ABSTRACT
Interaction of leukemia blasts with the bone marrow extracellular matrix often results in protection of leukemia cells from chemotherapy and in persistence of the residual disease which is on the basis of subsequent relapses. The adhesion signaling pathways have been extensively studied in adherent cells as well as in mature haematopoietic cells, but the adhesion structures and signaling in haematopoietic stem and progenitor cells, either normal or malignant, are much less explored. We analyzed the interaction of leukemia cells with fibronectin (FN) using interference reflection microscopy, immunofluorescence, measurement of adherent cell fraction, real-time microimpedance measurement and live cell imaging. We found that leukemia cells form very dynamic adhesion structures similar to early stages of focal adhesions. In contrast to adherent cells, where Src family kinases (SFK) belong to important regulators of focal adhesion dynamics, we observed only minor effects of SFK inhibitor dasatinib on leukemia cell binding to FN. The relatively weak involvement of SFK in adhesion structure regulation might be associated with the lack of cytoskeletal mechanical tension in leukemia cells. On the other hand, active Lyn kinase was found to specifically localize to leukemia cell adhesion structures and a less firm cell attachment to FN was often associated with higher Lyn activity (this unexpectedly occurred also after cell treatment with the inhibitor SKI-1). Lyn thus may be important for signaling from integrin-associated complexes to other processes in leukemia cells.
Introduction
Ten enzymes with homology to c-Src have been identified up to date and are collectively referred to as Src family kinases (SFK). The expression of 5 members of this family tends to be restricted to haematopoietic cells and increased activity of these haematopoietic cell-related SFK is often associated with worse prognosis in leukemias.
Involvement of the founding member of the family, c-Src, in the regulation of adhesion and migration of adherent cells is well documented. The expression and the activity of c-Src are correlated with advanced malignancy, higher invasiveness and poor prognosis in a variety of human cancers. Historically, it was the transformation of fibroblasts with the constitutively active form of Src from Rous sarcoma virus, which induced formation of invadosomes.Citation1 It is known that c-Src interacts with focal adhesion kinase (FAK), binds to adhesion complexes and phosphorylates a large number of their components. The amount of phosphorylated tyrosine at adhesion sites decreases with maturation and stabilization of focal adhesions. The signaling from c-Src promotes the activity of Rac1/Cdc42 which are required e.g. for the formation of membrane protrusions, and reduces that of RhoA which is responsible for the mechanical tension of cytoskeletal fibers and thereby for adhesion complex maturation.Citation2 It is not clear, however, if the kinase activity of c-Src is necessary for the assembly or disassembly of adhesion complexes. A recent proteomic analysis of integrin adhesion complexes has shown that the composition of these structures is largely insensitive to SFK or FAK inhibition.Citation3 The architecture of adhesion complexes seems to be modulated mainly by mechanical force which alters the composition of protein complexes through mechanosensors like talin or Cas.Citation4-Citation6
Although SFK have partially overlapping functions, some of their activities are highly specific. Both transactivation and mutual inhibition between individual members of the family have been described. SFK can be divided to 2 categories according to their sequence homology: Lyn-related (Lyn, Hck, Lck, Blk), which are expressed mainly in haematopoietic cells, and Src-related (Src, Yes, Fyn, Fgr), which are expressed ubiquitiously.Citation7 The sequence homology between c-Src on one hand and Lyn, Hck or Lck on the other hand is relatively lowCitation8 and the role of these haematopoietic cell-related SFK in adhesion signaling is much less explored than that of c-Src. It is known, however, that Lyn kinase influences adhesion and migration in different cell types. Lyn depletion leads to decreased migration rate in neutrophilesCitation9 and macrophages.Citation8 Lyn is also required for long-term engraftment of trasplanted haematopoietic stem and progenitor cellsCitation10 as well as for platelet activation and thrombus formation.Citation11 Several papers suggested a role for Lyn in migration and adhesion of adherent cells, too.Citation12-Citation14 Although Lyn function in haematopoietic cell adhesion and migration often resembles that of c-Src in adherent cells, opposing effects of these 2 kinases have been described in endothelial cells where Lyn strengthens cell-cell junctions and inhibits vascular leakage.Citation15 Lyn also acts in opposition to Hck in mastocyte activation.Citation16
Interaction of immature haematopoietic cells with the bone marrow microenvironment is important for many processes, including proliferation and differentiation of haematopoietic progenitor cells, progenitor mobilization in blood cell donors, homing of transplanted progenitors in recipients, or persistence of residual disease in leukemias which is associated with protective effects of the bone marrow on leukemia blasts during chemotherapy. Kinases of the Src family represent possible therapeutic targets in many types of cancer and their inhibition may have impact on several physiologic and patophysiological processes. In this work, we focused on the role of SFK, and more specifically Lyn, in leukemia cell interaction with a canonical bone marrow protein, fibronectin.
Results
In screening experiments involving different proteins of the extracellular matrix (ECM), we identified fibronectin (FN) as the most general interacting partner for leukemia cells (see e.g., Supplementary Fig. S1) and we thus focused to this component of the bone marrow ECM when characterizing adhesion structures of leukemia cells. The use of a specific fibronectin fragment (120 K cell-binding fragment) further increased cell binding to FN-coated surfaces. To evaluate the firmness of cell attachment to the surface, we determined the adherent cell fraction (ACF), i.e. the percentage of cells that remain on FN layer after PBS Ca2+/Mg2+ wash. To analyze in detail cell interaction with FN-coated surfaces, we used immunofluorescence (IF), live cell imaging, interference reflection measurement (IRM), and real-time monitoring of impedance (Electric Cell-substrate Impedance Sensing, ECIS). To analyze the role of kinases of the Src family (SFK) we used dasatinib, a potent clinically used SFK inhibitor. We have previously found that EC50 value for SFK inhibition in Bcr-Abl-negative leukemia cells is about 20 nM.Citation17 In the present study, we used 100 nM dasatinib which provides nearly complete inhibition of SFK. For comparison, we also tested the effects of an alternative SFK inhibitor, SKI-1. Potential cytotoxicity of the inhibitors used was assessed by counting cells stained with Trypan blue and by flow-cytometry analysis of propidium iodide-stained samples during 48h incubation with 100 nM dasatinib or 20 µM SKI-1. We detected no significant effect of the inhibitors on the cell viability in this time frame. We only noted a decreased proliferation rate of HEL and OCI-AML3 cells treated with SKI-1.
Cell morphology on FN-coated surface
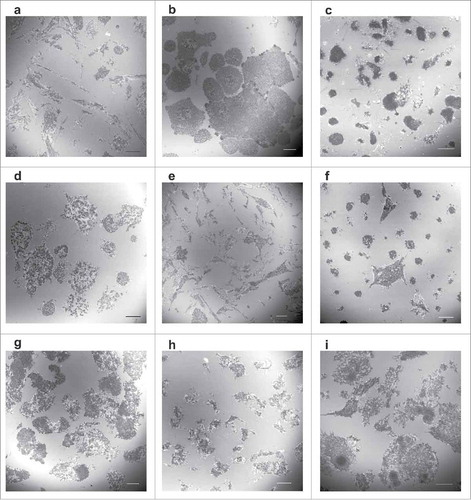
The morphology of leukemia cells and of their adhesion structures on a FN layer was found to be variable in leukemia cell lines as well as in primary cells from patients with acute myeloid leukemia (AML). Examples of IRM images shown in illustrate different extent of cell spreading, various cell shapes as well as different size and arrangement of adhesion points. The percentage of adhered cells (ACF) ranged from 2–4% in CML-T1 cells to 60–90% in the most adherent cell types (OCI-AML3, HEL) and was also variable in primary cells (range from 7 to 67%, samples from 14 AML patients were analyzed). Cell interaction with the coated surface was very dynamic and although the cells did not extensively migrate on the surface, they were continuously forming „exploratory“ protrusions. Multiple transient small contact points were visible in IRM imaging of living cells. We have tested several canonical markers of adhesion structures known from adherent cell types (focal adhesions) or from normal mature haematopoietic cells (podosomes), but none of them specifically localized to the adhesion points (dark areas seen in IRM) although talin was usually present. Paxillin and vinculin were found in some of them only, depending on the cell type and conditions. Frequently, a nice correlation between immunofluorescence and IRM images was observed for a phospho-specific antibody detecting the autophosphorylated (active) form of Src family kinases (pTyr416 in c-Src and the corresponding residues in Lyn, Hck, Lck, Fyn and Yes). Examples of immunofluorescence images are given in Supplementary Fig. S2. Although no actin stress fibers are usually found in leukemia cells, we detected such structures in cells treated with phorbol 12-myristate 13-acetate (Supplementary Fig. S3). In this case, the adhesion points were similar to those of adherent cells and colocalized very well with vinculin and paxillin.
Figure 1. Morphology of contact areas between leukemia cells and fibronectin-coated surface. Leukemia cell lines (a-f) or primary cells from leukemia patients (g-i) were seeded on FN-coated slides and incubated for 1 h at 37°C. The cell parts which are very close to the surface were visualized by interference reflection microscopy. The mean diameter of cells in suspension is 12–16 µm for cell lines and 6–9 µm for primary cells. Scale bars: 10 µm. Characteristic IRM images for leukemia/lymphoma cell lines OCI-AML3 (a), HEL (b), HL-60 (c), K562 (d), MOLM-7 (e), Karpas-299 (f). Primary cells from 3 different patients with AML are shown in (g-i).
Expression of Src family members in leukemia cells
The amount of c-Src, and of other Src family members which are known to be expressed in haematopoietic cells, was assessed by western-blotting (). As the different members of the family slightly differ in their molecular weights, it was possible to assign the bands detected by the phospho-specific antibody (denoted as pSFK in ) to the corresponding kinases (see Supplementary Fig. S4). With the exception of CML-T1 cells which express mainly Lck, the predominant active Src family kinase detected by pSFK antibody in haematopoietic cell lines was Lyn (double band at 53/56 kDa). Although c-Src (60 kDa) was also expressed in haematopoietic cells, its activity seems to be rather low judged from pSFK blots. The overall SFK activity in adherent cells (HeLa, HEK293T) was low in comparison with leukemia cells.
Figure 2. Expression levels of Src family kinases and of their active forms. Expression of c-Src, Lyn, Hck and Lck was assessed in human leukemia/lymphoma cell lines as well as in 2 human adherent cell lines (HeLa, HEK293T). The phospho-specific antibody against pSFK (Tyr416) recognizes an autophosphorylation site shared by all these kinases which serves as a marker of kinase activity. The same number of cells was harvested for all samples, actin was used as a control for total protein load.
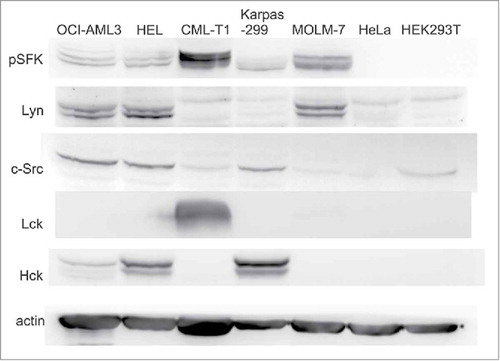
Based on our previous findingsCitation17 and on the literature data, we focused on Lyn as possible regulator of adhesion structures in haematopoietic cells and we selected cell lines with predominant Lyn activity (OCI-AML3, HEL, Karpas-299 and MOLM-7) for further analyses.
Effect of kinase inhibitors on adherent cell fraction
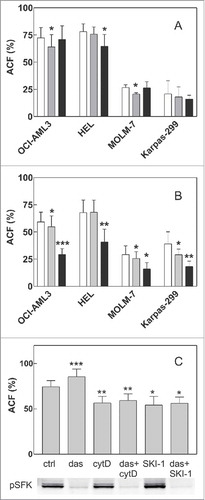
Effect of 2 well-known inhibitors of Src family kinase activity, i.e., dasatinib and SKI-1 (Src kinase inhibitor 1), was analyzed in 2 settings: (i) cells were pretreated with the inhibitors for 1 h, then seeded on FN and left adhere for 1 h before PBS/Ca2+/Mg2+ wash, or (ii) cells were seeded on FN, left adhere and only then treated with the inhibitors, incubated and washed. Cell pretreatment with dasatinib or SKI-1 had no or small impact on cell ability to bind to FN (). Dasatinib had only limited effect also when it was added to cells on FN (). On the other hand, a larger decrease in ACF occurred after 30 min treatment of pre-adhered HEL and OCI-AML3 cells with SKI-1 (). Although dasatinib had no significant effect on HEL cells after 30 min (), it strengthened HEL cell adhesion after longer incubation time (90 min, ). This indicated that SFK inhibition may result in firmer cell attachment to the coated surface. However, no such effect was observed in the other leukemia cell lines. As SFK are supposed to be involved in the disassembly of adhesion structures, we tested if the inhibition of SFK activity by dasatinib affected cell detachment induced by cytochalasin D, an inhibitor of actin polymerization which is known to reduce the adhesivity of adherent cells through disruption of mechanical tension.Citation18 Cytochalasin D (5 µM, 1 h) lowered the fraction of adhered HEL cells by 27% (average from 4 experiments) and this decrease in ACF remained almost unaffected by cell pretreatment with 100 nM dasatinib (). No effect of cytD on cell adhesivity to FN was observed in the other cell lines (data not shown). Dasatinib pretreatment also failed to prevent the decrease in ACF induced by SKI-1, both in HEL () and OCI-AML3 cells (data not shown), although dasatinib efficiently inhibited SFK activity (western-blot in ). The results presented in were analyzed using paired t-test. The increase of ACF after 90 min dasatinib treatment as well as the decrease of ACF due to SKI-1 or cytD treatment was statistically significant. On the other hand, no significant effect of dasatinib pretreatment was found for cytD or SKI-1-treated samples. Similar results were obtained using ANOVA test: a significant difference of variation was found for SKI-1 or cytD treatment (p less than 0.001) whereas no significant interaction was detected between dasatinib and SKI-1 or cytD (p = 0.131).
Figure 3. Effect of SFK inhibitors and cytochalasin D on cell attachment to fibronectin. A: Cells were pretreated with 100 nM dasatinib or 20 µM SKI-1 for 1 h and then seeded to FN-coated wells. The fraction of adhered cells was determined after 1 h incubation of the plate at 37°C. White bars: controls, gray bars: dasatinib, black bars: SKI-1. B: Cells were seeded on FN and left adhere for 1.5 h. Thereafter, inhibitors were added for additional 30 min at the same concentrations as in A. White bars: controls, gray bars: dasatinib, black bars: SKI-1. C: HEL cells were seeded into FN-coated wells. Dasatinib was added after 1.5 h to the corresponding wells, then 5 µM cytochalasin D or 20 µM SKI-1 was added after additional 30 min. The plate was further incubated for 1 h and the adhered cell fraction was determined thereafter. The level of phosphorylated SFK was assessed in parallel by western-blotting. All graphs show means and standard deviations from 3 to 7 independent experiments for each condition, each of them performed in quadruplets. The treated samples were compared with untreated controls using paired t-test: *p less than 0.05, **p less than 0.01, ***p less than 0.001.
Effect of kinase inhibitors on Lyn activity
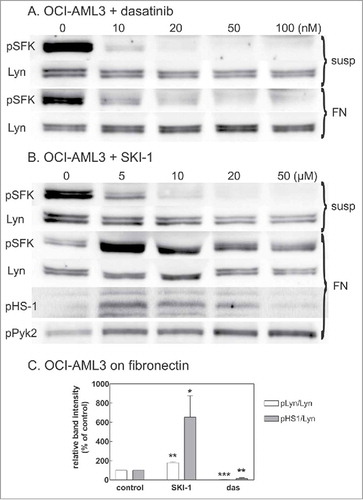
To verify the extent of kinase inhibition in the different conditions used in , OCI-AML3 cells in suspension or adhered to FN were treated with dasatinib or SKI-1 and Lyn phosphorylation at the autophosphorylation site was monitored by western-blotting (). When the cells were treated in suspension, both inhibitors reduced Lyn phosphorylation to negligible values. The effect of dasatinib was comparable on cells in suspension and on pre-adhered cells (), but SKI-1 induced an unexpected increase in Lyn autophosphorylation when added to cells on FN (, ). To confirm that the increased phosphorylation was actually associated with an increase in the kinase activity, we also analyzed the phosphorylation status of 2 known Lyn substrates, HS-1 (haematopoietic -specific analog of cortactinCitation19) and Pyk2 (haematopoietic analog of focal adhesion kinase). Indeed, the phosphorylation of Lyn targets (Tyr 397 in HS-1 and Tyr402 in Pyk2) increased upon treatment with SKI-1. Similar results were obtained for HEL cell line (Supplementary Fig. S5).
Figure 4. Effect of SFK inhibitors on Lyn activity. OCI-AML3 cells were treated with dasatinib or SKI-1, either in suspension (susp) or after adhesion to FN-coated surface (FN). Lyn activity was assessed from the extent of phosphorylation at Lyn autophosphorylation site (Tyr397) and on 2 known Lyn substrates, HS-1 and Pyk2. Lyn autophosphorylation was analyzed from bands at 53 and 56 kDa on western-blots incubated with pan anti-pSFK antibody. A-B: Representative western-blots from cells treated for 1 h with increasing concentrations of dasatinib (A) or SKI-1 (B). C: Summary results from 4 experiments performed with 20 µM SKI-1 or 100 nM dasatinib treatment of pre-adhered OCI-AML3 cells (cell binding to FN for 1.5 h, then 30 min inhibitor treatment). Note that the exposition time for different membranes was not the same and was optimized to show the changes in pSFK level under different conditions.
Microimpedance analysis
Changes in cell interaction with FN induced by treatment with Src family kinase inhibitors were further studied using real-time measurement of microimpedance. This technique allows for monitoring of changes in the cell spreading or in the distance of the cell ventral membrane from the coated surface. The instrument enables measurement at multiple frequencies of the electric field and calculates the components of impedance, i.e., resistance and capacitance. The capacitance at high field frequencies (40 kHz or more) involves transcellular currents and mirrors essentially the surface coverage (i.e., cell spreading in our experiments).Citation18,Citation20 The resistance at lower frequencies (400 Hz to 5 kHz) involves paracellular currents which are influenced both by the intercellular area and by cell closeness to the coated surface. We reported previously that the microimpedance signal progressively increases for 1 to 1.5 h following leukemia cell addition to FN-coated wells, reflecting the cell attachment and spreading.Citation21
shows representative examples of extensive changes induced in adherent cells upon treatment with dasatinib or with cytochalasin D (cytD), an inhibitor of actin polymerization which releases the cytoskeletal tension and promotes disassembly of focal adhesions. Panels B-D show the effect of dasatinib, cytD and SKI-1 on OCI-AML3 cells, as a representative example of leukemia cell lines, and on HEL cells, an erythroleukemia cell line which is to some extent similar to adherent cells. Pretreatment with dasatinib did not significantly affect the ability of leukemia cells to attach to FN (, left). The amplitude of changes in the resistance was reduced by dasatinib treatment in HEL cells (, right), probably due to reduced cell spreading. However, the rate of the attachment was unchanged indicating that the cell capacity to form adhesion structures is retained in the absence of SFK activity. Unlike the adherent cells, leukemia cells were also largely insensitive to dasatinib addition (cf with , left) whereas the response of HEL cells was closer to the adherent cells (cf with , right). CytD treatment resulted in a slow increase in the microimpedance signal in leukemia cells, in contrast to adherent cells and HEL cells. Finally, SKI-1 produced a transient marked increase in the resistance in OCI-AML3 cells and concentration-dependent changes different from those induced by dasatinib in HEL cells (). SKI-1 induced changes in OCI-AML3 cells (, left) were not markedly influenced by the frequency of the electric field and thus probably reflect a transient increase in the mean cell area.
Figure 5. Monitoring of cell-FN interaction through microimpedance measurement. The effect of dasatinib (100 nM), cytochalasin D (5 µM) and SKI-1 (20 or 50 µM) is shown for adherent (HeLa, HEK293T) and leukemic (OCI-AML3, HEL) cells. The arrows indicate the time of inhibitor addition to cells on FN. The curves are means from well duplicates. The baseline values (before cell addition) were subtracted. In A, C and D, the resistance was normalized to 1 at a time point just before addition of inhibitors. In B, non-normalized values are shown. A: Effect of dasatinib or cytochalasin D addition on the microimpedance signal from adherent cells (left – HeLa, right – HEK293T). B: Effect of 1h dasatinib pretreatment on the microimpedance signal from attaching leukemia cells (left – OCI-AML3, right – HEL). C: Effect of dasatinib or cytochalasin D addition on the signal from leukemic cells (left – OCI-AML3, right – HEL). D: Effect of SKI-1 on the signal from leukemic cells (left – OCI-AML3, right – HEL).
Effect of kinase inhibitors on leukemia cell morphology and dynamics
We failed to detect any effect of 100 nM dasatinib treatment on cell morphology and dynamics, regardless of leukemia cell type and of experimental settings (cell pretreatment or addition to adhered cells). As expected, the signal of the phospho-specific (Tyr416) antibody against SFK disappeared from immunofluorescence images in the presence of dasatinib. However, the cells still formed membrane protrusions and short-lived contacts with the surface as monitored using IRM, with no apparent change in their morphology and assembly/disassembly rate.
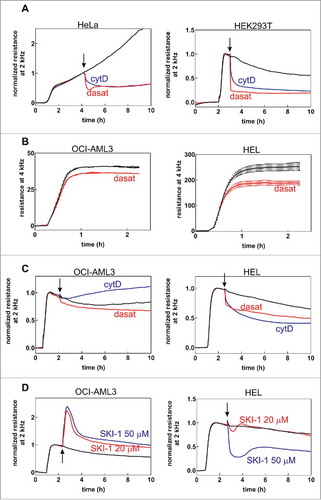
Similarly, SKI-1 did not seem to induce marked changes in the morphology and dynamics of the treated cells and of their adhesion structures. In agreement with the results of western-blots, this inhibitor enhanced the signal of the phospho-specific antibody against SFKs in cells plated on FN, specifically in adhesion sites. The anti-pSFK (Tyr416) antibody was nearly undetectable in control OCI-AML3 cells and SKI-1 treatment resulted in a marked increase of the fluorescence signal which was clearly localized to adhesion sites detected in IRM (). In MOLM-7 cells, which have higher level of active Lyn even without treatment, the signal was distributed both in adhesion sites and in multiple small foci in the cytoplasm. SKI-1 treatment highlighted the staining in the adhesion sites while attenuating the signal from the other cell parts (). Lyn activation by SKI-1 is thus limited to the proximity of adhesion complexes. Additional examples of changes in pSFK signal after cell treatment with SKI-1 are included in Supplementary Fig. S6.
Figure 6. Localization of autophosphorylated Lyn kinase in cells treated with SKI-1. The cells were incubated for 1 h on fibronectin, then treated with 20 µM SKI-1 for 30 to 60 min. The sample was fixed and the active Lyn was detected using anti-phospho-SFK (Tyr416) and Alexa-488-conjugated secondary antibody. Red: actin polymers visualized by phalloidin, blue: nuclei (DAPI). IRM and IF from the same visual field are shown in each pair of images. A: OCI-AML3 cells, B: MOLM-7 cells. Scale bars: 10 µm.
Effect of kinase inhibitors on HeLa cells
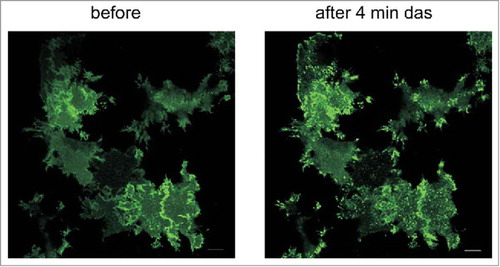
To investigate Lyn behavior in adherent cells, we transfected HeLa cells with plasmids coding for Lyn kinase fused with the green fluorescent protein (Lyn-eGFP). Although the fluorescence signal was spread over the whole cell, focal adhesions were usually enriched (). We detected no difference in the morphology of focal adhesions between control and transfected cells as well as between successfully transfected cells (with eGFP signal, 8–15% of cells) and cells without Lyn-eGFP expression. Cells treated with 100 nM dasatinib had larger focal adhesions and appeared to be more tensioned in comparison with the untreated controls, independently of the presence of Lyn-eGFP. In parallel, dasatinib treatment resulted in a fast (in several minutes) accumulation of Lyn-eGFP in focal adhesions (, right panel, Supplementary Fig. S7). Similar effects were observed after cell treatment with SKI-1, but the changes were slower and less apparent.
Figure 7. Changes in the distribution of Lyn-eGFP signal upon treatment of HeLa cells with dasatinib. HeLa cells were transfected with plasmid coding for Lyn-eGFP. The figure shows the fluorescence intensity from eGFP before and 4 min after treatment of cells with 100 nM dasatinib. Scale bars: 10 µm.
Discussion
Abnormal function of cell adhesion signaling contributes to aberrant behavior of leukemia blast cells. A typical example is the chronic myelogenous leukemia (CML) which is characterized by expression of the fusion protein BCR-ABL with tyrosine kinase activity, SH3 domain and actin-binding domain. CML cells display altered ß1-integrin-dependent adhesion and adhesion-mediated growth regulationCitation22 as well as increased ability to survive in the absence of interactions with the bone marrow. A number of other adhesion receptors, including CD44 and L-selectin have also been implicated in abnormal trafficking and growth of CML blasts.Citation23 In addition, BCR-ABL downregulates the expression of the chemokine receptor CXCR4 and thereby alters CML cell response to SDF-1.Citation24,Citation25 It has also been reported that p190 form of Bcr-Abl (expressed in acute lymphocytic leukemia) but not p210 form (expressed in CML) induces invadopodia formation upon transfection into BaF3 cells.Citation26 Treatment with tyrosine kinase inhibitors used in CML therapy may restore the interaction of CML cells with the bone marrow niches which in turn results in acquisition of stroma-mediated chemoresistence and may aggravate the residual disease.Citation17,Citation27,Citation28 Similarly, multiple reports have already described altered expression of adhesion receptors (e.g., CXCR4, CD44, VLA-4, CD166) in AML cellsCitation29,Citation30 as well as differences in interaction with bone marrow components between primary AML blasts and normal progenitor cells.Citation31 Targeting of cell-matrix interactions is considered to be a promising tool for eradication of the residual disease in AML. In B-cell chronic lymphocytic leukemia (CLL), increased levels of cortactin, which is involved in regulation of cell motility, was found to correlate with negative prognostic factors.Citation32
Increased expression or activity of haematopoietic cell-related SFK is often associated with leukemia progression and resistance to treatment. For example, SFK have been described to play a role in CML,Citation33 acute lymphoblastic leukemia,Citation34 AML,Citation35 multiple myelomaCitation36 and B-cell CLL.Citation37 Increased levels of Lyn kinase were associated with enhanced cytoskeletal activity in B-cell CLL.Citation38 Dasatinib, a dual inhibitor of BCR-ABL and SFK, is used in CML treatment, especially in cases of resistance to imatinib mesylate, a specific BCR-ABL inhibitor. Targeting of SFK is also considered as a part of combined anti-leukemia therapy.Citation35
Cell binding to fibronectin is mediated by integrins and associated protein complexes. In adherent cells, the composition and the architecture of these complexes evolve during cell attachment and spreading and the mature adhesion structures are connected to actin-myosin fibers as well as to intermediate filaments. A large set of integrin-associated proteins (so-called consensus integrin adhesome) has been defined on the basis of the composition of adhesion complexes from 7 different cell types, including one leukemia cell line (K562).Citation39 In comparison with adherent cells, the integrin adhesome of K562 cells lacked in particular proteins with force-recognition domains and the authors suggested that K562 data set likely represented a higher proportion of immature structures that form before applied myosin-II-generated cytoskeletal forces. In agreement with this result, we found that adhesion structures of leukemia cells were very dynamic and the cells usually did not contain actin stress fibers. Also, the contact points seen in IRM did not colocalize with markers which are generally used to visualize focal adhesions. Indeed, paxillin did not form part of K562 integrin adhesome although it was present in all data sets from adherent cells.Citation39 On the other hand, Lyn was found exclusively in the adhesome of K562 cells. In our experiments, SFK localization in contact points was clearly evident mainly for the active (autophosphorylated) form, in several leukemia cell lines as well as in primary cells from AML patients (Supplementary Fig. S6).
Dasatinib is a potent SFK inhibitor used in CML treatment and we confirmed nearly complete Lyn inhibition by 100 nM dasatinib in our cell models ( and ). Our results show that SFK are not required for leukemic cell attachment to FN as the adherent cell fraction was not very different in the presence of dasatinib () and the kinetics of cell binding monitored through the microimpedance signal were also nearly the same (, left). A minor effect of dasatinib on the impedance signal could be attributed to a small decrease in the mean cell area. It is known that c-Src attenuates RhoA activity, limits the actinomyosin contractility and allows for cell spreading. Cell shrinking due to SFK inhibition is thus not surprising and suggests that Lyn could have similar role in HEL cells where dasatinib induced a more marked reduction of the resistance signal ( and , right).
Suprisingly, Lyn kinase was activated following cell treatment with another SFK inhibitor SKI-1 when the cells were on FN layer (, Supplementary Fig. S5). The increase in Lyn activity correlated with a significant decrease of adherent cell fraction () as well as with changes in microimpedance signal which were cell type-dependent (). In HEL cells, lower resistance could reflect looser cell-surface contact corresponding to lower ACF, but the effect was already apparent at 20 µM SKI-1 concentration on ACF () whereas 50 µM was needed to produce a significant change in the microimpedance signal (). In OCI-AML3 cells, Lyn activity may stimulate membrane blebbing and transient cell spreading on the surface, which would mask the decrease in resistance due to weakening of the cell attachment to the surface. The increase in Lyn activity in adhesion points after treatment with SKI-1 is probably due to the inhibition of an unspecific target in addition to SFK. To further confirm this assumption, we also tested a third SFK inhibitor, saracatinib. In our cell systems, the effect of saracatinib on Lyn activity was similar to that of dasatinib, i.e., it gradually reduced the bands seen on anti-pSFKs western-blots, both in suspension and in cells on FN, with EC50 of about 1 µM. No increase in Lyn activity was noted in the range from 30 nM to 20 µM (data not shown).
Lyn kinase has been repeatedly reported to affect adhesion and migration in mature haematopoietic cells. Lyn knockout resulted in hyperadhesivity and impaired migration in macrophages,Citation8 migration defects were also observed in neutrophiles after Lyn knockdown using siRNA.Citation9 Our results indicate that in leukemia cells, the kinase aktivity of SFK including Lyn is dispensable for cell attachment to fibronectin. On the other hand, active Lyn localizes to adhesion structures and increased Lyn activity is associated with looser cell binding: cell treatment with SKI-1 induced simultanously an increase in Lyn activity (, Supplementary Fig. S5) and a decrease in adherent cell fraction (). We have previously reported that leukemic cell treatment with histone deacetylase inhibitors resulted in an increase in adherent cell fraction in parallel with a decrease of Lyn activity.Citation21 We have also noted that cell lines with lower SFK activity (OCI-AML3, HEL and adherent cell lines) are more adherent than cells with higher SFK activity (MOLM-7, CML-T1).
Transfection of fluorescently labeled Lyn into adherent HeLa cells showed that Lyn localizes in focal adhesions of these cells, too (). In agreement with previous studies,Citation6,Citation40,Citation41 SFK inhibition by dasatinib induced an increase in focal adhesion size suggesting that focal adhesions are rapidly stabilized in the absence of SFK signaling. On the contrary, we failed to detect any effect of dasatinib on adhesion structure morphology and dynamics in leukemia cells and an increase in ACF was observed only in HEL cells treated with dasatinib for a longer time (90 min, ). In addition, dasatinib pretreatment did not prevent the decrease in cell adhesivity induced by cytochalasin D or SKI-1 () which means that cells can detach from FN in the absence of SFK signaling. This indicates that Lyn does not directly participate in the adhesion structure disassembly and that the decrease in cell adhesivity following SKI-1 treatment is not causally related to Lyn activation.
Interestingly, one leukemia cell line (HEL) resembles to some extent to adherent cells both by more firm attachment to FN in the presence of dasatinib and by partial sensitivity to cytochalasin D (, ). Formation of actin stress fibers and of integrin-containing focal adhesions in HEL cells stimulated with PMA and seeded on FN has already been described previously.Citation42,Citation43 However, according to our results, HEL cell line is not a representative example of leukemia cells as to the adhesion structures and signaling. This infers the hypothesis that the main raison for difference between adherent and leukemia cells is the absence of cytoskeletal fibers which precludes focal adhesion maturation and stabilization. In adherent cells, SFK would act in opposition to mechanical forceCitation41 whereas no space for SFK activity would be provided in the absence of this force in leukemia cells.
In conclusion, Lyn kinase localizes in adhesion structures of leukemia cells as well as of adherent HeLa cells. In leukemia cells, its increased activity correlates with lower cell adhesivity to fibronectin. However, SFK activity is not required for adhesion structure assembly or function and, in contrast to adherent cells, its impact on the stability of the attachment is limited. With regard to documented involvement of Lyn in many important processes in immature haematopoietic cells,Citation10,Citation44 we suppose that the role of Lyn in adhesion structures of these cells is to link the cell-matrix interaction to signaling regulating cell growth and survival.
Material and methods
Chemicals and antibodies
Fibronectin fragment (120 kDa cell attachment region) was purchased from Merck (#F1904) and used at 20 µg/ml concentration for surface coating. Dasatinib was obtained from Selleckchem (#S1021), 200 µM stock solution was made in sterile dimethylsulfoxide (DMSO). Src Kinase Inhibitor I (SKI-1) was from Abcam (#ab120839), 50 mM stock solution was prepared in sterile DMSO. The stock solution of cytochalasin D (Sigma, #C8273) was 10 mM in DMSO. Further dilutions of all compounds were made with RPMI-1640 medium.
Antibodies against the following targets were used: phospho-Src Family (Tyr416) (Cell Signaling, #2101), c-Src (Calbiochem, #OP07), Lyn (BD Biosciences, #610003), Hck (BD Biosciences, #610278), Lck (Abcam, #ab 32149), vinculin (Abcam, #ab129002), paxillin (BD Biosciences, #610051), talin 1 (Abcam, #ab157808), phospho-HS-1 (Tyr397) (Cell Signaling, #8714S), phospho-Pyk2 (Tyr402) (Life Technologies, #44–618G), β-actin (Sigma, #A5441).
Cell isolation and culture
Cell lines were purchased from DSMZ (Braunschweig, Germany – OCI-AML3, JURL-MK1, Karpas-299, CML-T1) or ECACC (Salisbury, UK – HL-60, K562). HEL, HeLa and HEK293T cells were obtained as a gift and authenticated using analysis of short tandem repeats (May 2016), the results were compared with ATCC database. The cell line MOLM-7 which is not commercially available was obtained from Dr J. Minowada.Citation45 All the purchased cell lines were shortly expanded after receipt and stored in frozen aliquots in liquid nitrogen. Freshly resuscitated aliquots were used for experiments and the cells were not passaged for more than 6 months. The majority of cell lines were cultured in RPMI-1640 medium with 10% fetal calf serum, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in 5% CO2 humidified atmosphere, except for OCI-AML3 (α-MEM medium, 20% FCS) and HEK293T (DMEM medium).
Primary cells from patients with acute myeloid leukemia (AML) were obtained from leukapheresis at diagnosis. All patients provided their written informed consent as to the use of their biologic material for research purposes, in accordance with the Helsinki Declaration. The leukapheretic products were diluted 20-fold in PBS and the mononuclear cell fraction was then separated using the standard protocol for Histopaque-1077 (Sigma, #H8889). Analysis by flow cytometry (CD45/SSC dotplots) confirmed high prevalence of leukemia blasts in mononuclear cell samples.
Measurement of adherent cell fraction (ACF)
The end-point method for assessment of cellular adhesivity to fibronectin-coated surface has been descibed previously.Citation46 Briefly, the cells (1 × 104) were seeded into fibronectin-coated wells on a microtitration plate and incubated for 1 h at 37°C. Then, the cells were washed wih PBS/Ca2+/Mg2+ and the remaining cells were quantified by means of fluorescent labeling (CyQuant Cell Proliferation Assay Kit; Molecular Probes, #C7026). The adherent cell fraction (ACF) was calculated using the fluorescence signal from fibronectin-coated plate and the signal obtained from a reference plate that contained the total cell number.
Immunofluorescence microscopy (IF)
The cells were plated on fibronectin-coated glass bottom dishes, incubated for 60 minutes in CO2 incubator, fixed with 2% paraformaldehyde, permeabilized in 0.3% Triton/PBS and incubated with primary and secondary antibodies. F-actin was labeled with Alexa Fluor® 647 Phalloidin (Molecular Probes, #A22287). Samples were analyzed using an Olympus FluoView FV1000 confocal laser scanning microscope, focusing on the sample layer adjoining to the FN-coated substrate.
Interference reflection measurement (IRM) and live cell imaging
Cells were incubated for 1 h on fibronectin-coated coverslip and used for live cell imaging and time-lapse microscopy or fixed with 2% paraformaldehyde. The interference in reflected light was observed by means of FV-1000 confocal microscope (Olympus), using 405 nm laser beam and focusing to the coated glass surface.
Real-time monitoring of electric cell-substrate impedance (ECIS)
Impedance measurements were performed using the ECIS Ztheta apparatus (Applied Biophysics). The wells of a 8W10E+ plate were filled with 150 µl FN solution (20 µg/ml in 0.15 M NaCl), incubated for 30 min at the room temperature, washed with RPMI medium without calf serum and filled with 200 µl medium with serum. The baseline was monitored for about 1 h before addition of cells (150 to 200 thousands per well in 200 µl). The subsequent progressive impedance increase lasted for 1 to 1.5 h until the cell attachment to the well bottom reached an equilibrium state. The instrument automatically decomposes the impedance signal into resistance and capacitance.
Immunoblotting
The cells (5 × 106) were pelleted by centrifugation and lysed for 15 min / 4°C in modified RIPA lysis buffer (50 mM HEPES; 0,15 M NaCl; 2 mM EDTA; 0,1% NP-40; 0,05% sodium deoxycholate) with freshly added protease and phosphatase inhibitors. Cellular debris was disposed by centrifugation (16.000 g / 4°C / 15 min), and the lysate was mixed 1:1 (v/v) with 2x Laemmli sample buffer.
In the experiments involving cell harvest from FN, the cells (1 × 106) were seeded into FN-coated wells of a 12-well microtitration plate. After incubation with inhibitors, the fraction of cells in suspension was collected by aspirating the media from the wells and pelleted by centrifugation. The remaining adhered cells were simultaneously scrapped into lysis buffer (500 µl of modified RIPA lysis buffer with phosphatase and protease inhibitors), on ice. The 2 cell fractions were combined and incubated in RIPA for 30 min at 4°C. The lysate was then concentrated using Amicon Ultra centrifugal filters (Sigma, cat. no. Z677108, 10 kDa cutoff) to the final volume of 50 µl. The concentrate was mixed 1:1 (v/v) with 2x Laemmli sample buffer.
An equivalent of 20 µg of total protein was resolved on polyacrylamide gel and transferred to a nitrocellulose membrane. The membrane was incubated with primary antibodies overnight at 4°C in 3% BSA in PBS with 0,1% Tween-20 (PBST), washed in PBST 6 times, and incubated with corresponding HRP-conjugated secondary antibodies for 1 h. Chemiluminiscence signal was developed by Pierce SuperSignal® Substrate (Thermo Scientific, #34080F), and detected and assessed by G:BOX iChemi XT-4 (Syngene).
Cell transfection with Lyn-eGFP plasmid
Lyn kinase-coding cDNA was amplified from cDNA library (Jurkat cells, Origene) by PCR using merged primers containing appropriate restriction sites (Fw: AAAAAACTCGAGCATGGGATGTATAAAATCAAAAGGG, Rv: AAAAAAGGATCCCAGGCTGCTGCTGGTATTG). By standard methods of molecular cloning, the cDNA fragment was inserted into vector peGFP-N2 (originally Clontech) designed for expression of target protein tagged with the fluorescent protein at its C-terminus. The construct was amplified in E. coli and purified with the PureYield Plasmid Miniprep System (Promega). This purified plasmid was then transfected into HeLa cells using the jetPRIME transfection reagent (Polyplus Transfection) following the manufacturer's instructions.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_materials.zip
Download Zip (2.3 MB)Acknowledgments
The authors wish to thank M.Voráčová and H.Pilcová for expert technical assistance.
Funding
This work was supported by the Grant Agency of the Czech Republic under grant No 16-16169S and the Ministry of Health of the Czech Republic under project for conceptual development of the research organization No 00023736.
References
- Guarino M. Src signaling in cancer invasion. J Cell Physiol 2010; 223:14-26; PMID:20049846
- Huveneers S, Danen EH. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci 2009; 122:1059-69; PMID:19339545; https://doi.org/10.1242/jcs.039446
- Horton ER, Humphries JD, Stutchbury B, Jacquemet G, Ballestrem C, Barry ST, Humphries MJ. Modulation of FAK and Src adhesion signaling occurs independently of adhesion complex composition. J Cell Biol 2016; 212:349-64; PMID:26833789; https://doi.org/10.1083/jcb.201508080
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science 2009; 323:638-41; PMID:19179532; https://doi.org/10.1126/science.1162912
- Yao M, Goult BT, Klapholz B, Hu X, Toseland CP, Guo Y, Cong P, Sheetz MP, Yan J. The mechanical response of talin. Nat Commun 2016; 7:11966; PMID:27384267; https://doi.org/10.1038/ncomms11966
- Teckchandani A, Cooper JA. The ubiquitin-proteasome system regulates focal adhesions at the leading edge of migrating cells. Elife 2016; 5:pii: e17440; PMID:27656905; https://doi.org/10.7554/eLife.17440
- Ingley E. Functions of the Lyn tyrosine kinase in health and disease. Cell Commun Signal 2012; 10:21; PMID:22805580; https://doi.org/10.1186/1478-811X-10-21
- Abram CL, Lowell CA. The diverse functions of Src family kinases in macrophages. Front Biosci 2008; 13:4426-50 [pii]; PMID:18508521; https://doi.org/10.2741/3015
- He Y, Kapoor A, Cook S, Liu S, Xiang Y, Rao CV, Kenis PJ, Wang F. The non-receptor tyrosine kinase Lyn controls neutrophil adhesion by recruiting the CrkL-C3G complex and activating Rap1 at the leading edge. J Cell Sci 2011; 124:2153-64; PMID:21628423; https://doi.org/10.1242/jcs.078535
- Orschell CM, Borneo J, Munugalavadla V, Ma P, Sims E, Ramdas B, Yoder MC, Kapur R. Deficiency of Src family kinases compromises the repopulating ability of hematopoietic stem cells. Exp Hematol 2008; 36:655-66; PMID:18346837; https://doi.org/10.1016/j.exphem.2008.01.002
- Zhi H, Dai J, Liu J, Zhu J, Newman DK, Gao C, Newman PJ. Platelet Activation and Thrombus Formation over IgG Immune Complexes Requires Integrin alphaIIbbeta3 and Lyn Kinase. PLoS One 2015; 10:e0135738; PMID:26291522; https://doi.org/10.1371/journal.pone.0135738
- Penzes K, Baumann C, Szabadkai I, Orfi L, Keri G, Ullrich A, Torka R. Combined inhibition of AXL, Lyn and p130Cas kinases block migration of triple negative breast cancer cells. Cancer Biol Ther 2014; 15:1571-82; PMID:25482942; https://doi.org/10.4161/15384047.2014.956634
- Roseweir AK, Qayyum T, Lim Z, Hammond R, MacDonald AI, Fraser S, Oades GM, Aitchison M, Jones RJ, Edwards J. Nuclear expression of Lyn, a Src family kinase member, is associated with poor prognosis in renal cancer patients. BMC Cancer 2016; 16:229, 016-2254-9; PMID:26984511; https://doi.org/10.1186/s12885-016-2254-9
- Pham H, Birtolo C, Chheda C, Yang W, Rodriguez MD, Liu ST, Gugliotta G, Lewis MS, Cirulli V, Pandol SJ, et al. Essential role of lyn in Fibrosis. Front Physiol 2016; 7:387; PMID:27630579; https://doi.org/10.3389/fphys.2016.00387
- Han J, Zhang G, Welch EJ, Liang Y, Fu J, Vogel SM, Lowell CA, Du X, Cheresh DA, Malik AB, et al. A critical role for Lyn kinase in strengthening endothelial integrity and barrier function. Blood 2013; 122:4140-9; PMID:24108461; https://doi.org/10.1182/blood-2013-03-491423
- Hong H, Kitaura J, Xiao W, Horejsi V, Ra C, Lowell CA, Kawakami Y, Kawakami T. The Src family kinase Hck regulates mast cell activation by suppressing an inhibitory Src family kinase Lyn. Blood 2007; 110:2511-9; PMID:17513616; https://doi.org/10.1182/blood-2007-01-066092
- Obr A, Roselova P, Grebenova D, Kuzelova K. Real-time analysis of imatinib- and dasatinib-induced effects on chronic myelogenous leukemia cell interaction with fibronectin. PLoS One 2014; 9:e107367; PMID:25198091; https://doi.org/10.1371/journal.pone.0107367
- Michaelis S, Wegener J, Robelek R. Label-free monitoring of cell-based assays: combining impedance analysis with SPR for multiparametric cell profiling. Biosens Bioelectron 2013; 49:63-70; PMID:23711901; https://doi.org/10.1016/j.bios.2013.04.042
- Scielzo C, Bertilaccio MT, Simonetti G, Dagklis A, ten Hacken E, Fazi C, Muzio M, Caiolfa V, Kitamura D, Restuccia U, et al. HS1 has a central role in the trafficking and homing of leukemic B cells. Blood 2010; 116:3537-46; PMID:20530793; https://doi.org/10.1182/blood-2009-12-258814
- Wegener J, Keese CR, Giaever I. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp Cell Res 2000; 259:158-66; PMID:10942588; https://doi.org/10.1006/excr.2000.4919
- Obr A, Roselova P, Grebenova D, Kuzelova K. Real-time monitoring of hematopoietic cell interaction with fibronectin fragment: The effect of histone deacetylase inhibitors. Cell Adh Migr 2013; 7:275-82; PMID:23567296; https://doi.org/10.4161/cam.24531
- Prosper F, Verfaillie CM. Regulation of hematopoiesis through adhesion receptors. J Leukoc Biol 2001; 69:307-16; PMID:11261776
- Martin-Henao GA, Quiroga R, Sureda A, Gonzalez JR, Moreno V, Garcia J. L-selectin expression is low on CD34+ cells from patients with chronic myeloid leukemia and interferon-a up-regulates this expression. Haematologica 2000; 85:139-46
- Jin L, Tabe Y, Konoplev S, Xu Y, Leysath CE, Lu H, Kimura S, Ohsaka A, Rios MB, Calvert L, et al. CXCR4 up-regulation by imatinib induces chronic myelogenous leukemia (CML) cell migration to bone marrow stroma and promotes survival of quiescent CML cells. Molecular Cancer Therapeutics 2008; 7:48-58; PMID:18202009; https://doi.org/10.1158/1535-7163.MCT-07-0042
- Chen YY, Malik M, Tomkowicz BE, Collman RG, Ptasznik A. BCR-ABL1 alters SDF-1alpha-mediated adhesive responses through the beta2 integrin LFA-1 in leukemia cells. Blood 2008; 111:5182-6; PMID:18339898; https://doi.org/10.1182/blood-2007-10-117705
- Daubon T, Rochelle T, Bourmeyster N, Genot E. Invadopodia and rolling-type motility are specific features of highly invasive p190(bcr-abl) leukemic cells. Eur J Cell Biol 2012; 91:978-87; PMID:22717125; https://doi.org/10.1016/j.ejcb.2012.04.006
- Konig H, Holtz M, Modi H, Manley P, Holyoake TL, Forman SJ, Bhatia R. Enhanced BCR-ABL kinase inhibition does not result in increased inhibition of downstream signaling pathways or increased growth suppression in CML progenitors. Leukemia 2008; 22:748-55; PMID:18273048; https://doi.org/10.1038/sj.leu.2405086
- Weisberg E, Wright RD, McMillin DW, Mitsiades C, Ray A, Barrett R, Adamia S, Stone R, Galinsky I, Kung AL, et al. Stromal-mediated protection of tyrosine kinase inhibitor-treated BCR-ABL-expressing leukemia cells. Mol Cancer Ther 2008; 7:1121-9; PMID:18445657; https://doi.org/10.1158/1535-7163.MCT-07-2331
- Becker PS. Dependence of acute myeloid leukemia on adhesion within the bone marrow microenvironment. ScientificWorldJournal 2012; 2012:856467; PMID:22346731; https://doi.org/10.1100/2012/856467
- Brault L, Rovo A, Decker S, Dierks C, Tzankov A, Schwaller J. CXCR4-SERINE339 regulates cellular adhesion, retention and mobilization, and is a marker for poor prognosis in acute myeloid leukemia. Leukemia 2014; 28:566-76; PMID:23817178; https://doi.org/10.1038/leu.2013.201
- Hanke M, Hoffmann I, Christophis C, Schubert M, Hoang VT, Zepeda-Moreno A, Baran N, Eckstein V, Wuchter P, Rosenhahn A, et al. Differences between healthy hematopoietic progenitors and leukemia cells with respect to CD44 mediated rolling versus adherence behavior on hyaluronic acid coated surfaces. Biomaterials 2014; 35:1411-9; https://doi.org/10.1016/j.biomaterials.2013.11.011
- Gattazzo C, Martini V, Frezzato F, Trimarco V, Tibaldi E, Castelli M, Facco M, Zonta F, Brunati AM, Zambello R, et al. Cortactin, another player in the Lyn signaling pathway, is overexpressed and alternatively spliced in leukemic cells from patients with B-cell Chronic Lymphocytic Leukemia. Haematologica 2014; 99(6):1069-77; https://doi.org/10.3324/haematol.2013.090183
- Li S. Src-family kinases in the development and therapy of Philadelphia chromosome-positive chronic myeloid leukemia and acute lymphoblastic leukemia. Leuk Lymphoma 2008; 49:19-26; PMID:18203007; https://doi.org/10.1080/10428190701713689
- Hu Y, Liu Y, Pelletier S, Buchdunger E, Warmuth M, Fabbro D, Hallek M, Van Etten RA, Li S. Requirement of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia. Nat Genet 2004; 36:453-61; PMID:15098032; https://doi.org/10.1038/ng1343
- Dos Santos C, McDonald T, Ho YW, Liu H, Lin A, Forman SJ, Kuo YH, Bhatia R. The Src and c-Kit kinase inhibitor dasatinib enhances p53-mediated targeting of human acute myeloid leukemia stem cells by chemotherapeutic agents. Blood 2013; 122:1900-13; PMID:23896410; https://doi.org/10.1182/blood-2012-11-466425
- Gertz MA. New targets and treatments in multiple myeloma: Src family kinases as central regulators of disease progression. Leuk Lymphoma 2008; 49:2240-5; PMID:19052970; https://doi.org/10.1080/10428190802475311
- Veldurthy A, Patz M, Hagist S, Pallasch CP, Wendtner CM, Hallek M, Krause G. The kinase inhibitor dasatinib induces apoptosis in chronic lymphocytic leukemia cells in vitro with preference for a subgroup of patients with unmutated IgVH genes. Blood 2008; 112:1443-52; PMID:18550857; https://doi.org/10.1182/blood-2007-11-123984
- ten Hacken E, Scielzo C, Bertilaccio MT, Scarfò L, Apollonio B, Barbaglio F, Stamatopoulos K, Ponzoni M, Ghia P, Caligaris-Cappio F. Targeting the LYN/HS1 signaling axis in chronic lymphocytic leukemia. Blood 2013; 121:2264-73; PMID:23325840; https://doi.org/10.1182/blood-2012-09-457119
- Horton ER, Byron A, Askari JA, Ng DH, Millon-Fremillon A, Robertson J, Koper EJ, Paul NR, Warwood S, Knight D, et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat Cell Biol 2015; 17:1577-87; PMID:26479319; https://doi.org/10.1038/ncb3257
- Kaplan KB, Bibbins KB, Swedlow JR, Arnaud M, Morgan DO, Varmus HE. Association of the amino-terminal half of c-Src with focal adhesions alters their properties and is regulated by phosphorylation of tyrosine 527. EMBO J 1994; 13:4745-56; PMID:7525268
- Fincham VJ, Frame MC. The catalytic activity of Src is dispensable for translocation to focal adhesions but controls the turnover of these structures during cell motility. EMBO J 1998; 17:81-92; PMID:9427743; https://doi.org/10.1093/emboj/17.1.81
- Jarvinen M, Ylanne J, Vartio T, Virtanen I. Tumor promoter and fibronectin induce actin stress fibers and focal adhesion sites in spreading human erythroleukemia (HEL) cells. Eur J Cell Biol 1987; 44:238-46; PMID:3319626
- Ylanne J, Cheresh DA, Virtanen I. Localization of beta 1, beta 3, alpha 5, alpha v, and alpha IIb subunits of the integrin family in spreading human erythroleukemia cells. Blood 1990; 76:570-7; PMID:1696147
- O'Laughlin-Bunner B, Radosevic N, Taylor ML, Shivakrupa DeBerry C, Metcalfe DD, Zhou M, Lowell C, Linnekin D. Lyn is required for normal stem cell factor-induced proliferation and chemotaxis of primary hematopoietic cells. Blood 2001; 98:343-50; PMID:11435302; https://doi.org/10.1182/blood.V98.2.343
- Tsuji-Takayama K, Kamiya T, Nakamura S, Matsuo Y, Adachi T, Tsubota T, Imanishi J, Minowada J. Establishment of multiple leukemia cell lines with diverse myeloid and/or megakaryoblastoid characteristics from a single Ph1 positive chronic myelogenous leukemia blood sample. Hum Cell 1994; 7:167-71; PMID:7873501
- Kuzelova K, Pluskalova M, Brodska B, Otevrelova P, Elknerova K, Grebenova D, Hrkal Z. Suberoylanilide hydroxamic acid (SAHA) at subtoxic concentrations increases the adhesivity of human leukemic cells to fibronectin. J Cell Biochem 2010; 109:184-95; PMID:19911379; https://doi.org/10.1002/jcb.22397
