ABSTRACT
Tumor extracellular vesicles (EVs), including exosomes, emerged as key drivers of the pro-tumorigenic dialog between the tumor mass and its microenvironment by mediating long and short distance communication. In vitro studies defined the capacity of tumor EVs to modify the phenotypes of stromal and tumor cells. These studies are now supported by a growing number of functional in vivo experiments. Remarkably, they allowed the identification of a new role for tumor EVs in priming the pre-metastatic niches (PMN). Several molecules transported in tumor EVs (RNAs and proteins) have recently been found to be essential for tumor progression and metastasis in vivo. In parallel, novel EV labeling and tracking strategies have very recently allowed the first descriptions of tumor EVs in vivo and pave the way for a better understanding of their function in realistic pathophysiological contexts. Here, we review the functional approaches and the recent progress in in vivo imaging of EVs, which have refined our understanding of the role played by tumor EVs. Finally, we emphasize the remaining challenges and open questions related to the biology of tumor EVs.
Cancer progression and metastasis formation rely on the bi-directional and dynamic interactions between mutated tumor cells and their complex tumorigenic microenvironment.Citation1 Tumor cells have the capacity to physically and biochemically modify their surrounding extracellular matrix and the phenotype of stromal cells (which include fibroblasts, endothelial cells, mesenchymal stem cells, macrophages and other types of immune cells) for their own benefit. Reciprocally, the neighboring tumor microenvironment reacts and shapes the tumor to sustain its growth, eventually fostering its aggressiveness.Citation2 This educated tumor ecosystem will support tumor growth at a primary site, during invasion and throughout the metastasis cascade. In addition, tumor-secreted factors modify the host microenvironment in distant organs, thereby promoting the formation of a premetastatic niche (PMN) and favoring the colonization of metastatic cells.Citation3 Some studies however suggest that the microenvironment, such as the well described tumor-associated fibroblasts and extracellular matrix components, can have adverse behavior on tumor progression and potentially mediate anti-tumorigenic immune surveillance.Citation2,4 Better understanding of this constant dialog within the tumor ecosystem is instrumental in developing appropriate and efficient therapeutic strategies.
In addition to direct cellular interaction or action of soluble factors, communication between a tumor cell and its microenvironment can also be mediated by the secretion and uptake of extracellular vesicles (EVs). Since the late 90s, and the functional characterization of a particular subtype of EV, exosomes,Citation5 interest in EVs as mediator of cell-cell communication has grown exponentially. EVs are secreted lipid bilayered vesicles encapsulating mRNA, miRNA, proteins and, in some cases DNA fragments, by active sorting mechanisms, which can be uptaken by distant cells and transduce a message.Citation6 EV is a generic term for vesicles secreted through different mechanisms: exosomes, microvesicles and apoptotic bodies.Citation6,7 Exosomes arise from the fusion of an endosomal compartment, the multi-vesicular body (MVB), with the plasma membrane, while microvesicles and apoptotic bodies are secreted through shedding of the plasma membrane (for a review on exosome secretion mechanisms see Citationref.8). Because the biogenesis and sorting of the cargo involve different molecular machineries, exosomes and microvesicles have different contents and diameters (50–100 nm for exosomes; 10 to 1000 nm for microvesicles). The absence of specific markers and consensual purification methods account for the controversy on the nature of extracellular vesicles.Citation9 Importantly, recent data have demonstrated the existence of content heterogeneity within exosomes, suggesting the existence of multiple subpopulations of exosomes.Citation10-13 Identification of new markers defining specific subtypes of EVs is therefore needed to understand their respective functions.Citation12
EVs play a pivotal role in cancer. Initially, EVs secreted by tumor cells were shown to induce an anti-tumoral immune response through the activation of dendritic cells and T lymphocytes.Citation14,15 These studies initiated a new therapeutic approach using EVs as anti-tumoral vaccines (for review, see ref. Citation16). However, since this initial discovery, many studies have established that tumor EVs can promote tumor growth through different pro-tumoral activities. The current model suggests that tumor EVs, including exosomes, promote tumor growth and metastasis by modulating most of the hallmarks of cancer such as tumor cell proliferation, stromal cell differentiation, angiogenesis, the immune response and the properties of the surrounding extracellular matrix.Citation17 Tumor EVs are now being treated as potent promoters of metastasis and are used as promising targets in cancer diagnosis.Citation18-20 Over the past couple of years significant efforts have been made to assess the functions of tumor EVs in vivo and expand insights obtained from important yet limited in vitro experimental studies. These new findings lead to a giant leap toward the understanding of the roles of tumor EVs in tumor progression, in particular in the evolution to metastasis.
Here, focusing on EVs secreted by tumor cells, we will first briefly describe their main functions identified through in vitro experiments, by providing a few examples selected from the large number of publications in the field. We will then describe how the function of tumor EVs has been investigated in vivo and how new molecular players contained in tumor EVs are emerging. Finally, we will describe recent imaging strategies, which now allow to follow EVs in vivo and open new avenues to tackle open questions regarding their function in cancer progression.
Tumor EVs in vitro: Transferring malicious information to tumor, stroma, and beyond
In vitro experiments with EVs purified from tumor cells allowed the identification of several properties of tumor EVs, which, for some of them, have been confirmed in vivo (see next section). In a seminal paper, Skog and colleagues demonstrated that human glioblastoma EVs contain functional pro-tumoral mRNA, miRNA and proteins, which can be endocytosed by endothelial cells and can promote angiogenesis in vitro.Citation21 They elegantly showed that recipient cells could efficiently translate EV-derived mRNA, providing a valid proof for their ability to deliver active information within the tumor microenvironment. The mechanisms by which cargoes are selectively enriched in EVs have started to be unraveled. Interestingly, sorting of miRNAs relies on the oncogene KRAS which is mutated in many human cancers.Citation22,23 Besides, a recent study showed that cancer EVs are enriched in specific miRNA compared with non cancer EVs. EVs carry cell-independent miRNA editing abilities, which could allow EVs to efficiently modify the phenotype of target cells by rapidly silencing selected mRNA.Citation24 Importantly, further insights show that in a population of EVs the average number of copy of a given miRNA per EV is lower than one.Citation25 Given the reported heterogeneity of EVs in terms of protein cargoes, this suggests that only a subpopulation of EVs is enriched in specific miRNA molecules and carry the functional miRNA message. Furthermore, while the transfer of miRNA activity from macrophages EVs to endothelial cells has been nicely demonstrated in vitro, it was also shown to have a modest impact on target gene repression.Citation26 Future methods allowing to track EVs-loaded miRNAs and assess their activity in the target cell will undoubtedly help solving the complex and heteregenous nature of EVs.
In culture, tumor EVs can be ingested by tumor cells and confer them more robust tumoral traits. For instance, tumor cells can be transformed through the exchange of EVs containing the truncated and oncogenic form of the epidermal growth factor receptor (EGFRvIII) in the case of glioma cells,Citation27 or the oncoprotein KRAS in the case of colon cancer cells.Citation28,29 Similarly, transfer of EVs between tumor cells can stimulate their proliferation and migration, as it has been shown for prostate cancer cells.Citation30 Tumor EVs have also been shown to promote drug resistance in vitro. Breast cancer cells exchange EVs mediating chemoresistance,Citation31 and tumor cells can directly expel drugs via EVs.Citation32-34
In vitro experiments suggest that tumor EVs can also be delivered to multiple types of stromal cells and thus modify their phenotypes. One of the most documented examples of this exchange is the effect of tumor EVs on endothelial cells. Tumor-derived EVs of different originsCitation21,35-38 have the capacity to induce proliferation and tubulogenesis of endothelial cells in vitro and could therefore promote angiogenesis during tumorigenesis. Interestingly, tumor EVs were also shown to destabilize the endothelial barrier and affect its permability in vitro.Citation38-40 Besides, tumor EVs can be internalized by other cell types and induce their differentiation into potent and well-known pro-tumorigenic actors such as CAFs (Cancer-associated fibroblasts),Citation41,42 myofibroblasts Citation43-46 and macrophages.Citation47 In addition, tumor EVs can also promote immunosuppression in different ways. For instance, EVs purified from carcinoma or melanoma cells lines or purified from blood serum of patients with ovarian or colorectal cancer can induce the death of T-lymphocytes in vitro.Citation48-51 Alternatively, EVs originating from different tumor cells can activate in vitro a population of cells, the myeloid-derived suppressor cells, known to suppress T-cell activation.Citation52-54 EVs from breast tumor, melanoma or colorectal cancer cell lines added to monocytes inhibit their differentiation into dendritic cells.Citation52,55 Therefore, tumor EVs appear as potent immunomodulators presumably shaping tumor escape from immunosurveillance (for a review, see ref. Citation56).
In addition tumor EVs may also have an impact on the extracellular matrix (ECM) by either carrying and depositing ECM or by delivering ECM degrading enzymes such as MMPs. In a systematic study of the interaction between ECM and tumor EVs, EVs were shown to be enriched in several proteases, including active MMP2 and MMP9 that, efficiently degraded different molecules of ECM.Citation57 Furthermore, the MT1-MMP metalloprotease is present in EVs secreted by different tumor cells and can efficiently degrade collagen or laminin in vitro.Citation58,59 Recently, tumor EVs carrying MT1-MMP were shown to function in a coordinate fashion with invadosomes to promote matrix degradation and cellular invasion.Citation60 Finally, EVs are capable of carrying important ECM molecules and deposit them in close vicinity to focal adhesions and thus inducing directional cell migration.Citation61 In addition, fibronectin present at the surface of EVs mediates their interaction with heparin sulfate present on receiving cells, triggering the cell response.Citation62
Overall, these data demonstrate that tumor EVs have a functional impact in vitro and suggest that they could promote tumorigenesis by acting at different levels in vivo. Although these findings rely on the use of relatively high concentrations of EVs whose physiologic relevance has been questioned,Citation63 they are now being supported by several evidences in vivo. Animal studies complement in vitro assays by allowing to follow EVs in their complex natural environment and, permit to assess their range of action, behavior in body fluids and their response to diverse challenges including blockade by epithelial/endothelial barriers, trapping by ECM and depletion by clearance mechanisms.
Pro-tumoral role of tumor EVs in vivo
Studies describing the function of tumor exosomes in vivo fall into 2 categories. In a first category of experiments, genetically engineered tumor cells allowing tuning of EV secretion levels are injected into mice and their capacity to form metastasis is assessed (). Using this approach, it was demonstrated that depletion of Rab27A in mammary carcinoma or melanoma cells decreases the levels of secreted EVs in vitro and hampers in vivo tumor growth and metastasis.Citation45,64,65 Exosomes isolated from advanced metastatic melanoma were shown to carry high concentrations of TYRP2, VLA4, HSP70, MET and Rab27a proteins and could instigate BDMCs (Bone marrow-derived cells) to secrete increased levels of MET thereby favoring the establishment of a pro-metastatic niche.Citation64 In mammary carcinomas, secreted EVs, together with soluble proteins, repress the tumor-prone and systemic accumulation of neutrophils.Citation65 These studies allowed the identification of Rab27A as a key driver of EV biogenesis and tumorigenesis. Surprisingly however, although overexpressing Rab27A expectedly leads to increased secretion of exosomal proteins in a lung cancer cell line, in vivo immunization of mice with such EVs leads to reduced tumorigenesis by eliciting an antitumoral immune surveillance.Citation66 Whether overexpressing Rab27a also increases the amount of secreted EVs is not clear and needs to be determined. The role of Rab27b, originally involved in EV expressed in EV from Hela cells,Citation67 is more complex. Whereas its depletion does not affect EV secretion in 2 mammary tumor cell lines,Citation65 Rab27b overexpression increases the secretion of HSP90, a known marker of EVs, and stimulates breast tumor growth, invasion and metastasis.Citation68
Figure 1. Functional analysis of tumor EVs. (A) The impact of tumor-secreted EVs on tumor growth and metastasis relies on the amounts of EVs and their loading in specific RNA or protein cargoes. Here, expression levels of genes involved in EV biogenesis or encoding EV cargoes are tuned (si/shRNA or overexpression) in tumor cells, which are grafted into mice. Subsequent tumor growth (T) and metastasis (M) formation is tracked over time. (B) Alternatively, isolated EV (where expression of cargoes has also been tuned) are used for priming a metastatic niche. For this, repeated intravenous injection of EVs purified from cultured tumor cells are performed, favoring the formation of a pre-metastatic niche. Metastasis onset and growth is then assessed upon subsequent intravenous injection of tumor cells.
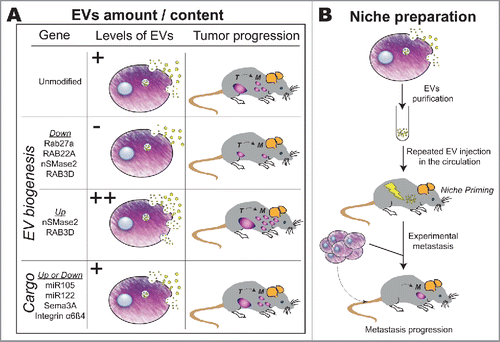
Two other RAB GTPases RAB22A and RAB3D, known to regulate EV formation, have been similarly used to determine the role of EVs in tumor progression.Citation69,70 Depletion of RAB22A in MDA-MB231 breast cancer cells decreases their capacity to secrete EVs under hypoxia in vitro and impairs their ability to form lung metastasis in mice.Citation69 RAB3D overexpression increases EVs secretion in breast cancer cells in vitro, and in parallel promotes breast cancer cell invasion and lung metastasis in mice, while its downregulation has the opposite effect.Citation70 Similarly, breast cancer cells overexpressing the neutral Sphingomyelinase nSMase2 secrete more EVs in vitro and develop more lung metastasis through an increase in angiogenesis once injected in mice, while cells depleted for nSMase2 have an opposite phenotype.Citation71,72 Together, these studies generally correlate the levels of secreted EVs by tumor cells and the capacity of these cells to form tumors and metastasis in mice. However, one important drawback of this approach is that all the genes known to affect EV secretion so far also have other cellular functions, which can contribute to cancer progression independently of EV secretion.
In the second category of studies, EVs are purified from cultured tumor cells and subsequently injected into animals (before or together with tumor cells), before assessment of tumor growth and metastasis (). For instance, repeated intraperitoneal injection of exosomes purified from mammary tumor cells before subcutaneous injection of tumor cells in the mammary gland, enhances tumor growth, through inhibition of immune NK cell growth and activity.Citation73 Similarly, EVs purified from rat pancreatic adenocarcinoma or from human renal carcinoma cells promoted lung metastasis of tumor cells in rats Citation74 or mice.Citation36 Also, orthotopic co-injection of human mammary tumor cells with exosomes from breast cancer patients, enhanced tumor growth in nude mice which was not seen with EVs from healthy individuals.Citation24 Very recently, melanoma EVs repeatedly injected in the circulation were shown to ‘activate’ alveolar epithelial cells, potentially through non-coding small nuclear RNAs, thereby favoring the neutrophil-dependent education of PMN.Citation75 Over the past couple of years, this strategy has notably been used to identify a new role for tumor EVs in preparing a pre-metastatic niche at distance from the primary tumor.Citation36 Using different models, the group of David Lyden has shown that injection of tumor exosomes in the mouse circulation modifies the microenvironment of specific organs, notably in the absence of tumor cells.Citation64,76,77 In a seminal study by Peinado and colleagues, lethally irradiated mice were transplanted with bone marrow from GFP-expressing mice that were previously treated with exosomes purified from melanoma cells.Citation64 Upon bone marrow reconstitution, growth, angiogenesis and metastatic potency of orthotopic tumors were enhanced in mice whose bone marrow had been ‘educated’ by tumor EVs (versus synthetic vesicles or PBS).Citation64 This groundbreaking discovery was later confirmed using pancreatic cancer EVs, whose injection in mice leads to a stroma-dependent fibrotic niche, to the benefit of colonizing tumor cells.Citation76 Here, exosomes from pancreatic tumor cells were shown to be loaded with MIF (Migration Inhibitory Factor) that drives a pro-fibrotic secretion of cytokines, such as TGF-ß, by targeted Kupffer cells leading to a pro-metastatic deposition of Fibronectin by local Hepatic Stellate cells.Citation76 Similarly, injection of cancer EVs can modify the phenotype of fibroblasts in the lungs, or affect Kupffer cells in the liver, depending on the cellular origin of the EVs and their integrin repertoire at their surface.Citation77
Altogether, these experiments point to a model where tumor cells secrete EVs, which can be ingested by stromal cells either locally or at distance. This will induce local alterations in the tumor microenvironment as well as generate a metastatic niche at distant sites. More specifically, tumor EVs can modulate the immune response, modify the ECM or alter the phenotype of stromal cells. Although very informative, these studies would strongly benefit from in vivo tracking of EVs, using reliable markers and state-of-the-art intravital imaging, with a resolution adapted to EVs (see last section). Nevertheless, these studies paved the ground for the identification of important EV cargoes in cancer progression.
Function of individual EV cargoes in tumor progression
Over the past couple of years, several tumor EV cargoes, miRNAs and proteins, have emerged as essential modulators of the function of tumor EVs. Two factors present in tumor EVs, the protein Sem3A and the miRNA miR105, were shown to promote endothelial permeabilization, thereby favor tumor metastasis.Citation38,40 The transmembrane protein Semaphorin3A, when present at the surface of glioblastoma stem cell EVs, promotes vascular permeability in the murine brain by destabilizing VE-cadherin.Citation40 The microRNA miR105, which is only expressed by metastatic breast cancer cells, localizes in EVs and induces a destabilization of the endothelium through a disruption of tight junctions via ZO1 downregulation, thus promoting metastasis in mice.Citation38
In addition to miR105, several miRNAs present in tumor EVs are now known to functionally contribute to tumor progression in vivo through distinct processes. EV transfer of miR122 to stromal cells reprograms glucose metabolism in PMN in mice, thereby promoting metastasis,Citation78 while miR155 delivered by the tumor EVs promotes drug resistance in a neuroblastoma model.Citation79 Meanwhile, two other microRNAs, miR192 and miR29a/c, were shown to have an opposite effect and decrease tumor progression when present in tumor EVs.Citation80,81 miR192, whose expression is lower in highly metastatic human adenocarcinoma cells, inhibits angiogenesis in vitro when overexpressed in EVs. Repeated injection of EVs containing miR192 in mice represses osseous colonization.Citation80 miR29a/c, which can target VEGFR, can be transferred to endothelial cells via EVs in vitro, and subsequently decreases angiogenesis. Treatment of mice with intravenous injections of miR29a/c EVs inhibits tumor growth and angiogenesis in vivoCitation81 which may suggest therapeutic opportunities.
In addition, several transmembrane proteins, present at the surface of tumor Evs, contribute to their function and to their distribution in the organism. The tetraspanins Tspan8 and CD151 promote PMN formation in a rat model of pancreatic adenocarcinoma and promote metastases in lungs and bone marrow, most likely through extracellular matrix remodeling.Citation82 Interestingly, EVs deprived of Tspan8 have a reduced capacity to cross the blood-brain barrier in vivo. Finally, integrins, which are transmembrane proteins better known for mediating important cell-ECM and cell-cell contacts, are present on the surface of EVs and were shown to dictate the organotropism of tumor EVs and subsequent metastasis in mice.Citation77 Indeed, while tumor EVs derived from pancreatic or breast cancer cells, carrying integrins α6β4 and α6β1 specifically target lungs, EVs with the αvβ5 integrin specifically target the liver.
Successful identification of important cargoes often relies on the ability to track labeled EVs in the animal, which is a challenging task due to their small size, absence of bonafide markers and the lack of adapted imaging technologies. Therefore, recent development in EV tagging, tracking and imaging technologies is largely advancing the field of tumor EVs biology.
Following tumor EVs in vivo
Following EVs in living multi-cellular organisms is challenging, due to the small size of these vesicles and to their rapid dispersion in body fluids. Different strategies have been developed to label EVs, allowing to follow them in the animal at different scales (from whole body to cellular level) and with different specificities (global labeling of EVs or specific targeting of subpopulations) (, ). Two approaches have been used to track, at the animal scale, large amounts of EVs injected at once in the circulation of mice: bioluminescence and radiolabeling. In the first case, the luciferase is directed to EVs, either randomlyCitation83 or through a fusion with a transmembrane peptide,Citation84,85 while in the second case, radiolabeling is achieved after EV purification, either in subpopulations of EVs expressing a specific fusion proteinCitation86 or in the global population of EVs through the introduction of radiolabeled probes.Citation87 These approaches allow the non-invasive tracking of EVs at the animal scale and identification of the tissues that received most EVs, although they lack further resolution.
Figure 2. Following EVs in vivo. Directing luciferase into EVs allow to follow their organ distribution at the animal scale. Different lipid inserting dyes have been used to label EVs and track them back in the animal. Alternatively, tumor cells secreting labeled EVs (directed to EVs via fusions between a fluorescent protein and diverse peptides or proteins such as a palmitoylation signal (Palm) or CD63 can be injected in mice. Notably this allowed the first intravital imaging of tumor EVs in the living mouse. In addition, the mRNA cargo contained in EVs has been tracked in vivo, using the cre-lox system.
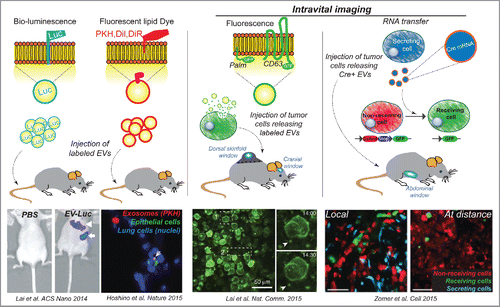
Alternatively, many studies have used fluorescent lipophilic dyes. In particular long chain carbocyanines such as PKH, DiL or DiR, which insert into the EVs lipid bilayer, have been used to label EVs post-purification (examples: refs. Citation77, 85, 88, 89). However, their labeling specificity and reliability over time has been questionedCitation84,90 More specific labeling can be obtained through the expression of fluorescent proteins fused to proteins or peptides present within the EVs (such as CD63, the C1C2 domains of lactadherin or a palmitoylation signal) in the secreting cells.Citation90-92 However, this approach restricts the labeling to a subpopulation of EVs, may create artifacts due to overexpression of the marker. In addition, the signal intensity of this fluorescent fusion protein depends on the expression levels of the reporter protein.Citation84,90 Further studies will be needed for designing methods and live-compatible dyes capable to reliably and stably label distinct EV populations.
Finally, methods have been developed to follow the fate of mRNA cargoes transported by EVs. These include luciferase mRNA, which will be translated by the receiving cell and therefore label it in an enzymatic assay Citation90 or Cre mRNA.Citation83,93,94 whose translation can be observed in the receiving cells using lox-reporter transgenic mice or cells. This latter approach turned out to be a very potent tool to identify the tumor EV receiving cells Citation93,94
All these different approaches provided a first insight into the behavior of tumor EVs in vivo. Interestingly, although EVs seem to be extremely stable in body fluids ex-vivo,Citation84,95 they are cleared from the circulation in only a few minutes following their intravenous injection in mice.Citation85,86,96 The biodistribution of EVs in organs varies between studies, as they have been reported to mainly reach the spleen, liver, lungs, bladder or kidneys. In addition, EVs injected intradermally in the mouse tail rapidly reach the lymphatic vessels and accumulate in the lymph nodes where they are stably retained for a couple of days, in addition to other organs (kidney, liver and thymus).Citation97 These differences could be due to the distinct cellular origins of the EVs, the different labeling methods or the different modes or doses of administration.Citation84,98,99 Similar discrepancies have been reported when testing the tumor-targeting potential of tumor EVs injected in animals already hosting a mammary tumor. In particular, it has been reported that these EVs are massively incorporated in the tumor,Citation84,89 suggesting a strong therapeutic potential,Citation89 while another study showed only minimal accumulation in tumors.Citation87 Although these studies have brought important insights in the biology of EVs, they rely on the bulk injection of tumor EVs in the circulation, which might not necessarily represent what happens upon natural progressive release of EVs by a tumor.
The identity of the cells incorporating tumor EVs is a central question. Several studies have demonstrated the existence of a cell specific uptake in vitro (for instance.Citation100,101). Several in vivo studies demonstrate that EVs of different origins are mainly incorporated by specialized phagocytic cells, such as dendritic cells or macrophages in mice.Citation77,94,96,97,102-104 This is the case for instance of pancreatic cancer EVs injected in the circulation, which are uptaken by Kupffer cells in the liver.Citation76 Strikingly, tumor EVs are much more stable in the circulation and in several organs, once macrophages have been depleted in mice, demonstrating that macrophages might be the main cell type absorbing tumor EVs upon bulk injection.Citation103 It will be important to confirm whether these cells are also targeted by EVs released in physiologic levels. This however requires first the establishment of stable and genetically-encoded EV markers in relevant in vivo models.Citation105 Nevertheless, other cell types have been shown to incorporate tumor EVs in vivo, including endothelial cells,Citation77,94,103 alveolar epithelial cells,Citation75 fibroblasts,Citation77 neurons and microglia Citation94 and tumor cells themselves.Citation93,106 The molecular mechanisms underlying potential tumor EV organotropism and cell-type specific uptake, start to be unraveled where integrins seem to play an important role.Citation77 The fate of uptaken EVs (degradation vs signaling) and the functional consequences of this uptake on the receiving cell phenotype and on cancer progression deserve particular attention.
The secretion of EVs by tumor cells and their transfer to stromal cells has been witnessed in vivo in recent groundbreaking studies using intravital imaging, opening the door to the dynamic analysis of such transfer. Using time-lapse multiphoton imaging, the group of X. Breakfield has been able to follow tumor EVs labeled with a palmitoylation signal fused to GFP (Palm-GFP) that had been secreted either by glioblastoma (using a cranial windowCitation107) or by thymoma cells (using a dorsal skinfold windowCitation90) in living mice. In both cases, tumor EVs were mostly stationary in the center of the tumor and rather mobile on its edges, where interactions and exchange with stromal cells could be visualized. Meanwhile, Zomer et al. used an elegant genetic approach to follow the fate of tumor EVs containing Cre mRNA in the living mouse.Citation93 This demonstrated the transfer of mRNA contained in EVs secreted by carcinoma and melanoma cells to tumoral cells (both local and systemic exchanges) and non-tumoral cells (immune and non-immune) as well as the transfer of migratory and metastatic properties. It is important to note that these studies have essentially described rather large EVs in vivo. Whether this is due to the labeling methods used, or to the limits of optical resolution, is not clear.
The recent studies described above demonstrate that combining different labeling and imaging strategies to follow EVs naturally released by a primary tumor in a living animal provides unique insights into their behavior and function in vivo. It could for instance allow to follow the natural dispersion of tumor EVs from a primary tumor, which might lead to different observations than upon bulk injection of EVs in the circulation. This has been recently illustrated by Pucci et al. who combined multiple strategies of EV labeling and imaging to study tumor EVs released by melanoma in mice.Citation104 They showed that most EVs naturally released by tumor cells end up in the lymph nodes where their uptake by macrophages inhibits tumor growth, by preventing them to interact with B-cells. Using similar combination of strategies will certainly allow to better describe the fate and function of tumor EVs in a near future.
Another exciting field of investigation that could be approached using pioneer imaging technologies concerns the cellular mechanisms of secretion, uptake and sorting of tumor EVs in vivo. Determining the fate of tumor EVs in receiving cells, in particular identifying in which compartment they are sorted, will be instrumental in understanding how their message is delivered. Besides, intriguing mechanisms have recently been described in vitro, but their existence in vivo is so far unknown. It has for instance been shown that exosome secretion is coupled to the formation of an actin rich protrusion involved in tumor cell invasion, the invadopodia.Citation60 Moreover, EVs were shown to be taken up by filopodia from the receiving cell, shuttled in endosomes where they scan the endoplasmic reticulum before being directed to lysosomes.Citation108 The precise characterization of this mechanism relied on the use of the exosomal tetraspanin CD63 fused to GFP to monitor its behavior by time-lapse confocal microscopy and to the APEX2 tag allowing to precisely locate it by electron microscopy. Ideally, the study of EVs at the cellular to subcellular scale would require intravital Correlated Light and Electron Microscopy (CLEM). This challenging approach, which allows to follow an event at the fluorescent scale in the living animal and retrieve it at electron microscopy scale, could permit the precise characterization of tumor EV secretion uptake and sorting.Citation109,110 There is no doubt that applying this methodology to relevant animal models mimicking the metastasis cascade will unravel the exact subcellular mechanisms underlying the delivery of exosome-carried messages.
Outstanding open questions
In a near future, the imaging strategies described in the paragraph before will certainly help to address general open questions, such as how EVs cope with the extracellular matrix, how they cross the endothelial barrier or how they behave in fluids such as the blood and lymphatic circulation. These issues need to be resolved to fully understand the relevance of tumor EVs in cancer progression. They could benefit from alternative model organisms, which allow nice intravital imaging and constitute emerging cancer models in vivo, such as Drosophila (where EVs function has been described in a developmental contextCitation111-114). In addition, a recent study has used the zebrafish embryo model for testing their potential in drug delivery.Citation115,116 Although preliminary, these studies suggest that the zebrafish embryo could be a very useful model for studying the biology of exosomes in the near future.
In addition, several important questions regarding tumor EVs remain to be addressed in vivo. While in vitro studies show that more aggressive cancer lines secrete more EVs (e.g.Citation64), it is currently not clear whether tumor cells continuously secrete similar levels and populations of EVs, or whether they vary following tumor progression. Similarly, tumors have been shown to be composed of heterogeneous populations of cells, but how this is linked to different amounts of EVs and EVs with different content is unknown in vivo, although there are some hints from in vitro studies.Citation117 Furthermore, the heterogeneity of EVs, which has been demonstrated in vitro, still awaits validation in vivo and the functional significance of this diversity remains to be addressed. A recent study used intravital lung imaging in mice to show that melanoma cells arriving in the lung capillaries release large cytoplasmic blebs (5 μm average diameter), which are uptaken by myeloid cells and promote the formation of a successful metastasis.Citation118 This study reveals that exchange of cellular material cellular can occur via diverse mechanisms, going beyond small extracellular vesicles, and thereby control the efficient formation of metastatic niches in vivo.
Importantly, stromal cells also secrete EVs which contribute to tumor progression, as it has been shown for EVs secreted by stromal cells,Citation119,120 and astrocytes.Citation121 Therefore, EVs mediate the communication between tumor and stromal cells in both directions and it might be interesting to determine how one influences the other, through positive or negative feedback loops.
Finally, future studies should aim at identifying cargoes whose function could be exploited for the design of effective therapeutic approaches. Understanding the involvement of individual EVs cargoes in vivo could benefit from a recently developed tool allowing a temporal control of cargo loading in EVs through optogenetics which functions in vivo.Citation122 There is no doubt that the fascinating and fast evolving field of extracellular vesicles has been booming over the past years. Nevertheless, this field requires the design of studies dedicated to refine the role of EVs in cancer progression and to validate their potential targeting in anti-tumor therapies. Those aspects would therefore strongly benefit from establishing relevant in vivo models, experimental strategies, adapted imaging approaches and associated quantification of EV levels and delivery according to tumor stage and progression.
Table 1. Summary of the different approaches used to study tumor EVs in vivo in mice. Representative references are given as examples.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We apologize to the people whose work has not been cited, due to space constraints. We thank G. Orend for critical reading of the manuscript.
Funding
Research in the Goetz lab is supported by funding from University of Strasbourg (IdEx), INSERM, the French National Cancer Institute (INCa), the Canceropole Grand-Est, the Plan Cancer, the Ligue contre le Cancer and by ROCHE. V.H. received support from the French National Cancer Institute (INCa).
References
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21:309-22; PMID:22439926; http://dx.doi.org/10.1016/j.ccr.2012.02.022
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19:1423-37.
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005; 438:820-7; PMID:16341007; http://dx.doi.org/10.1038/nature04186
- Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014; 25:719-34; PMID:24856586; http://dx.doi.org/10.1016/j.ccr.2014.04.005
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996; 183:1161-72; PMID:8642258; http://dx.doi.org/10.1084/jem.183.3.1161
- Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol 2013; 200:373-83; http://dx.doi.org/10.1083/jcb.201211138
- Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin I V, Mathivanan S, Quesenberry P, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 2014; 3:26913; PMID:25536934; http://dx.doi.org/10.3402/jev.v3.26913
- Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol 2014; 29C:116-25; http://dx.doi.org/10.1016/j.ceb.2014.05.004
- Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles 2013; 2:3-5; http://dx.doi.org/10.3402/jev.v2i0.20389
- Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles 2012; 1:18397; http://dx.doi.org/10.3402/jev.v1i0.18397
- Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Théry C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 2013; 126:5553-65; PMID:24105262; http://dx.doi.org/10.1242/jcs.128868
- Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A 2016; 113:E968-77; PMID:26858453; http://dx.doi.org/10.1073/pnas.1521230113
- Willms E, Johansson HJ, Mäger I, Lee Y, Blomberg KEM, Sadik M, Alaarg A, Smith CIE, Lehtiö J, El Andaloussi S, et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep 2016; 6:22519; PMID:26931825; http://dx.doi.org/10.1038/srep22519
- Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 2001; 7:297-303; PMID:11231627; http://dx.doi.org/10.1038/85438
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 1998; 4:594-600; PMID:9585234; http://dx.doi.org/10.1038/nm0598-594
- Pitt JM, Charrier M, Viaud S, Andre F, Besse B, Chaput N, Zitvogel L. Dendritic Cell-Derived Exosomes as Immunotherapies in the Fight against Cancer. J Immunol 2014; 193:1006-11; PMID:25049431; http://dx.doi.org/10.4049/jimmunol.1400703
- Syn N, Wang L, Sethi G, Thiery JP, Goh BC. Exosome-Mediated Metastasis: From Epithelial–Mesenchymal Transition to Escape from Immunosurveillance. Trends Pharmacol Sci 2016; 37(7):606-17; PMID:27157716
- Martins VR, Dias MS, Hainaut P. Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr Opin Oncol 2013; 25:66-75; PMID:23165142; http://dx.doi.org/10.1097/CCO.0b013e32835b7c81
- Lässer C. Exosomes in diagnostic and therapeutic applications: biomarker, vaccine and RNA interference delivery vehicle. Exp Opin Biol Ther 2015; 15:103-17; PMID:25363342; http://dx.doi.org/10.1517/14712598.2015.977250
- Melo S a., Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, Lebleu VS, Mittendorf E a., Weitz J, Rahbari N, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015; 523:177-82; PMID:26106858; http://dx.doi.org/10.1038/nature14581
- Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008; 10:1470-6; PMID:19011622; http://dx.doi.org/10.1038/ncb1800
- Cha DJ, Franklin JL, Dou Y, Liu Q, Higginbotham JN, Beckler MD, Weaver AM, Vickers K, Prasad N, Levy S, et al. KRAS-dependent sorting of miRNA to exosomes. Elife 2015; 4:1-22; http://dx.doi.org/10.7554/eLife.07197
- McKenzie AJ, Hoshino D, Hong NH, Cha DJ, Franklin JL, Coffey RJ, Patton JG, Weaver AM. KRAS-MEK Signaling Controls Ago2 Sorting into Exosomes. Cell Rep 2016; 15:978-87; PMID:27117408; http://dx.doi.org/10.1016/j.celrep.2016.03.085
- Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et al. Cancer Exosomes Perform Cell-Independent MicroRNA Biogenesis and Promote Tumorigenesis. Cancer Cell 2014; 26(5):707-21.
- Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, Cheng HH, Arroyo JD, Meredith EK, Gallichotte EN, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A 2014; 111:14888-93; PMID:25267620; http://dx.doi.org/10.1073/pnas.1408301111
- Squadrito ML, Baer C, Burdet F, Maderna C, Gilfillan GD, Lyle R, Ibberson M, De Palma M. Endogenous RNAs Modulate MicroRNA Sorting to Exosomes and Transfer to Acceptor Cells. Cell Rep 2014; 8:1432-46; PMID:25159140; http://dx.doi.org/10.1016/j.celrep.2014.07.035
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 2008; 10:619-24; PMID:18425114; http://dx.doi.org/10.1038/ncb1725
- Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, Kremers GJ, Piston DW, Ayers GD, McConnell RE, Tyska MJ, et al. Amphiregulin exosomes increase cancer cell invasion. Curr Biol 2011; 21:779-86; PMID:21514161; http://dx.doi.org/10.1016/j.cub.2011.03.043
- Demory Beckler M, Higginbotham JN, Franklin JL, Ham A-J, Halvey PJ, Imasuen IE, Whitwell C, Li M, Liebler DC, Coffey RJ. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics 2013; 12:343-55; http://dx.doi.org/10.1074/mcp.M112.022806
- Di Vizio D, Kim J, Hager MH, Morello M, Yang W, Lafargue CJ, True LD, Rubin MA, Adam RM, Beroukhim R, et al. Oncosome formation in prostate cancer: Association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res 2009; 69:5601-9; PMID:19549916; http://dx.doi.org/10.1158/0008-5472.CAN-08-3860
- Chen W, Liu X, Lv M, Chen L, Zhao J, Zhong S, Ji M, Hu Q, Luo Z, Wu J, et al. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PloS One 2014; 9:e95240; PMID:24740415; http://dx.doi.org/10.1371/journal.pone.0095240
- Safaei R, Larson BJ, Cheng TC, Gibson M a, Otani S, Naerdemann W, Howell SB. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther 2005; 4:1595-604; PMID:16227410; http://dx.doi.org/10.1158/1535-7163.MCT-05-0102
- Federici C, Petrucci F, Caimi S, Cesolini A, Logozzi M, Borghi M, D'Ilio S, Lugini L, Violante N, Azzarito T, et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PloS One 2014; 9:e88193; http://dx.doi.org/10.1371/journal.pone.0088193
- Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Advances in brief expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res 2003; 63(15):4331-7.
- Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A 2009; 106:3794-9; PMID:19234131; http://dx.doi.org/10.1073/pnas.0804543106
- Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res 2011; 71:5346-56; PMID:21670082; http://dx.doi.org/10.1158/0008-5472.CAN-11-0241
- Kim CW, Lee HM, Lee TH, Kang C, Kleinman HK, Gho YS. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res 2002; 62:6312-7; PMID:12414662
- Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O'Connor STF, Chin AR, et al. Cancer-Secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014; 25:501-15; PMID:24735924; http://dx.doi.org/10.1016/j.ccr.2014.03.007
- Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, Lötvall J, Nakagama H, Ochiya T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood–brain barrier. Nat Commun 2015; 6:6716; PMID:25828099; http://dx.doi.org/10.1038/ncomms7716
- Treps L, Edmond S, Galan-moya EM, Harford-wright E, Schmitt A, Citerne A, Bidere N, Ricard D, Gavard J. Extracellular vesicle-transported Semaphorin3A promotes vascular permeability in glioblastoma. Oncogene 2015; 35(20):2615-23.
- Gu J, Qian H, Shen L, Zhang X, Zhu W, Huang L, Yan Y, Mao F, Zhao C, Shi Y, et al. Gastric Cancer Exosomes Trigger Differentiation of Umbilical Cord Derived Mesenchymal Stem Cells to Carcinoma-Associated Fibroblasts through TGF-β/Smad Pathway. PloS One 2012; 7:e52465
- Paggetti J, Haderk F, Seiffert M, Janji B, Distler U, Ammerlaan W, Kim YJ, Adam J, Lichter P, Solary E, et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fi broblasts. Blood 2015; 126:1106-18; PMID:26100252; http://dx.doi.org/10.1182/blood-2014-12-618025
- Cho JA, Park H, Lim EH, Kim KH, Choi JS, Lee JH, Shin JW, Lee KW. Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecol Oncol 2011; 123:379-86; PMID:21903249; http://dx.doi.org/10.1016/j.ygyno.2011.08.005
- Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol 2012; 40:130-8; PMID:21904773
- Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, Wymant J, Jones AT, Kynaston H, Mason MD, et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene 2014; 34(3):290-302.
- Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res 2010; 70:9621-30; PMID:21098712; http://dx.doi.org/10.1158/0008-5472.CAN-10-1722
- Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, Chin AR, Ren X, Gugiu BG, Meng Z, et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κB. Sci Rep 2014; 4:5750; http://dx.doi.org/10.1038/srep05750
- Klibi J, Niki T, Riedel A, Pioche-durieu C, Souquere S, Rubinstein E, Moulec S Le, Hirashima M, Guemira F, Adhikary D, et al. Blood diffusion and Th1-suppressive effects of galectin-9 – containing exosomes released by Epstein-Barr virus – infected nasopharyngeal carcinoma cells. Blood 2009; 113:1957-66; PMID:19005181; http://dx.doi.org/10.1182/blood-2008-02-142596
- Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med 2002; 195:1303-16; PMID:12021310; http://dx.doi.org/10.1084/jem.20011624
- Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, Zaccheddu A, Colone M, Arancia G, Gentile M, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: Role in immune escape. Gastroenterology 2005; 128:1796-804; PMID:15940614; http://dx.doi.org/10.1053/j.gastro.2005.03.045
- Taylor DD, Gerçel-taylor Ç, Lyons KS, Stanson J, Whiteside TL. T-Cell Apoptosis and Suppression of T-Cell Receptor / CD3- ζ by Fas Ligand-Containing Membrane Vesicles Shed from Ovarian Tumors. Clin Cancer Res 2003; 9:5113-9
- Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-?-mediated suppressive activity on T lymphocytes. Cancer Res 2006; 66:9290-8; PMID:16982774; http://dx.doi.org/10.1158/0008-5472.CAN-06-1819
- Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, et al. Membrane associated Hsp72 from tumor derived exosomes mediates STAT3 depedent immunosuppressive function of mouse and human myeloid derived suppressor cells. J Clin Invest 2010; 120:467-71.
- Liu Y, Xiang X, Zhuang X, Zhang S, Liu C, Cheng Z, Michalek S, Grizzle W, Zhang HG. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol 2010; 176:2490-9; PMID:20348242; http://dx.doi.org/10.2353/ajpath.2010.090777
- Zhang HG, Kimberly R, Grizzle WE, Falkson C, Liu Y, Zhang L, Li C, Cong Y, Yu S, Liu C, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol 2007; 178:6867-75.
- Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest 2016; 126:1216-23; PMID:26927673; http://dx.doi.org/10.1172/JCI81136
- Mu W, Rana S, Zöller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia (New York, NY) 2013; 15:875-87; http://dx.doi.org/10.1593/neo.13786
- Hakulinen J, Sankkila L, Sugiyama N, Lehti K, Keski-Oja J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J Cell Biochem 2008; 105:1211-8; PMID:18802920; http://dx.doi.org/10.1002/jcb.21923
- Ngora H, Galli U. Membrane-bound and exosomal metastasis-associated C4. 4A promotes migration by associating with the α6β4 integrin and MT1-MMP. Neoplasia (New York, NY) 2012; 14:95-107; http://dx.doi.org/10.1593/neo.111450
- Hoshino D, Kirkbride K, Costello K, Clark E, Sinha S, Grega-Larson N, Tyska M, Weaver A. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep 2013; 5:1159-68; PMID:24290760; http://dx.doi.org/10.1016/j.celrep.2013.10.050
- Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun 2015; 6:7164; PMID:25968605; http://dx.doi.org/10.1038/ncomms8164
- Purushothaman A, Bandari SK, Liu J, Mobley JA, Brown EA, Sanderson RD. Fibronectin on the surface of myeloma cell-derived exosomes mediates exosome-cell interactions. J Biol Chem 2016; 291:1652-63; PMID:26601950; http://dx.doi.org/10.1074/jbc.M115.686295
- Sverdlov ED. Amedeo Avogadro's cry: What is 1 µg of exosomes? BioEssays 2012; 34:873-5; PMID:22815202; http://dx.doi.org/10.1002/bies.201200045
- Peinado H, Aleč ković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar CM, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012; 18:883-91; PMID:22635005; http://dx.doi.org/10.1038/nm.2753
- Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Théry C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 2012; 72:4920-30; PMID:22865453; http://dx.doi.org/10.1158/0008-5472.CAN-12-0925
- Li W, Mu D, Tian F, Hu Y, Jiang T, Han Y, Chen J, Han G, Li X. Exosomes derived from Rab27a-overexpressing tumor cells elicit efficient induction of antitumor immunity. Mol Med Rep 2013; 8:1876-82; PMID:24146068
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010; 12:19-30; PMID:19966785; http://dx.doi.org/10.1038/ncb2000
- Hendrix A, Maynard D, Pauwels P, Braems G, Denys H, Van den Broecke R, Lambert J, Van Belle S, Cocquyt V, Gespach C, et al. Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis. J Natl Cancer Inst 2010; 102:866-80; PMID:20484105; http://dx.doi.org/10.1093/jnci/djq153
- Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, Chaturvedi P, Green JJ, Semenza GL. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U S A 2014; 111:E3234-42; PMID:24938788; http://dx.doi.org/10.1073/pnas.1410041111
- Yang J, Liu W, Lu X, Fu Y, Li L, Luo Y. High expression of small GTPase Rab3D promotes cancer progression and metastasis. Oncotarget 2015; 6:11125-38; PMID:25823663; http://dx.doi.org/10.18632/oncotarget.3575
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 2010; 285:17442-52; PMID:20353945; http; http; http://dx.doi.org/10.1074/jbc.M110.107821
- Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic micrornas regulate cancer cell metastasis. J Biol Chem 2013; 288:10849-59; PMID:23439645; http://dx.doi.org/10.1074/jbc.M112.446831
- Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, Kappes JC, Barnes S, Kimberly RP, Grizzle WE, et al. Murine Mammary Carcinoma Exosomes Promote Tumor Growth by Suppression of NK Cell Function. J Immunol 2006; 176:1375-85; PMID:16424164; http://dx.doi.org/10.4049/jimmunol.176.3.1375
- Jung T, Castellana D, Klingbeil P, Cuesta Hernández I, Vitacolonna M, Orlicky DJ, Roffler SR, Brodt P, Zöller M. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia (New York, NY) 2009; 11:1093-105; http://dx.doi.org/10.1593/neo.09822
- Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X, Huang B, Xu X, Zheng J, Cao X. Tumor Exosomal RNAs Promote Lung Pre-metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer Cell 2016; 30:243-56; PMID:27505671; http://dx.doi.org/10.1016/j.ccell.2016.06.021
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 2015; 17:1-7; PMID:25679028
- Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015; 527:1-19.
- Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, Chow A, O'Connor ST, Li S, Chin AR, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol 2015; 17:183-94; PMID:25621950; http://dx.doi.org/10.1038/ncb3094
- Challagundla KB, Wise PM, Neviani P, Chava H, Murtadha M, Xu T, Kennedy R, Ivan C, Zhang X, Vannini I, et al. Exosome-Mediated Transfer of microRNAs Within the Tumor Microenvironment and Neuroblastoma Resistance to Chemotherapy. J Natl Cancer Inst 2015; 107:1-13; http://dx.doi.org/10.1093/jnci/djv135
- Valencia K, Luis-Ravelo D, Bovy N, Antón I, Martínez-Canarias S, Zandueta C, Ormazábal C, Struman I, Tabruyn S, Rebmann V, et al. miRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol Oncol 2014; 8:689-703; PMID:24593875; http://dx.doi.org/10.1016/j.molonc.2014.01.012
- Zhang H, Bai M, Deng T, Liu R, Wang X, Qu Y, Duan J, Zhang L, Ning T, Ge S, et al. Cell-derived microvesicles mediate the delivery of miR-29a/c to suppress angiogenesis in gastric carcinoma. Cancer Lett 2016; 375:331-9; PMID:27000664; http://dx.doi.org/10.1016/j.canlet.2016.03.026
- Yue S, Mu W, Erb U, Zöller M. The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding. Oncotarget 2015; 6:2366-84; PMID:25544774; http://dx.doi.org/10.18632/oncotarget.2958
- Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci U S A 2015; 112(12):E1433-42.
- Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire CA, Chen JW, Tannous BA, Breakefield XO. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 2014; 8:483-94; PMID:24383518; http://dx.doi.org/10.1021/nn404945r
- Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, Takakura Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol 2013; 165:77-84; PMID:23562828; http://dx.doi.org/10.1016/j.jbiotec.2013.03.013
- Morishita M, Takahashi Y, Nishikawa M, Sano K, Kato K, Yamashita T, Imai T, Saji H, Takakura Y. Quantitative analysis of tissue distribution of the B16BL6-derived exosomes using a streptavidin-lactadherin fusion protein and Iodine-125-Labeled biotin derivative after intravenous injection in mice. J Pharm Sci 2015; 104:705-13; PMID:25393546; http://dx.doi.org/10.1002/jps.24251
- Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release 2015; 199:145-55; PMID:25523519; http://dx.doi.org/10.1016/j.jconrel.2014.12.013
- Hood JL, San RS, Wickline SA. Exosomes Released by Melanoma Cells Prepare Sentinel Lymph Nodes for Tumor Metastasis. Cancer Res 2011; 71:3792-801; PMID:21478294; http://dx.doi.org/10.1158/0008-5472.CAN-10-4455
- Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol Ther 2012; 21:185-91; PMID:23032975; http://dx.doi.org/10.1038/mt.2012.180
- Lai CP, Kim EY, Badr CE, Weissleder R, Mempel TR, Tannous BA, Breakefield XO. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat Commun 2015; 6:7029; PMID:25967391; http://dx.doi.org/10.1038/ncomms8029
- Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González MÁ, Bernad A, Sánchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2011; 2:282; PMID:21505438; http://dx.doi.org/10.1038/ncomms1285
- Suetsugu A, Honma K, Saji S, Moriwaki H, Ochiya T, Hoffman RM. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv Drug Deliv Rev 2013; 65:383-90; PMID:22921594; http://dx.doi.org/10.1016/j.addr.2012.08.007
- Zomer A, Maynard C, Verweij FJ, Kamermans A, Sch?fer R, Beerling E, Schiffelers RM, De Wit E, Berenguer J, Ellenbroek SIJ, et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 2015; 161:1046-57; PMID:26000481; http://dx.doi.org/10.1016/j.cell.2015.04.042
- Ridder K, Sevko A, Heide J, Dams M, Rupp A-K, Macas J, Starmann J, Tjwa M, Plate KH, Sültmann H, et al. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. OncoImmunology 2015; 4:e1008371; PMID:26155418; http://dx.doi.org/10.1080/2162402X.2015.1008371
- Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, Hulett MD, Mathivanan S. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics 2013; 13:3354-64; PMID:24115447; http://dx.doi.org/10.1002/pmic.201300282
- Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood 2014; 123:208-16; PMID:24255917; http://dx.doi.org/10.1182/blood-2013-03-489732
- Srinivasan S, Vannberg FO, Dixon JB. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci Rep 2016; 6:24436; PMID:27087234; http://dx.doi.org/10.1038/srep24436
- Wiklander OPB, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 2015; 4:26316; PMID:25899407; http://dx.doi.org/10.3402/jev.v4.26316
- Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C, Camussi G. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med 2014; 33:1055-63; PMID:24573178
- Hazan-Halevy I, Rosenblum D, Weinstein S, Bairey O, Raanani P, Peer D. Cell-specific uptake of mantle cell lymphoma-derived exosomes by malignant and non-malignant B-lymphocytes. Cancer Lett 2015; 364:59-69; PMID:25933830; http://dx.doi.org/10.1016/j.canlet.2015.04.026
- Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF. Cellular internalization of exosomes occurs through phagocytosis. Traffic 2010; 11:675-87; PMID:20136776; http://dx.doi.org/10.1111/j.1600-0854.2010.01041.x
- Morelli AE, Larregina AT, Shufesky WJ, Sullivan MLG, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 2004; 104:3257-66; PMID:15284116; http://dx.doi.org/10.1182/blood-2004-03-0824
- Imai T, Takahashi Y, Nishikawa M, Kato K, Morishita M, Yamashita T, Matsumoto A, Charoenviriyakul C, Takakura Y. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J Extracell Vesicles 2015; 4:26238; PMID:25669322; http://dx.doi.org/10.3402/jev.v4.26238
- Pucci F, Garris C, Lai CP, Newton A, Pfirschke C, Engblom C, Alvarez D, Sprachman M, Evavold C, Magnuson A, et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 2016; 1328:aaf1328.
- Yoshimura A, Kawamata M, Yoshioka Y, Katsuda T, Kikuchi H, Nagai Y, Adachi N, Numakawa T, Kunugi H, Ochiya T, et al. Generation of a novel transgenic rat model for tracing extracellular vesicles in body fluids. Sci Rep 2016; 6:31172; PMID:27539050; http://dx.doi.org/10.1038/srep31172
- Ricklefs F, Mineo M, Rooj AK, Nakano I, Charest A, Weissleder R, Breakefield XO, Chiocca EA, Godlewski J, Bronisz A. Extracellular Vesicles from High-Grade Glioma Exchange Diverse Pro-oncogenic Signals That Maintain Intratumoral Heterogeneity. Cancer Res 2016; 76:2876-81; PMID:27013191; http://dx.doi.org/10.1158/0008-5472.CAN-15-3432
- van der Vos KE, Abels ER, Zhang X, Lai C, Carrizosa E, Oakley D, Prabhakar S, Mardini O, Crommentuijn MHW, Skog J, et al. Directly visualized glioblastoma-derived extracellular vesicles transfer RNA to microglia/macrophages in the brain. Neuro Oncol 2015; 18:244; PMID:26289590
- Heusermann W, Hean J, Trojer D, Steib E, von Bueren S, Graff-Meyer A, Genoud C, Martin K, Pizzato N, Voshol H, et al. Exosomes surf on filopodia to enter cells at endocytic hot spots and shuttle within endosomes to scan the ER. J Cell Biol 2016; 213:173-84 Final revised manuscript submitted; PMID:27114500; http://dx.doi.org/10.1083/jcb.201506084
- Karreman MA, Hyenne V, Schwab Y, Goetz JG. Intravital Correlative Microscopy: Imaging Life at the Nanoscale. Trends Cell Biol 2016; 26(11):848-863
- Karreman MA, Mercier L, Schieber NL, Solecki G, Allio G, Winkler F, Ruthensteiner B, Goetz JG, Schwab Y. Fast and precise targeting of single tumor cells in vivo by multimodal correlative microscopy. J Cell Sci 2016; 129:444-56; PMID:26659665; http://dx.doi.org/10.1242/jcs.181842
- Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol 2012; 14:1036-45; PMID:22983114; http://dx.doi.org/10.1038/ncb2574
- Matusek T, Wendler F, Polès S, Pizette S, D'Angelo G, Fürthauer M, Thérond PP. The ESCRT machinery regulates the secretion and long-range activity of Hedgehog. Nature 2014; 516(7529):99-103; PMID:25471885
- Gradilla AC, González E, Seijo I, Andrés G, Bischoff M, González-Mendez L, Sánchez V, Callejo A, Ibáñez C, Guerra M, et al. Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat Commun 2014; 5:5649; PMID:25472772; http://dx.doi.org/10.1038/ncomms6649
- Corrigan L, Redhai S, Leiblich A, Fan SJ, Perera SMW, Patel R, Gandy C, Mark Wainwright S, Morris JF, Hamdy F, et al. BMP-regulated exosomes from Drosophila male reproductive glands reprogram female behavior. J Cell Biol 2014; 206:671-88; PMID:25154396; http://dx.doi.org/10.1083/jcb.201401072
- Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in danio rerio. Pharm Res 2015; 32(6):2003-14.
- Yang T, Fogarty B, LaForge B, Aziz S, Pham T, Lai L, Bai S. Delivery of small interfering RNA to inhibit vascular endothelial growth factor in zebrafish using natural brain endothelia cell-secreted exosome nanovesicles for the treatment of brain cancer. AAPS J 2016; 1-12 ; http://dx.doi.org/10.1208/s12248-016-0015-y
- Sánchez CA, Andahur EI, Valenzuela R, Castellón EA, Fullá JA, Ramos CG, Triviño JC. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget 2015; 7:3993-4008.
- Headley MB, Bins A, Nip A, Roberts EW, Looney MR, Gerard A, Krummel MF. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature 2016; 531:513-7; PMID:26982733; http://dx.doi.org/10.1038/nature16985
- Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012; 151:1542-56; PMID:23260141; http://dx.doi.org/10.1016/j.cell.2012.11.024
- Shimoda M, Principe S, Jackson HW, Luga V, Fang H, Molyneux SD, Shao YW, Aiken A, Waterhouse PD, Karamboulas C, et al. Loss of the Timp gene family is sufficient for the acquisition of the CAF-like cell state. Nat Cell Biol 2014; 16:889-901; PMID:25150980; http://dx.doi.org/10.1038/ncb3021
- Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015; 527:100-4; PMID:26479035; http://dx.doi.org/10.1038/nature15376
- Yim N, Ryu SW, Choi K, Lee KR, Lee S, Choi H, Kim J, Shaker MR, Sun W, Park JH, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat Commun 2016; 7:12277; PMID:27447450; http://dx.doi.org/10.1038/ncomms12277
