ABSTRACT
The unc-53/NAV2 gene encodes for an adaptor protein required for cell migrations along the anteroposterior (AP) axes of C. elegans. This study identifies unc-53 as a novel component of signaling pathways regulating Distal tip cell (DTC) migrations along the AP and dorsoventral (DV) axes. unc-53 negatively regulates and functions downstream of ced-10/Rac pathway genes; ced-10/Rac and mig-2/RhoG, which are required for proper DTC migration. Moreover, unc-53 exhibits genetic interaction with abl-1 and unc-5, the 2 known negative regulators of ced-10/Rac signaling. Our genetic analysis supports the model, where abl-1 negatively regulates unc-53 during DTC migrations and requirement of unc-53 function during both AP and DV DTC migrations could be due to unc-53 mediated regulation of unc-5 activity.
KEYWORDS:
Introduction
Cell migration is a crucial aspect of metazoan development, organ morphogenesis, nervous system patterning, immune response, and pathogenesis. Cells are steered by responding to extracellular cues through transmembrane receptors by initiating actin cytoskeleton modulation.Citation1 Genetic studies in Drosophila and C. elegans have shown that Rac and Rho subfamily GTPases transduce the signal received at the membrane via effector proteins to initiate actin cytoskeleton remodeling.Citation2,Citation3,Citation4,Citation5,Citation6 Activity of small GTPases is regulated by diverse set of proteins such as guanine nucleotide exchange factors (GEFs), GTPase activating factors (GAPs), and adaptor proteins.
unc-53/NAV2, an adaptor protein, is required during cell and growth cone migration primarily along the AP axes of C. elegans.Citation7,Citation8 Functional genomics, genetics, and biochemical approaches in C. elegans have identified several components of unc-53 mediated signaling including sem-5/GRB-2 (Growth factor receptor bound protein-2), an adaptor protein,Citation9 abi-1/ABI-1, Abelson interactor protein,Citation10 unc-73/Trio, a RhoGEF,Citation11 mig-10/Lpd, an adaptor protein,Citation12 and fft-2, the C. elegans homolog of 14–3–3ϵ.Citation13 Recent studies in Drosophila demonstrated that sickie, the fly homolog of unc-53 functions via a non-canonical Rac-cofilin pathway requiring Slingshot phosphatase (ssh) activity to regulate cofilin dependent axon outgrowth.Citation14 Interestingly, 14–3–3ϵ plays a key role in Slingshot phosphatase (Ssh) function.Citation15
C. elegans adult gonads are composed of 2 U-shaped arms generated by migration of Distal tips cells (DTCs) in a 3 phased migratory route along the AP and DV axes of the animals. Functional genomics and genetics approaches have identified several component of signaling pathways regulating DTC migrations,Citation16,Citation17 which regulate reorganization of the components of the extracellular matrix (ECM), basement membrane (BM), and the actin cytoskeleton.Citation18 Out of the 3 C. elegans rac GTPases, mig-2 and ced-10 exhibit DTC migration defects.Citation2,Citation19 The other components of ced-10/Rac pathway include unc-73/Trio, a GEF protein, ced-2/CrkII, an adaptor protein, ced-5/Dock180 and ced-12/ELMO, an atypical GEF.Citation20,Citation2,Citation21,Citation19 Cabello et al.Citation22 reported that ced-10/Rac pathway is activated by mom-5/Frizzled a Wnt receptor, whereas abl-1/Abl kinase and unc-5/UNC-5 receptor, function as negative regulators.Citation23,Citation24
This work reports the requirement of unc-53 gene function during DTC migration. unc-53(n152) and unc-53(e2432), the 2 null alleles display phase 2 and phase 3 DTC migration defects primarily comprising path-finding defects. Analysis of double mutants support unc-53 functions as a negative regulator of rac GTPases; ced-10 and mig-2. Our conclusion, that unc-53 might regulate unc-5 activity is based on 2 observations first unc-53 suppresses the DTC phase 3 AP polarity reversal defect of the ced-10 and mig-2 mutants and second unc-53(lf) mutants display DTC phase 3 AP polarity reversal defect, which is the hallmark phenotype of ced-10/ Rac signaling pathway genes. Moreover, unc-53 exhibits genetic interaction with abl-1/Abl Abelson Kinase and unc-5/UNC-5 receptor, which are the 2 known negative regulators of ced-10/Rac signaling during DTC migration.
Results
unc-53 gene activity is required during Distal tip cell migrations
In this study 2 null alleles; unc-53(n152) and unc-53(e2432) were analyzed for DTC migration defects.Citation9 DTCs are a pair of gonadal leader cells present at the distal extending edge of the 2 gonad arms and undergo a 3-phased migratory route post-embryonically to generate the adult gonad morphology (). DTC migration commences in the L2 stage and is completed in the L4 stage, generating the U-shaped gonads with 2 bilaterally symmetric arms; the anterior arm (AA) and the posterior arm (PA) (). Aberrant DTC migration changes the morphology of the adult gonads, which can be visualized using Differential Interference Contrast (DIC) microscopy. DTC migration defects can be broadly categorized into DTC movement defects where the DTCs fail to migrate and DTC path-finding defects comprising misdirected migrations. As a control, N2, wild type L4 stage animals were assessed for DTC migration defects, where 94% of the animals displayed proper U-shaped gonad morphology ( and ). Analysis of unc-53(n152) and unc-53(e2432) mutant animals displayed normal migration during phase 1, whereas phase 2 and 3 exhibited path-finding defects. The defects observed can be classified into DTC phase 3 AP polarity reversal defect (), a hallmark phenotype of ced-10/Rac signaling pathway genesCitation2 and DTC phase 2 path-finding defects (). Quantitative analysis supported the requirement of unc-53 activity during DTC migration as 15.3% of n152 and 15.5% of e2432 animals exhibited defects (). Both the unc-53 mutant alleles displayed marginally more defects in the posterior arm (5% in e2432 and 5% in n152) than the anterior arm (1% for e2432 and 0.3% for n152).
Figure 1. unc-53 gene activity is required for proper Distal tip cell migrations: (A) The graphic depicts the U-shaped morphology of the adult gonads and the three migratory phases of DTCs. Phase 1 commences during the L2 stage along the anteroposterior (AP) axes followed by Phase 2 in the L3 stage along the dorsoventral (DV) axes. Cessation of migration occurs in the L4 stage in the midbdoy region, where the two gonad arms migrate centripetally along the AP axes. Representative Differential Interference Contrast (DIC) micrograph of L4 stage animal, where midbody region () is on the left, with dorsal side up, displaying the normal U-shaped adult gonad morphology. (B) N2, wild type animal with proper U-shaped morphology of adult gonad posterior arm exhibiting the three migratory phases. (C) unc-53(n152) animal, exhibiting DTC phase 3 AP polarity reversal path-finding defect, where during phase 3 the posterior gonad arm migrates centrifugally. (D) unc-53(n152) animal, exhibiting DTC phase 2 path-finding defect. Scale bar: 50 μm.
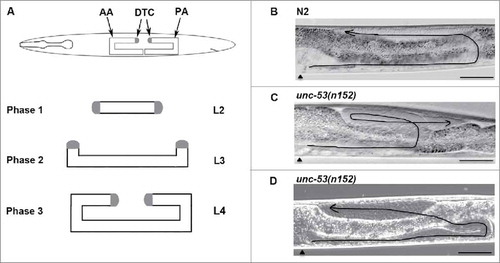
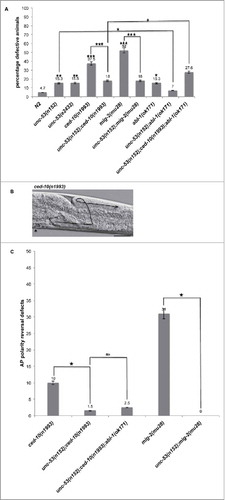
Figure 2. unc-53 Mutation suppresses DTC phase 3 AP polarity defects of ced-10 and mig-2. (A) L4 stage mutant animals were assessed for DTC migration defects using DIC microcopy as described in Materials and Methods. The bars represent percentage of animals with misshaped gonad arms. p values for single mutations are given on the outside end of the bars and were calculated by comparing with N2. p values for double mutants were calculated by pairwise comparison with single mutations. (B) Representative DIC micrograph of ced-10(n1993) L4 stage animals with anterior and midbody () on the left, dorsal side up, displaying DTC phase 3 AP polarity reversal defect in the posterior arm. Scale bar: 50 μm. (C) The graph shows the percentage of animals exhibiting DTC phase 3 AP polarity reversal defects. Statistical analysis used TTEST. *p < 0.05, **p < 0.005, and ***p < 0.0005.
unc-53 acts as a negative regulator of rac GTPases mediated signaling during DTC migration
To place unc-53 in the signaling pathways described previously to regulate DTC migration we investigated the genetic interaction with ced-10/Rac and mig-2/RhoG, which are known to be required for proper DTC migration.Citation2,Citation19 In this study ced-10(n1993) a partial loss-of-function allele, harboring a missense Val190 to Gly mutationCitation2 and mig-2(mu28) a null allele with a substitution from W60 to opalCitation25 were assessed for DTC migration defects and interaction with unc-53(lf) mutants.
DIC microscopy of the rac GTPases revealed DTC movement and path-finding defects. Quantitative analysis supported requirement of rac GTPase function for proper DTC migration as 37.5% of ced-10 and 52% of mig-2 animals exhibited misshapen gonads (). Out of the total migration defects observed 10% of ced-10 and 31% of mig-2 animals exhibited DTC phase 3 AP polarity reversal defect ( and ). We did not observed any bias for the requirement of ced-10(n1993) function between the anterior arm and the posterior arm. Whereas, for mig-2(mu28) animals the anterior arm (17%) exhibited less defects when compared with the posterior arm (44%). mig-2 and ced-10 have been previously shown to function redundantly during DTC migration or movement and in the same pathway to regulate DTC phase 3 AP polarity reversals.Citation19
To investigate the possibility of a genetic interaction between unc-53 and ced-10 in regulation of DTC migration, a double mutant was constructed. unc-53(n152);ced-10(n1993) animals displayed significant suppression of DTC migration defects with 18% of the animals exhibiting defects (). Notably, unc-53 mutation suppressed the DTC phase 3 AP polarity reversal defect observed in ced-10(n1993) animals as only 1.5% of the animals displayed the defect (). Unlike ced-10 mutant animals for the double mutant the defect observed in the posterior arm (14%) exceeded that of anterior arm (5%).
Similarly, unc-53(n152);mig-2(mu28) double mutant was generated to assess genetic interaction between unc-53 and mig-2 during DTC migration. DIC microscopy of L4 stage animals revealed suppression of mig-2 mediated DTC migration defects where only 18% of the animals exhibited defects (). unc-53 suppressed the DTC phase 3 AP polarity reversal defect observed in mig-2 mutant animals (). The percentage of DTC migration defects observed in the anterior arm (7%) were less than that observed in the posterior arm (13%).
abl-1 negatively regulates unc-53 mediated signaling during DTC migration
Previously, abl-1/Abl has been shown to act as a negative regulator of ced-10/Rac signaling pathway during DTC migration.Citation23 In this study abl-1(ok171) a null mutant allele,Citation26 harboring a deletion from exon 8 to exon 12 was used to investigate genetic interaction with unc-53 during DTC migration. 15.3% of abl-1(ok171) L4 stage animals exhibited DTC migration defects mainly comprising path-finding defects (). Whereas, unc-53(n152);abl-1(ok171) double mutant when analyzed for DTC migration defects exhibited significant suppression of DTC migration defects. These observations support that either of the gene could negatively regulate the other during DTC migration since both unc-53(n152) and abl-1(ok171) exhibited DTC migration defects. To examine whether unc-53 and abl-1 functions in the same pathway to regulate ced-10/Rac signaling, unc-53;ced-10;abl1 triple mutant was generated and assessed for DTC migration defects. The triple mutant exhibited enhancement in DTC migration defects including extension of both the arms on the same side of the animals (loss of symmetry), short gonad arms, and abnormal morphology (distended arms) (), however no significant enhancement was observed for the DTC phase 3 AP polarity reversal defect when compared with unc-53;ced-10 double mutant animals ().
unc-5 and unc-53 function in same signaling pathway to regulate DTC migration
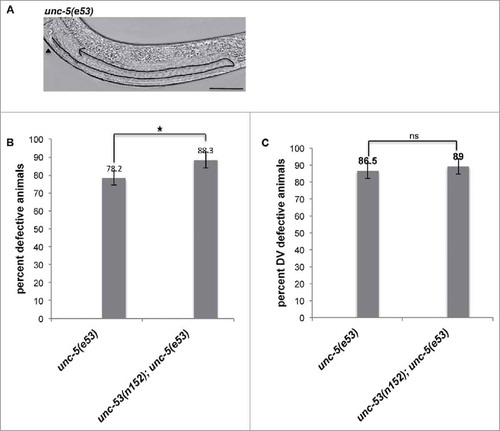
It has been shown that chemorepulsive activity of UNC-6 via its receptors UNC-5 and UNC-40 guide DTCs to complete the phase 2 migrations.Citation27,Citation28 More recently, it has been shown that unc-5 activity is inhibited by mom-5/frizzled and ced-10/Rac pathway during DTC migration and unc-5 negatively regulates the ced-10/Rac pathway genes.Citation24 Since unc-53(lf) mutations exhibited DTC phase 3 AP polarity reversal defect, we investigated possibility of a genetic interaction with unc-5. unc-5(e53) mutant allele, harboring a substitution to a stop codon (W28STOP) in the extracellular domain was assessed for DTC migration defects.Citation28 unc-53(e53) animals revealed DTC phase 2 DV defect (), where 78.2% of the animals exhibited DTC migration defects (). Of the total defective animals, 86.5% of animals displayed DTC phase 2 DV defect where the phase 3 was completed on the ventral muscle (). A bias was observed for the defects observed in posterior arm (65%) than for the anterior arm (29%). unc-53(n152);unc-5(e53) double mutant animals exhibited an enhancement in DTC migration defects (91% animals exhibited defects) (), though no significant enhancement in DTC phase 2 DV migration defects was observed over the unc-5(e53) single mutant animals (). For the double mutant the posterior arm (81%) exhibited more defects than the anterior arm (37%), a trend similar to unc-53(n152) and unc-5(e53) single mutant animals.
Figure 3. (A) Representative DIC micrograph of unc-5(e53) L4 stage animals with anterior and midbody () on the left, displaying DTC phase 2 DV defect. Scale bar: 50 μm. (B) L4 stage animals were assessed for DTC migration defects using DIC microcopy as described in Materials and Methods. The bars represent percentage of animals with misshaped gonad arms. (C) The graph shows the percentage of animals exhibiting DTC phase 2 DV defects. Statistical analysis used TTEST. *p < 0.05, **p < 0.005, and ***p < 0.0005.
Discussion
UNC-53 is an adaptor protein and C. elegans homolog of human Neuron navigator 2 (NAV2), harboring multiple domains, which enables the protein to bind to actin and complex with other proteins to regulate migrations of diverse cell types along the AP axes of C. elegans.Citation9,Citation8 This research work shows the requirement of unc-53 gene activity for proper migration of the DTCs, which are a pair of gonadal leader cells present at the extending end of the 2 gonad arms. The DTCs under go a 3-phased migratory route to generate the adult gonad morphology. The 2 mutant alleles: unc-53(n152) and unc-53(e2432) when assessed for DTC migration defects, exhibited DTC path-finding defects including DTC phase 3 AP polarity reversal defect, where the gonad arm take an extra turn and migrate centrifugally to complete migration and DTC phase 2 associated path-finding defects. Detection of DTC phase 2 defects, which occurs along the DV axes of the animal is an interesting observation and supports previous report, where unc-53 has been proposed to play a minor role in dorsal process outgrowth of DA and AS motoneurone.Citation9 Observation of defects associated with both phase 2 and phase 3 DTC migration implicates that unc-53 gene activity might be either required in more than one signaling pathway or regulating a common signaling component, which is required for both phase 2 and phase 3 DTC path finding and migrations.
The DTC phase 3 AP polarity reversal defect observed in unc-53(lf) mutant animals is reminiscent of the defects observed in the ced-10/Rac signaling pathway genes such as ced-2/CrkII, ced-5/Dock180, ced-12/Elmo, unc-73/Trio, ced-10/Rac, mig-2/RhoG, and mom-5/Frizzled.Citation2,Citation19 Also, it has been previously shown that gonadal expression of UNC-5 causes AP polarity reversal defect and removal of unc-5 activity suppresses mom-5, ced-12, ced-10, and mig-2 associated DTC phase 3 AP polarity reversal defect.Citation22,Citation24 Taken together, these findings implicated ced-10/Rac signaling pathway in inhibiting unc-5 activity during phase 3 for proper back to midbody DTC migration. To test the possibility of existence of a genetic interaction between unc-53 and signaling components required during DTC migration, double mutants were generated and analyzed for DTC migration and path-finding defects.
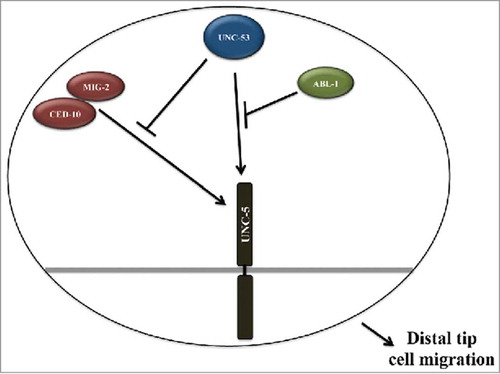
The 2 C. elegans rac GTPases, ced-10 and mig-2, previously shown to regulate DTC migrations, exhibited DTC Phase 3 AP polarity reversal and migration defects. ced-10 and mig-2 have been shown to act redundantly to regulate migration but act in the same pathway to regulate path-finding. Assessment of DTC migration defects in unc-53;ced-10 and unc-53;mig-2 double mutant animals revealed partial suppression of the DTC migration defects, particularly the DTC phase 3 AP polarity reversal defect. These results support unc-53 functioning as a negative regulator of ced-10 and mig-2 signaling. Our analysis suggest that unc-53 functions downstream of mig-2 since unc-53(lf) mediated suppression did not required functional mig-2 allele. Similar conclusion cannot be drawn for ced-10 since the ced-10(n1993) is not a null allele, although the possibility that unc-53 might function in conjunction with ced-10 to regulate DTC migration cannot be excluded. These results support a model where UNC-53 negatively regulates a downstream component of CED-10/ Rac signaling pathway, which is activated by CED-10/Rac, hence when both UNC-53 and Rac GTPase function are removed the negative regulation is relieved resulting in proper DTC migration (). Since unc-53 suppresses the DTC phase 3 AP reversals this negative regulation might be by regulating unc-5 activity, however this regulation of unc-5 activity occurs via a downstream signaling component of ced-10/Rac pathway and not by direct inhibition of unc-5 activity because if unc-53 was acting independent of rac GTPase pathway there would be an increase in DTC phase 3 AP polarity reversals due to increased unc-5 activity, as 2 pathways will be simultaneously compromised, the rac GTPase and the unc-53 pathways. Our conclusion is further supported by a recent report in Drosophila, where Sickie, a fly homolog of UNC-53, functions downstream of Rac GTPase via Slingshot phosphatase in a Pak-independent non-canonical pathway to activate coffilin dependent axon growth.Citation14 However, recent studies have shown that the RhoGEF specific isoform and not the RacGEF isoform of unc-73/Trio is associated with unc-53 in excretory canal extension.Citation11 unc-73/Trio function has also been shown to be required during DTC migrations exhibiting DTC phase 3 AP polarity reversals activating the Rac GTPases. Previuosly, unc-73 was shown to regulate the 3 GTPases; mig-2, ced-10, and rho-1 during P cell migration.Citation29,Citation30 It is possible that during DTC migration unc-53 might act via the both RhoGEF and RacGEF specific isoforms of unc-73 or preferentially with RhoGEF isoform. Currently, we are performing experiments to identify other components of unc-53 mediated signaling during DTC migrations.
Figure 4. Molecular model depicting UNC-53 mediated regulation of UNC-5 in Rac GTPase dependent and independent pathways required for proper DTC migration: The genetic data supports UNC-53 functioning in Rac GTPase dependent and independent pathways for proper DTC migration by regulating the activity of UNC-5 receptor. We propose that UNC-53 primarily regulate UNC-5 activity by 2 pathways, first by negatively regulating CED-10 and MIG-2 mediated signaling and second pathway includes ABL-1 and most probably ABI-1.
Observation of low penetrance DTC phase 3 AP reversals in unc-53(lf) mutants indicates the requirement of unc-53 activity in regulating unc-5 activity in a rac GTPase independent manner. This regulation is most probably by inhibiting unc-5 activity because when unc-53 function is removed the unc-5 activity increases resulting in DTC phase 3 AP reversals. unc-5/UNC-5 and unc-40/DCC genes are known to regulate DTC phase 2 migrations occurring along DV axes in response to unc-6/Netrin guidance cue. Loss of unc-5 function causes failure of DTC phase 2 DV migrations,Citation28 whereas overexpression of UNC-5 in gonads results in precocious DTC phase 2 DV migration and DTC phase 3 AP reversals.Citation24 Since both the mutant alleles of unc-53 exhibit DTC phase 3 AP reversals, which from previous studies have been associated to increased unc-5 activity we examined the possibility of genetic interaction between unc-53 and unc-5. We propose that unc-53 and unc-5 might function in the same pathway, where unc-53 regulates unc-5 activity based on the following observations. Firstly, assessment of DTC migration defects in unc-53;unc-5 mutant animals revealed no enhancement in the DTC phase 2 DV defects when compared with unc-5 single mutant animals, placing unc-53 and unc-5 in the same genetic pathway to regulate DTC migrations, in particular phase 2 DV migrations. Secondly, the double mutant did not exhibited DTC phase 3 AP reversals, which were observed in unc-53(lf) animals, supporting unc-53 might inhibit unc-5 activity. Although an overall enhancement in DTC migration defects was observed, which can be explained by assuming that unc-53 and unc-5 might regulate certain other aspects of DTC migration or regulation of unc-40 activity by unc-53. The model for UNC-53 mediated regulation of UNC-5 is further supported by previous reports, where UNC-53 has been implicated in receptor trafficking supported by 2 independent studies where unc-53(lf) mutant display defects in receptor uptake including the coelomocyte uptake (CUP) assay and receptor-mediated endocytosis assays (7, Pandey and Garriga, unpublished results). This model is also supported by previous studies in which unc-53 and unc-6 double mutant animals exhibited ventral process outgrowth defects in AVM and HSN neurons. It was shown that this was caused by mislocalization of UNC-40:GFP. These studies showed a genetic model where unc-53 and unc-5 act in parallel pathways to inhibit unc-40 activity by affecting its distribution.Citation31 In our study we propose that unc-53 might inhibit unc-5 activity for proper phase 3 and phase 2 DTC migrations. Analysis of unc-53;unc-40 double mutant is being performed to furher support our hypothesis.
abl-1/ABL1, Abelson Kinase besides unc-5/UNC-5 is the other known negative regulator of ced-10/Rac signaling pathway. It has been shown that abl-1(lf) partially restores normal DTC migration in the ced-10/Rac pathway gene mutants.Citation23 The same study also proposed that abl-1 functions by inhibiting abi-1/ABI-1, an Abelson Interactor protein, where ABI-1 is required for proper DTC migration and has been previously shown to interact with actin modulatory proteins such as N-WASP, Scar/WAVE, and Ena/Mena.Citation32,Citation33,Citation34 In C. elegans, unc-53 and abi-1 have been shown to interact genetically and physically to regulate excretory canal extension.Citation10 Analysis of unc-53;abl-1 double mutant animals exhibited suppression of DTC migration defects. Since unc-53 and abl-1 mutant animals exhibit DTC migration defects, it is possible that either of the genes can inhibit the others function. We favor the conclusion that abl-1 might function as a negative regulator of unc-53 mediated DTC migration. Furthermore, this negative regulation might be through abi-1.Citation23 Introduction of abl-1 mutation in unc-53;ced-10 double mutant animals resulted in enhancement of DTC migration defects, although the path-finding DTC phase 3 AP polarity reversals did not exceeded to that observed in unc-53;ced-10 double mutant animals. These results suggest that unc-53 and abl-1 might function redundantly to regulate DTC migration but act in the same genetic pathway to regulate DTC path finding.
In summary, our data presents unc-53 as a novel regulator of ced-10/Rac signaling pathway during DTC migration. unc-53 regulates DTC path-finding by regulating unc-5 activity in a rac GTPase dependent and independent manner. UNC-53 might be regulating receptor levels by regulating rac GTPase function in a manner similar to VAB-8 and CRML-1, which regulate UNC-40 and SAX-3 levels at the membrane.Citation35,Citation36,Citation37 Additionally, we also propose that the unc-53 and abl-1 act in the same pathway to negatively regulate DTC path-finding and abl-1 functions a negative regulator of unc-53 in the rac GTPase independent pathway, which might occur via abi-1.
Materials and methods
Nematode strains
All nematode strains were cultured on nematode growth medium (NGM), fed OP50 (mutant of E. coli B and a uracil auxotroph), and maintained at 20°C as described.Citation38 The N2 wild type strain is Bristol and the other mutations used for this study include: LG II: unc-53(n152), LG II: unc-53(e2432), LG IV: ced-10(n1993), LGIV unc-5(e53), LG X: mig-2(mu28), LGX abl-1(ok171). Genotyping of the mutations containing a single base pair change was performed using polymerase chain reaction (PCR) using allele specific primers harboring a single base pair mismatch at the third last base at the 3′-end of the primers.Citation39 Genotyping of the deletion mutations was performed with primers flanking the deleted region. A list of the primers is provided in Supplementary Table 1.
Qualitative and quantitative analysis of DTC migration defects
Distal tip cells (DTCs) are present at the leading edge of the 2 U-shaped gonad arm (anterior arm and posterior arm) and the migratory route followed by the DTCs determine the final morphology of the adult gonads (). For qualitative analysis of DTC migration defects, L4-stage animals were anesthetized in M9 containing sodium azide (0.05%–0.1%) and visualized using Differential Interference Contrast (DIC) microscopy as described.Citation17 Olympus U-RFL-T fluorescent microscope was used for visualizing animals and imaged using ProgRes® Capture Pro 2.7.7 software. A total of 156 animals of each genotype for which both the gonad arms were completely visualized were scored for the DTC migration defects. Defective DTC migration was determined based on any change in the morphology of the gonads including gonad arms exhibiting extra turns, DTCs with abnormal trajectories, length of the gonad arms, and arms with abnormal morphologies. The animals were observed at 100X, 200X, and 400X magnification to confirm DTC migration defects. For comparative quantitative analysis, p-values for pairwise comparisons were calculated by TTEST (tails 2 and type 3).
Strains generated for this investigation are available upon request.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
CGC: Caenorhabditis Genetics Center for providing all mutant and the N2 wild type C. elegans strains, which is funded by NIH Office of Research Infrastructure Programs (P40OD010440).
DPMB: Department of Plant Molecular Biology, University of Delhi, for providing space and infrastructure to execute research work.
Supplemental_table.docx
Download MS Word (52.1 KB)Funding
All the authors in this manuscript extend thanks to the following organizations and people: Department of Biotechnology (DBT) Bio-CARe scheme for women scientists, for providing financial support for the research work for the project entitled, “Elucidation of mechanistic action of UNC-53 in cell migration and axon outgrowth in C. elegans and plant.” Sanction Order No. 102/IFD/SAN/2843/2014–2015.
References
- Bashaw GJ, Klein R. Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol 2010; 2:a001941; PMID:20452961; https://doi.org/10.1101/cshperspect.a001941
- Reddien PW, Horvitz HR. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat. Cell Biol 2000; 2:131-6; PMID:10707082; https://doi.org/10.1038/35004000
- Dickson BJ. Rho GTPases in growth cone guidance. Curr Opin Neurobiol 2001; 11(1):103-10; PMID:11179879; https://doi.org/10.1016/S0959-4388(00)00180-X
- Lundquist EA. Rac proteins and control of axon development. Curr Opin Neurobiol 2003; 13:384-90; PMID:12850224; https://doi.org/10.1016/S0959-4388(03)00071-0
- Bouquet C, Nothias F. Molecular mechanisms of axonal growth. Adv Exp Med Biol 2007; 621:1-16; PMID:18269207
- Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol 2010; 2:a001818; PMID:20182621; https://doi.org/10.1101/cshperspect.a001818
- Stringham E, Schmidt KL. Navigating the cell UNC-53 and the navigators, a family of cytoskeletal regulators with multiple roles in cell migration, outgrowth and trafficking. Cell Adhes Migr 2009; 3(4):342-6; https://doi.org/10.4161/cam.3.4.9451
- Pandey A, Pandey GK. The UNC-53 mediated interactome: Analysis of its role in generation of C. elegans connectome. SpringerBriefs Neuroscience 2014
- Stringham E, Pujol N, Vanderchove J, Bogaert T. unc-53 controls longitudinal migration in C. elegans. Development 2002; 129:3367-79; PMID:12091307
- Schmidt KL, Marcus-Gueret N, Adeleye A, Webber J, Baillie D, Stringham EG. The cell migration molecule UNC-53/NAV2 is linked to the ARP2/3 complex by ABI-1. Development 2009; 136:563-74; PMID:19168673; https://doi.org/10.1242/dev.016816
- Marcus-Gueret N, Schmidt KL, Stringham EG. Distinct cell guidance pathways controlled by the Rac and Rho GEF domains of UNC-73/TRIO in Caenorhabditis elegans. Genetics 2012; 190:129-42; PMID:21996675; https://doi.org/10.1534/genetics.111.134429
- McShea MA, Schmidt KL, Dubuke ML, Baldiga CE, Sullender ME, Reis AL, Zhang S, O'Toole SM, Jeffers MC, Warden RM, et al. Abelson interactor-1 (ABI-1) interacts with MRL adaptor protein MIG-10 and is required in guided cell migrations and process outgrowth in C. elegans. Dev Biol 2013; 373(1):1-13; PMID:23022657; https://doi.org/10.1016/j.ydbio.2012.09.017
- Marzinke MA, Mavencamp T, Duratinsky J, Clagett-Dame M. 14-3-3ϵ and NAV2 interact to regulate neurite outgrowth and axon elongation. Arch Biochem Biophys 2013; 540(1-2):94-100; PMID:24161943; https://doi.org/10.1016/j.abb.2013.10.012
- Abe T, Yamazaki D, Murakami S, Hiroi M, Yohei Nitta, Yuko Maeyama, Tabata T. The NAV2 homolog Sickie regulates F-actin-mediated axonal growth in Drosophila mushroom body neurons via the noncanonical Rac-Cofilin pathway. Development 2014; 141:4716-28; PMID:25411210; https://doi.org/10.1242/dev.113308
- Kligys K, Yao J, Yu D, Jones JCR. 14-3-3zeta/tau heterodimers regulate Slingshot activity in migrating keratinocytes. Biochem Biophys Res Commun 2009; 383:450-4; PMID:19371722; https://doi.org/10.1016/j.bbrc.2009.04.031
- Cram EJ, Shang H, Schwarzbauer JE. A systematic RNA interference screen reveals a cell migration gene network in C. elegans. J Cell Sci 2006; 119:4811-8; PMID:17090602; https://doi.org/10.1242/jcs.03274
- Nishiwaki K. Mutations affecting symmetrical migration of Distal tip cells in Caenorhabditis elegans. Genetics 1999; 152:985-97; PMID:10388818
- Wong M, Schwarzbauer JE. Gonad morphogenesis and Distal tip cell migration in the Caenorhabditis elegans hermaphrodite. Wiley Interdiscip Rev Dev Biol 2012; 1:519-31
- Lundquist EA, Reddien PW, Hartweig E, Horvitz HR, Bargmann CI. Three C. elegans Rac proteins and several Rac regulators control axon guidance, cell migration and apoptotic cell phagocytosis. Development 2001; 128:4475-88; PMID:23559979; https://doi.org/10.1002/wdev.45
- Wu YC, Horvitz HR. C. elegans phagocytosis and cell migration protein CED-5 is similar to human DOCK180. Nature 1998; 392:501-4; PMID:9548255; https://doi.org/10.1038/32195
- Gumienny TL, Brugnera E, Tosello-Trampnt AC, Kinchen JM, Haney LB, Nishiwaki K, Walk SF, Nemergut ME, Macara IG, Francis R, et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, required for phagocytosis and cell migration. Cell 2001; 107:27-41; PMID:11595183; https://doi.org/10.1016/S0092-8674(01)00520-7
- Cabello J, Neukomm LJ, Gunesdogan U, Burkart K, Charetle SJ, Lochnit G, Hengartner MO, Schnabel R. The Wnt pathway controls cell death engulfment, spindle orientation, and migration through CED-10/Rac. Plos Biol 2010; 8(2):e1000297; PMID:20126385; https://doi.org/10.1371/journal.pbio.1000297
- Hurwitz ME, Vanderzalm PJ, Bloom L, Goldman J, Garriga G, Horvitz HR. Abl kinase inhibits the engulfment of apoptotic [corrected] cells in Caenorhabditis elegans. PLoS Biol 2009; 7:e99; PMID:19402756; https://doi.org/10.1371/annotation/2259f958-a68e-4e57-92b5-2ef003070cf1
- Levy-Strumpf N, Meghan K, Zheng H, Brown L, Culotti JG. The Wnt frizzled receptor MOM-5 regulates the UNC-5 Netrin receptor through small GTPase-dependent signaling to determine the polarity of migrating cells. PloS Genet 2015; 11(8):e1005446; PMID:26292279; https://doi.org/10.1371/journal.pgen.1005446
- Zipkin ID, Kindt RM, Kenyon CJ. Role of new Rho family member in cell migration and axon guidance in C. elegans. Cell 1997; 90:883-94; PMID:9298900; https://doi.org/10.1016/S0092-8674(00)80353-0
- Deng X, Hofmann ER, Villanueva A, Hobert O, Capodieci P, Veach DR, Yin X, Campodonico L, Glekas A, Cordon-Cardo C, et al. Caenorhabditis elegans ABL-1 antagonizes p53-mediated germline apoptosis after ionizing irradiation. Nat Genet 2004; 36:906-12; PMID:15273685; https://doi.org/10.1038/ng1396
- Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis of C. elegans. Neuron 1990; 4(1):61-85; PMID:2310575; https://doi.org/10.1016/0896-6273(90)90444-K
- Su M, Merz DC, Killeen MT, Zhou Y, Zheng H, Kramer JM, Hedgecock EM, Culotti JG. Regulation of the UNC-5 netrin receptor initiates the first reorientation of migrating Distal tip cells in Caenorhabditis elegans. Development 2000; 127:585-94; PMID:10631179
- Spencer AG, Orita S, Malone CJ, Han M. A RHO GTPase-mediated pathway is required during P cell migration in Caenorhabditis elegans. Proc Natl Acad Sci USA 2001; 98:13132-7; PMID:11687661; https://doi.org/10.1073/pnas.241504098
- Steven R, Kubiseski TJ, Zheng H, Kulkarni S, Mancillas J, Ruiz Morales A, Hogue CW, Pawson T, Culotti J. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell 1998; 92:785-95; PMID:9529254; https://doi.org/10.1016/S0092-8674(00)81406-3
- Kulkarni G, Xu Z, Mohamed AM, Li H, Tang X, Limerick G, Wadsworth WG. Experimental evidence for UNC-6 (Netrin) axon outgrowth by stochastic fluctuation of intracellular UNC-40 (DCC) outgrowth activity. Biol Open 2013; 2(12):1300-12; PMID:24337114; https://doi.org/10.1242/bio.20136346
- Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Krischner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature 2002; 418:790-3; PMID:12181570; https://doi.org/10.1038/nature00859
- Tani K, Sato S, Sukezane T, Kojima H, Hirose H, Hanafusa H, Shishido T. Abl interactor 1 promotes tyrosine 296 phosphorylation of mammalian enabled (Mena) by c-Abl kinase. J Biol Chem 2003; 278:21685-92; PMID:12672821; https://doi.org/10.1074/jbc.M301447200
- Innocenti M, Gerboth S, Rottner K, Lai F, Hertzog M, Stradal TE, Frittoli E, Didry D, Polo S, Disanza A, et al. Abi1 regulates the activity of N-WASP and WAVE in distinct actin-based processes. Nat Cell Biol 2005; 7:969-76; PMID:16155590; https://doi.org/10.1038/ncb1304
- Levy-Strumpf N, Culotti JG. VAB-8, UNC-73 and MIG-2 regulate axon polarity and cell migration functions of UNC-40 in C. elegans. Nat Neurosci 2007; 10:161-8; PMID:17237777; https://doi.org/10.1038/nn1835
- Watari-Goshima N, Ogura K, Wolf FW, Goshima Y, Garriga G. C. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat Neurosci 2007; 10:169-76; PMID:17237778; https://doi.org/10.1038/nn1834
- Vanderzalm PJ, Pandey A, Hurwitz ME, Bloom L, Horvitz HR, Garriga G. C. elegans CARMIL negatively regulates UNC-73/Trio function during neuronal development. Development 2009; 136:1201-10; PMID:19244282; https://doi.org/10.1242/dev.026666
- Brenner S. The genetics of Caenorhabditis elegans. Genetics 1974; 77(1):71-94; PMID:4366476
- Ahmadian A, Gharizadeh B, O'Meara D, Odeberg J, Lundeberg J. Genotyping by apyrase-mediated allele-specific extension. Nucl Acids Res 2001; 29:e12; https://doi.org/10.1093/nar/29.24.e121
