can decrease E-Cad and ZO-1 while increase FN and Vim at mRNA levels (). Collectively, our result suggested that TGF-β can trigger the EMT of osteosarcoma cells.
Figure 2. TGF-β triggers the EMT of osteosarcoma cells. (A) MG-63 and HOS cells were treated with or without TGF-β (20 ng/ml) for 48 h, then the protein levels of E-cad, ZO-1, Vim, and FN were analyzed by Western blot analysis; (B) MG-63 and HOS cells were treated with or without TGF-β (20 ng/ml) for 24 h, then the mRNA levels of E-cad, ZO-1, Vim, and FN were analyzed by qRT-PCR. Data are presented as means ± SD of 3 independent experiments. **p < 0.01 compared with control.
Up regulation of Snail mediates TFG-β induced EMT of osteosarcoma cells
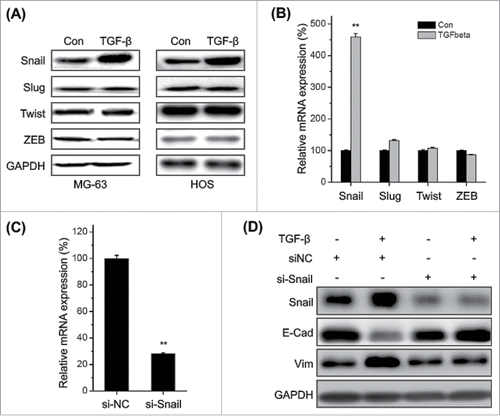
Transcription factors such as Snail, ZEB1, Twist and Slug play essential roles in regulating EMT,Citation5 then their roles in TFG-β induced EMT of osteosarcoma cells were investigated. Our data revealed that TFG-β treatment significantly increased the expression of Snail, while not Slug, Twist, and Zeb1 in both MG-63 and HOS cells (). This was confirmed by the results of qRT-PCR in MG-63 cells (). Furthermore, silencing of Snail via si-Snail () markedly attenuated TFG-β induced down regulation of E-Cad and up regulation of Vim in MG-63 cells (). Generally, these observations demonstrated that up regulation of Snail mediates TGF-β induced EMT of osteosarcoma cells.
Figure 3. Up regulation of Snail mediates TFG-β induced EMT of osteosarcoma cells. MG-63 and HOS cells were treated with TFG-β (20 ng/ml) for 24 h, the protein (A) and mRNA (B) levels of Snail, Slug, Twist, and ZEB were analyzed by Western blot analysis or qRT-PCR, respectively; (C) MG-63 cells were transfected with Snail specific si-RNA (si-Snail) or negative control si-RNA (si-NC) for 24 h, and then the mRNA of Snail were analyzed by qRT-PCR; (D) MG-63 cells transfected with si-Snail or si-NC were stimulated with or without TFG-β (20 ng/ml) for 48 h, the protein levels of Snail, E-Cad, and Vim were analyzed by Western blot analysis. Data were presented as means ± SD of 3 independent experiments. **p < 0.01 compared with control.
TGF-β increases the expression and nuclear localization of ERRα in osteosarcoma cells
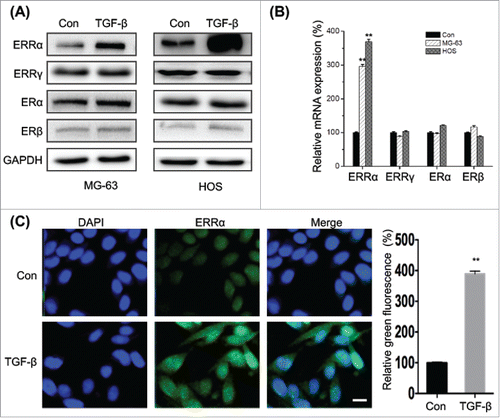
Recent studies suggested that TGF-β can trigger the EMT and cross talk with estrogenic signals.Citation2 Interestingly, our results revealed that TGF-β treatment can obviously increase the expression of ERRα in both MG-63 and HOS cells, while have limited effects on the protein expression of ERα, ERβ, or ERRγ (). This was confirmed by the result of qRT-PCR that TGF-β treatment can significantly trigger the mRNA expression of ERRα, while not others (). Furthermore, the results of immunofluorescence confirmed that TGF-β not only increased the expression of ERRα but also facilitated its nuclear translocation (). Collectively, the results suggested that TGF-β treatment can significantly increase the expression and nuclear translocation of ERRα in osteosarcoma cells.
Figure 4. TGF-β increases the expression and nuclear localization of ERRα in osteosarcoma cells. (A) MG-63 and HOS cells were treated with or without TGF-β (20 ng/ml) for 24 h, and then the express of protein (A) and mRNA (B) of ERRα, ERα, ERβ, or ERRγ were measured by use of Western blot analysis and qRT-PCR, respectively; (C) MG-63 cells were treated with or without TGF-β (20 ng/ml) for 24 h, the cellular location of ERRα (green) were examined by immunofluorescence staining and nuclei were stained with DAPI (blue). The quantification results were shown in the right column. Data were presented as means ± SD of 3 independent experiments. **p < 0.01 compared with control. Scale bar = 50 μm.
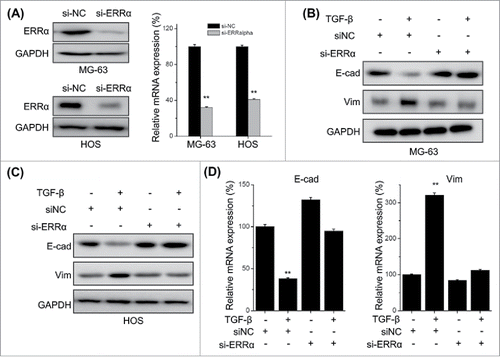
Knock down of ERRα attenuates TFG-β induced EMT of osteosarcoma cells
Previous studies indicated that ERRα can trigger the migration and invasion of cancer cells via induction of EMT.Citation16,29 We further evaluated the roles of ERRα in TGF-β induced EMT of osteosarcoma cells. Both MG-63 and HOS cells were transfected with si-ERRα and then treated with TGF-β. Both qRT-PCR and Western blot analysis revealed that ERRα was successfully silenced by si-ERRα (). The results showed that si-ERRα can obviously attenuate TGF-β induced down regulation of E-Cad and up regulation of Vim in both MG-63 () and HOS () cells. This was also confirmed by the results of qRT-PCR in MG-63 cells (), while showed that si-ERRα can significantly reverse TGF-β induced down regulation of E-Cad and up regulation of Vim at mRNA levels. These results suggested that ERRα mediates TGF-β induced EMT of osteosarcoma cells.
Figure 5. Knock down of ERRα attenuates TFG-β induced EMT of osteosarcoma cells. MG-63 and HOS cells were transfected with si-NC or si-ERRα for 24 h, and then the expression of ERRα was measured by use of Western blot analysis and qRT-PCR; MG-63 (B) and HOS (C) cells were transfected with si-NC or si-ERRα for 24 h and then further treated with TFG-β (20 ng/ml) for 48 h, the protein levels of E-cad and Vim were analyzed by Western blot analysis; (D) MG-63 cells were transfected with si-NC or si-ERRα for 24 h and then further treated with TFG-β (20 ng/ml) for 24 h, the mRNA levels of E-cad and Vim were analyzed by qRT-PCR. Data were presented as means ± SD of 3 independent experiments. **p < 0.01 compared with control.
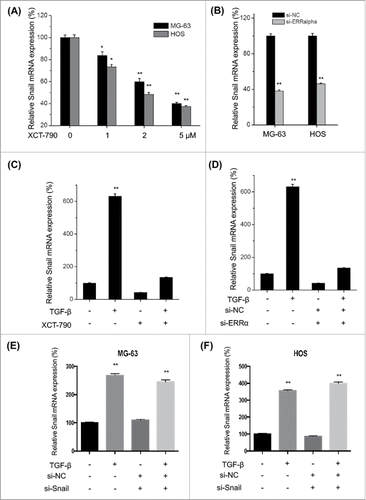
ERRα is involved in TFG-β induced transcription of Snail in osteosarcoma cells
ERRα can activate Snail via both transcriptional and posttranscriptional mechanisms in ovarian cancer cells.Citation16 To verify the roles of ERRα in TFG-β induced up regulation of Snail, we used XCT-790 and si-ERRα to treat MG-63 and HOS cells and then measured the mRNA levels of Snail. Our data showed that XCT-790 can decrease the transcription of Snail via a time dependent manner in both MG-63 and HOS cells (). This was confirmed by the use of si-ERRα, which also suppressed the mRNA of Snail in both MG-63 and HOS cells (). Furthermore, both XCT-790 () and silencing of ERRα () can significantly (p < 0.01) attenuate TFG-β induced up regulation of Snail in MG-63 cells. However, silencing of Snail had no significant effect on the mRNA expression of ERR α in either MG-63 (E) or HOS (F) cells. Generally, our data suggested that ERRα is involved in TFG-β induced transcription of Snail in osteosarcoma cells.
Figure 6. ERRα is involved in TFG-β induced transcription of Snail in osteosarcoma cells. MG-63 and HOS cells were treated with increasing concentrations of XCT-790 (A) or transfected with si-ERRα for 24 h, the expression of Snail mRNA were analyzed by qRT-PCR; (C) MG-63 cells were treated with TFG-β (20 ng/ml), XCT-790 (2 μM) or both of them for 24 h, the expression of Snail mRNA were analyzed by qRT-PCR; (D) MG-63 cells were transfected with si-ERRα or si-NC for 24 h and then treated with TFG-β (20 ng/ml) for 24 h, the expression of Snail mRNA were analyzed by qRT-PCR; MG-63 (E) or HOS (F) cells were transfected with siNC or si-Snail for 24 h and then further treated with TFG-β (20 ng/ml) for another 24 h, the mRNA expression of ERRα were measured by use of qRT-PCR. Data were presented as means ± SD of 3 independent experiments. **p < 0.01 compared with control.
Discussion
The molecular mechanisms underlying metastasis of osteosarcoma cells are still poorly understood. Our present study revealed that TGF-β treatment can increase the in vitro migration of invasion of osteosarcoma cells via induction of EMT. TFG-β treatment significantly increased the expression of Snail, while not Slug, Twist, and Zeb1 in both MG-63 and HOS cells. Silencing of Snail via si-Snail markedly attenuated TFG-β induced EMT of osteosarcoma cells. TGF-β treatment also increased the expression and nuclear localization of ERRα in osteosarcoma cells, while had no obvious effect on ERα, ERβ, or ERRγ. Knock down of ERRα significantly attenuated TFG-β induced EMT and transcription of Snail in osteosarcoma cells. Collectively, our present study revealed that TGF-β treatment can trigger the EMT of osteosarcoma cells via ERRα/Snail pathways.
TGF-β is one of the most abundant growth factors stored and released by bone. Numerous studies indicated that TGF-β can promote cancer metastasis by regulating the composition of extracellular matrix and induction of EMT.Citation14,20 As to osteosarcoma, TGF-β signaling is considered as a potential therapeutic target due to its involvement in cell proliferation and metastasis.Citation12,19 TGF-β can increase the proliferation of osteosarcoma cells via activation of the Raf/MAPK pathway or induction the expression of IGFBP-3.Citation23 Our present study revealed that TGF-β can promote the in vitro migration and invasion of osteosarcoma cells via induction of EMT. The treatment of TGF-β significantly decreased the expression of E-cad while up regulated the expression of Vim and FN. These data confirmed previous findings that TGF-β signals were involved in the EMT progression of osteosarcoma.Citation12,25
The underlying mechanisms for TGF-β induced EMT of osteosarcoma cells are unclear. Several studies indicated that TGF-β can trigger the EMT through induction of transcription factors, such as Snail, Slug, ZEB, and Twist, that downregulate epithelial and upregulate mesenchymal cell markers.Citation3,32 Of these, Snail was highly upregulated by TGF-β in both MG-63 and HOS cells in the present study. Silencing of Snail significantly attenuated TGF-β induced down regulation of E-Cad and up regulation of Vim. It suggested that Snail is essential for TGF-β induced EMT of osteosarcoma cells. Snail can bind to the E-box site in the promoter of E-cad and trigger the EMT of many types of cancer.Citation15 In support of our results, several reports demonstrated that Snail knockdown reverts TGF-β-induced EMT and cell migration in cancer cells.Citation17,25
TGF-β signals have been suggested to cross talk with estrogenic signal pathways during the tumorigenesis and development of cancers.Citation2,13 Our present study revealed for the first time that TGF-β treatment can increase the expression and nuclear translocation of ERRα in osteosarcoma cells, while not affect other molecules including ERα, ERβ, and ERRγ. While silencing or inhibiting of ERRα can significantly attenuated TGF-β induced EMT and transcription of Snail in osteosarcoma cells. ERRα has been suggested to positively regulate the EMT of cancer cells.Citation16,29 The primary function of ERRα is believed to be the regulation of energy metabolism. It is also expressed throughout osteoblast differentiation and regulates bone formation.Citation4 Our results confirmed the study that the expression of ERRα is correlated with osteosarcoma progression.Citation6 Considering that ERRα can activate Snail via both transcriptional and posttranscriptional mechanisms,Citation16 whether ERRα can regulate the stability of Snail protein also needs further studies in osteosarcoma cells.
In conclusion, we have demonstrated that TGF-β treatment can trigger the EMT of osteosarcoma cells via ERRα/Snail pathways. Although further studies about the detailed mechanisms responsible for TGF-β induced up regulation of ERRα, our study provided the first evidence that ERRα is essential for TGF-β induced EMT and Snail expression in osteosarcoma cells. Our data suggested that ERRα/Snail pathways might be potential therapeutic targets of osteosarcoma.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Ao A, Wang H, Kamarajugadda S, Lu J. Involvement of estrogen-related receptors in transcriptional response to hypoxia and growth of solid tumors. Proc Natl Acad Sci 2008; 105:7821-6; PMID:18509053; https://doi.org/10.1073/pnas.0711677105
- Band AM, Laiho M. Crosstalk of TGF-β and estrogen receptor signaling in breast cancer. J Mammary Gland Biol Neoplasia 2011; 16:109-15; PMID:21390570; https://doi.org/10.1007/s10911-011-9203-7
- Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, De Herreros AG. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Na Cell Biol 2000; 2:84-9; https://doi.org/10.1038/35000034
- Bonnelye E, Merdad L, Kung V, Aubin JE. The orphan nuclear estrogen receptor-related receptor α (ERRalpha) is expressed throughout osteoblast differentiation and regulates bone formation in vitro. J Cell Biol 2001; 153:971-84; PMID:11381083; https://doi.org/10.1083/jcb.153.5.971
- Cano A, Peinado H, Olmeda D. Snail, ZEB and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007; 7:415-28; PMID:17508028; https://doi.org/10.1038/nrc2131
- Chen P, Wang HB, Duan ZJ, Zou JX, Chen HW, He W, Wang JJ. Estrogen-related receptor α confers Methotrexate resistance via attenuation of reactive oxygen species production and p53 apoptosis pathway in osteosarcoma U2OS cells. J Bone Miner Res 2014; 29:S142-S; https://doi.org/10.1002/jbmr.2012
- Chen ZJ, Yang XL, Liu H, Wei W, Zhang KS, Huang HB, Giesy JP, Liu HL, Du J, et al. Bisphenol A modulates colorectal cancer protein profile and promotes the metastasis via induction of epithelial to mesenchymal transitions. Arch Toxicol 2015; 89:1371-81; PMID:25119493; https://doi.org/10.1007/s00204-014-1301-z
- Daw NC, Chou AJ, Jaffe N, Rao BN, Billups CA, Rodriguez-Galindo C, Meyers PA, Huh WW. Recurrent osteosarcoma with a single pulmonary metastasis: a multi-institutional review. Br J Cancer 2015; 112:278-82; PMID:25422914; https://doi.org/10.1038/bjc.2014.585
- Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguere V. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab 2007; 5:345-56; PMID:17488637; https://doi.org/10.1016/j.cmet.2007.03.007
- Fradet A, Sorel H, Bouazza L, Goehrig D, Depalle B, Bellahcene A, Castronovo V, Follet H, Descotes F, Aubin JE, et al. Dual function of ERRalpha in breast cancer and bone metastasis formation: implication of VEGF and osteoprotegerin. Cancer Res 2011; 71:5728-38; PMID:21734015; https://doi.org/10.1158/0008-5472.CAN-11-1431
- Grignani G, Palmerini E, Ferraresi V, D'Ambrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y, Sangiolo D, Marchesi E, et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol 2015; 16:98-107; PMID:25498219; https://doi.org/10.1016/S1470-2045(14)71136-2
- Hou CH, Lin FL, Tong KB, Hou SM, Liu JF. Transforming growth factor α promotes osteosarcoma metastasis by ICAM-1 and PI3K/Akt signaling pathway. Biochem Pharmacol 2014; 89:453-63; PMID:24685520; https://doi.org/10.1016/j.bcp.2014.03.010
- Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-β. Nat Med 1996; 2:1132-6; PMID:8837613; https://doi.org/10.1038/nm1096-1132
- Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol 2012; 25:76-84; https://doi.org/10.1097/CCO.0b013e32835b6371
- Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell 2009; 15:195-206; PMID:19249678; https://doi.org/10.1016/j.ccr.2009.01.023
- Lam SS, Mak AS, Yam JW, Cheung AN, Ngan HY, Wong AS. Targeting estrogen-related receptor α inhibits epithelial-to-mesenchymal transition and stem cell properties of ovarian cancer cells. Mol Ther 2014; 22:743-51; PMID:24419103; https://doi.org/10.1038/mt.2014.1
- Li H, Wang H, Wang F, Gu Q, Xu X. Snail involves in the transforming growth factor beta1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells. Plos One 2011; 6:e23322; https://doi.org/10.1371/journal.pone.0023322
- Luo J, Sladek R, Carrier J, Bader JA, Richard D, Giguere V. Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor α. Mol Cell Biol 2003; 23:7947-56; PMID:14585956; https://doi.org/10.1128/MCB.23.22.7947-7956.2003
- Matsuyama S, Iwadate M, Kondo M, Saitoh M, Hanyu A, Shimizu K, Aburatani H, Mishima HK, Imamura T, Miyazono K, et al. SB-431542 and Gleevec inhibit transforming growth factor-β-induced proliferation of human osteosarcoma cells. Cancer Res 2003; 63:7791-8; PMID:14633705
- Morrison CD, Parvani JG, Schiemann WP. The relevance of the TGF-β Paradox to EMT-MET programs. Cancer Lett 2013; 341:30-40; PMID:23474494; https://doi.org/10.1016/j.canlet.2013.02.048
- Raymond AK, Jaffe N. Osteosarcoma Multidisciplinary Approach to the Management from the Pathologist's Perspective. Pediatric and Adolescent Osteosarcoma 2009; 152:63-84; https://doi.org/10.1007/978-1-4419-0284-9_4
- Saitoh M. Epithelial-mesenchymal transition is regulated at post-transcriptional levels by transforming growth factor- signaling during tumor progression. Cancer Sci 2015; 106:481-8; PMID:25664423; https://doi.org/10.1111/cas.12630
- Schedlich LJ, Yenson VM, Baxter RC. TGF-β-induced expression of IGFBP-3 regulates IGF1R signaling in human osteosarcoma cells. Mol Cell Endocrinol 2013; 377:56-64; PMID:23831640; https://doi.org/10.1016/j.mce.2013.06.033
- Stein RA, Gaillard S, McDonnell DP. Estrogen-related receptor α induces the expression of vascular endothelial growth factor in breast cancer cells. J Steroid Biochem Mol Biol 2009; 114:106-12; PMID:19429439; https://doi.org/10.1016/j.jsbmb.2009.02.010
- Sung JY, Park SY, Kim JH, Kang HG, Yoon JH, Na YS, Kim YN, Park BK. Interferon consensus sequence-binding protein (ICSBP) promotes epithelial-to-mesenchymal transition (EMT)-like phenomena, cell-motility, and invasion via TGF-β signaling in U2OS cells. Cell Death Dis 2014; 5:e1224; https://doi.org/10.1038/cddis.2014.189
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2:442-54; https://doi.org/10.1038/nrc822
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871-90; PMID:19945376; https://doi.org/10.1016/j.cell.2009.11.007
- Wang LN, Wang Y, Lu Y, Yin ZF, Zhang YH, Aslanidi GV, Srivastava A, Ling CQ, Ling C. Pristimerin enhances recombinant adeno-associated virus vector-mediated transgene expression in human cell lines in vitro and murine hepatocytes in vivo. J Integr Med 2014; 12:20-34; PMID:24461592; https://doi.org/10.1016/S2095-4964(14)60003-0
- Wu YM, Chen ZJ, Liu H, Wei WD, Lu LL, Yang XL, Liang WT, Liu T, Liu HL, Du J, et al. Inhibition of ERRalpha suppresses epithelial mesenchymal transition of triple negative breast cancer cells by directly targeting fibronectin. Oncotarget 2015; 6(28):25588-601; https://doi.org/10.18632/oncotarget.4436
- Xu J, Lamouille S, Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res 2009; 19:156-72; PMID:19153598; https://doi.org/10.1038/cr.2009.5
- Yang G, Yuan J, Li K. EMT transcription factors: implication in osteosarcoma. Med Oncol 2013; 30:697; PMID:23975634; https://doi.org/10.1007/s12032-013-0697-2
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004; 117:927-39; PMID:15210113; https://doi.org/10.1016/j.cell.2004.06.006
- Yang JL, Zhang W. New molecular insights into osteosarcoma targeted therapy. Curr Opin Oncol 2013; 25:398-406; PMID:23666471; https://doi.org/10.1097/CCO.0b013e3283622c1b
