ABSTRACT
Aberrant activation of hepatocyte growth factor/scatter factor (HGF/SF) and its receptor, Met, is involved in the development and progression of many human cancers. In the screening assay of extracts from the root tuber of Tetrastigma hemsleyanum Diels et Gilg, isoquercitrin inhibited HGF/SF-Met signaling as indicated by its inhibitory activity on HGF/SF-induced cell scattering. Further analysis revealed that isoquercitrin specifically inhibited HGF/SF-induced tyrosine phosphorylation of Met. We also found that isoquercitrin decreased HGF-induced migration and invasion by parental or HGF/SF-transfected bladder carcinoma cell line NBT-II cells. Furthermore, isoquercitrin inhibited HGF/SF-induced epithelial mesenchymal transition in vitro and the invasion/metastasis of HGF/SF-transfected NBT-II cells in vivo. Our data suggest the possible use of isoquercitrin in human cancers associated with dysregulated HGF/SF-Met signaling.
Introduction
The c-met protooncogene encodes a transmembrane glycoprotein, Met tyrosine kinase receptor [Citation1,Citation2], and the ligand for Met is hepatocyte growth factor, also known as scatter factor (HGF/SF) [Citation3]. Both Met and HGF/SF are expressed in various tissues and signaling via this receptor-ligand pair affects a variety of biological activities, including cell growth [Citation4], cellular motility [Citation5], angiogenesis [Citation6], and morphogenesis [Citation7].
In addition, aberrant signaling driven by inappropriate activation of Met is frequently observed in human cancers and has been suggested to play a critical role in human tumorigenesis and metastasis [Citation8]. Most cases of Met activation in cancer have been reported to occur through ligand dependent autocrine or paracrine mechanisms. For instance, osteosarcomas and glioblastoma multiforme express both Met and HGF/SF, which results in Met activation in an autocrine manner. In many types of carcinoma, such as breast, gastric and colorectal cancers, overexpression of Met or HGF/SF predominates, resulting in the activation of HGF/SF-Met signaling in a paracrine manner [Citation8,Citation9]. Recently, the finding of activating mutants of Met in several human cancers such as renal papillary cancer [Citation10], early-onset hepatoma [Citation2] and gastric adenocarcinoma [Citation11] provides clear evidence implicating a causative role for Met in human carcinogenesis. Furthermore, HGF/SF-Met signaling has been reported to participate in tumor progression [Citation12].
Therefore, molecules that inhibit Met and/or HGF/SF may interfere with molecular causes of cancer formation and/or progression [Citation13]. In this regard, many efforts have been directed toward the development of inhibitors of HGF/SF and Met [Citation13]. Plant extracts are promising starting materials in the search for effective molecules. For example, an extract of bitter melon (Momordica charantia L.) pulp markedly induced HGF production and cell proliferation of human dermal fibroblasts by the activation of mitogen-activated protein kinases (MAPKs) signaling [Citation14]. The extract of traditional Chinese medicine YangZheng XiaoJi (YZXJ) has a significant role in reducing the migration, invasion and in vivo tumour growth of lung cancer and acts to inhibit the migratory and invasive effects induced by HGF [Citation15]. However, whether the extracts of Tetrastigma hemsleyanum Diels et Gilg (T.hemsleyanum) participate in HGF/SF signaling is still unclear.
T.hemsleyanum is a folk and rare medicinal plant and mainly distributed in south of China. The root tuber of T.hemsleyanum is commonly used as herb medicine for heat-clearing, toxicity–removing, dyspnea-relieving, promoting blood circulation and pain relief. More research found that T. hemsleyanum had significantly curative effects in terms of anti-tumor and immunomodulatory activities. The root tuber of T. hemsleyanum exhibited cytotoxic effects, triggered both extrinsic and intrinsic apoptotic pathways, and augmented oxidative stress in cervical carcinoma HeLa cells [Citation16]. The ethyl acetate extract from T. hemsleyanum evoked S phase arrest through the downregulation of cyclin A-CDK1 complex in human hepatoma HepG2 cells [Citation17]. Total flavonoids from T. hemsleyanum significantly inhibited tumor growth in C57BL/6 mice inoculated with Lewis lung carcinoma (LLC) cells [Citation18]. Moreover, several flavonoid ingredients isolated from the root tuber of T. hemsleyanum were reported to be associated with regulating epithelial mesenchymal transition (EMT) which had more recently been implicated as a driving force promoting the local invasion and distant dissemination of carcinoma [Citation19–Citation21]. Jo E et al found that kaempferol suppressed transforming growth factor β1 induced EMT and migration of A549 lung cancer cells by inhibiting akt1-mediated phosphorylation of smad3 at threonine-179 [Citation22]. Bhat FA et al reported that quercetin reversed epidermal growth factor (EGF) induced EMT and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway [Citation23]. Apigenin could inhibit migration and invasion via modulation of EMT in prostate cancer [Citation24].
In the present study, we demonstrate that isoquercitrin, extract from the root tuber of T. hemsleyanum, inhibits HGF/SF-Met signaling by decreasing the amount of tyrosine phosphorylation. This inhibition decreases HGF-induced migration and invasion both in vitro and in vivo, suggesting the possible use of isoquercitrin in human cancers with dysregulated HGF/SF-Met signaling.
Materials and methods
Extraction and isolation of T. hemsleyanum
The wild T.hemsleyanum were collected from Lishui City in Zhejiang province of China and identified by Prof. Ye Wang (College of Ecology of Lishui University, Zhejiang, China). The root tubers of T.hemsleyanum (5 kg) were dried and ground into fine powder, and extracted with ethanol under ultrasoniation (3 × 3 h) to yield a ethanol extract (207 g). The ethanol extract was suspended in water and partitioned with n-hexane (17.0 g), ethyl acetate (123.5 g), and n-butanol (19.1 g). The ethyl-acetate fraction was subjected to silica-gel (Merck) column chromatography with a step gradient elution of chloroform-methanol (20:1 to 1:1, v/v) to obtain four subfractions (fractions E1–E4). Fraction E3 (25 g) was further subjected to silica-gel column chromatography using an chloroform-methanol mixture (70:30 v/v) to give quercetin(32.1 mg), kaempferol(25.4 mg) and apigenin(21.7 mg). Fraction E4 (16 g) was further subjected to silica-gel column chromatography with chloroform-methanol system (60:40 v/v) to yield isoquercitrin(8.2mg), rutin(10.5 mg) and kaempferol-3-rutinoside(6.5 mg).
Cell lines
NBT-II cells were obtained from the American Type Culture Collection (ATCC) and cultured in DMEM (Gibco-BRL, Rockville, MD) supplemented with 10% (V/V) fetal bovine serum (FBS) (Gibco-BRL). The cells appeared to produce HGF when tested by scattering assay (data not shown).
Reagents, antibodies, constructs and transfection
Mouse anti-rat Met antibody was purchased from Santa Cruz (Santa Cruz, CA). Mouse anti-phosphotyrosine antibody was purchased from Invitrogen (Carlsbad, CA).
Recombinant human HGF was purchased from R&D systems (Minneapolis, MN). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was purchased from Duchefa (Haarlem,The Netherlands). Transfection of HGF was performed by using lipofectamine (Gibco-BRL). The transfected cells were cultured in DMEM/10% FBS supplemented with 800 μg/ml G-418 (Gibco-BRL) for two weeks. The clones were picked up and the expression of transfected HGF cDNAs was verified by RT-PCR. The positive clone was expanded and named as C2 cells.
Cell proliferation assay
The effect of isoquercitrin on NBT-II cell proliferation under stimulation with or without HGF was determined by MTT assay. In brief, cells growing in log-phase were trypsinized and seeded at 5000 cells per well into 96-well plates and allowed to attach overnight. Medium in each well was replaced with fresh medium. Various concentrations of isoquercitrin were added to the medium 2 h before the addition of HGF/SF (100 units/ml) in at least triplicate wells. Cells were cultured to another 24 h. After treatment, 1/10 volume of MTT solution (5 mg/ml) was added to each well, and the plate was incubated at 37°C for another 4 h. Two hundred microliters of DMSO was added to each well to solubilize the MTT-formazan product after removal of the medium. Absorbance at 595 nm was measured with a multi-well spectrophotometer (Thermo).
Immunoprecipitation
Monolayers of cells were washed twice with ice-cold PBS, lysed in ice-cold RIPA buffer [10 mM sodium phosphate (pH7.2), 150 mM NaCl, 1% (V/V) Nonidet P-40, 0.1% (W/V) SDS supplemented with 10mM sodium fluoride, 5 mM sodium orthovanadate] with complete protease inhibitor cocktail (Boehringer Mannheim, Germany), and centrifuged (15 min, 4oC, 14,000 g). After quantitation by using BCA protein assay reagent (Pierce, Rockford, IL), 400 μg of each lysate was pre-cleared with protein A/G-Sepharose and then incubated with anti-Met antibody for overnight at 4oC with rotation. The samples were then washed three times with ice-cold RIPA buffer. SDS gel-loading buffer (containing reducing agent) was added to each sample. After boiling and centrifugation, the resulting supernatants were resolved by SDS/PAGE and examined by Western blotting.
Pull down assay
Rac1-GTP pull down assay was carried out according to the manual (Pierce Biotechnology). Briefly, HGF induced NBT-II cells, HGF and isoquercitrin treated NBT-II cells and their control cells were washed with PBS at 4°C, harvested into 500 ml lysis buffer (200 mM NaCl, 50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 1% Triton X-100, 0.1% SDS, 0.5% deoxycholate, 5% glycerol, 1 mM phenylmethylsulfonyl fluoride, 9 nM pepstatin, 9 nM antipain, 10 nM leupeptin, and 10 nM chymostatin). Cell extracts were incubated with 30 mg of GST-GGA3 or GST immobilized on glutathione-Sepharose for 1 hour at 4°C. The pellets were washed three times with lysis buffer. Bound proteins were eluted by 30 ml elution buffer. The reactions were analysed by immunoblotting with a Rac1-specific monoclonal antibody.
Scratch assay
NBT-II cells were seeded in a 24-well plate in complete growth medium. After 24 h, cells were scratched using a micropipette tip and isoquercitrin was added at the indicated wells. Two hours later, HGF was treated to indicated wells. After additional incubation for 24 h in growth medium without fatal bovine serun (FBS), wells were observed under the light microscope. The distance was calculated by subtracting the average gap width after 24 h from the average gap width at 0 h divided by 2. The migratory ability was quanfified and normalized to NBT-II cells without HGF or isoquercitrin.
Invasion assay
Cell invasion assays were performed using 24-well transwell units with 8-mm polycarbonate filters (Costar). Briefly, 2 × 104 cells (in 100 ml DMEM+1% BSA with or without isoquercitrin) were plated onto the upper surface of the filter previously coated with Matrigel at a concentration of 20 mg/filter (BD Biosciences). The filter was then lowered into the lower compartment containing DMEM+1% BSA with or without HGF/SF (100 units/ml) with the indicated concentration of isoquercitrin. After 48 h of incubation at 37oC/5% CO2, cells were fixed in methanol and stained with Diff-Quick staining solution (Dade, Aguada, Puerto Rico). Non-migratory cells on the upper filter surface were removed using a cotton swab, and the total number of cells on each filter was counted at × 20 magnification using a phase-contrast microscope accommodated with an ocular grid.
In vivo metastasis assay
NBT-II cells or C2 cells (NBT-II cells expressing HGF) were labeled with luciferase tag and orthotopically injected into the bladder of 8-week-old syngenic nude mice by catheter. The mice were divided into two groups for 5 mice/group and injected intraperitoneally 6 times a week for 2 weeks with isoquercitrin (50 mg/kg) or saline, respectively. Four weeks after inoculation, the metastases of mice were detected under Xenogen machine. At the same time, mice that injected C2 cells were sacrificed and the metastasis into different organs was excised.
Immunocytochemistry
To analyze expression and localization of E-cadherin in indicated cells, tumor cells were prepared by culture in a slide chamber for 24 hour or by cytospin before staining. After treatment with Cytofix/Cytoperm solution (BD Pharmingen), tumor cells were stained with anti-E-cadherin mAb (BD Pharmingen). Alexa 488 (green)-conjugated secondary antibodies (Invitrogen) were used for visualization, and cells were observed using a confocal LSM 5 Pascal microscope (Carl Zeiss Meditec).
Stastasis
Statistical data analysis was performed using SPSS 17.0. Data are presented as means ± SD, based on at least three independent repeats. Comparisons between two groups were conducted using two-tailed Student's t test, and differences were considered to be statistically significant when the P value is less than 0.05.
Results
Isoquercitrin blocks HGf/SF-induced cell scattering
To better understand the antitumor effect of T.hemsleyanum, we isolated the ingredients from the root tuber of T.hemsleyanum by repeated column chromatography on silica gel. Six flavonoids were acquired and their structures were determined by 1H-Nuclear Magnetic Resonance Spectroscopy (1H-NMR) analysis ( and supplemental data). While searching these six ingredients for an inhibitor of HGF/SF-Met signaling, we observed that isoquercitrin efficiently blocked the HGF/SF induced cancer cell scatter. As shown in and supplemental videos, the bladder carcinoma cell line NBT-II cells formed compact colonies with well-defined cell contacts under in vitro cell culture medium. Under the stimulation of HGF, the NBT-II cells scattered and exhibited the lamellipodial activity at the free edge. However, the scatter ability of NBT-II cells was suppressed when isoquercitrin was added into the cell medium before being treated by HGF ( and supplemental videos). Next, we evaluated the activity of Rac1, a small GTPase well known to induce lamellipodia formation. Our data showed that HGF induced the activation of Rac1 while the activation effect of HGF was inhibited by isoquercitrin ( and ). We further investigated whether the inhibition of HGF/SF by isoquercitrin acted at the HGF/SF-Met level. Indeed, the phosphorylation of tyrosine residues in c-Met by HGF/SF was inhibited by isoquercitrin at concentration as low as 1 μg/ml, which was quite evident at a concentration of 5 μg/ml (). Together, these data suggest that isoquercitrin block the migration of NBT-II cells through HGF/SF –Met signaling.
Figure 1. structure of extracts from T.hemsleyanum The extracts of root tuber from T.hemsleyanum were acquired as described in materials and methods. Six flavonoids were isolated and their structures were determined by 1H-Nuclear Magnetic Resonance Spectroscopy (1H-NMR) analysis.
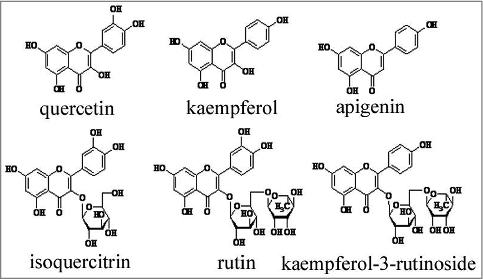
Figure 2. Isoquercitrin block HGf/SF-induced cell scattering A. The inhibitory activity of isoquercitrin against HGF-mediated NBT-II cell scattering was observed under the phase-contrast microscope (× 20). B. NBT-II cells were seeded in complete medium for 18 h, and then incubated in serum-free media overnight. Isoquercitrin was added to the media 2 h before the addition of HGF/SF (100 units/ml). Cells were incubated for 6 hours and lysed. The immobilized GST-GGA3-PBD beads were used to capture GTP-bound Rac1. Starting lysate and pull down samples were run on a Western and blotted for Rac1. Input indicated the total Rac1 and GAA3 showed activated Rac1. GST beads were used as negative control. C. Experiments in B were quantified by imaging system. Data were normalized by NBT-II control cells and shown as means ± standard error. D. NBT-II cells were seeded in complete medium for 18 h, and then incubated in serum-free media overnight. Isoquercitrin was added to the media 2 h before the addition of HGF/SF (100 units/ml). Fifteen minutes after the treatment, cells were harvested, and the lysates were subjected to immunoprecipitation using anti-phosphotyrosine antibody (upper panel), anti-Met antibody (middle panel) and anti-GAPDH antibody (lower panel). **P < 0.01, paired T test; p-c-Met, phosphorylation of c-Met; Ctr, control; H+D, HGF+DMSO; H+I,HGF+isoquercitrin.
Isoquercitrin inhibites HGF/SF-mediated in vitro cell motility and invasion
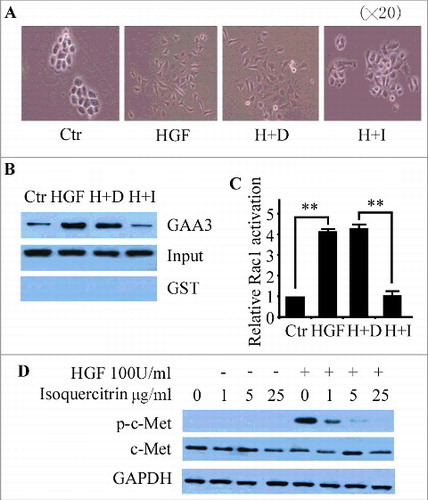
Since dysregulated HGF/SF-Met signaling is one of the principal changes not only in tumorigenesis but also in tumor progression, we tested the ability of isoquercitrin to inhibit HGF/SF-mediated motility and invasion in vitro. Our results showed that HGF/SF-induced migration of the cells in the “scratch wound assay” was clearly abolished by isoquercitrin in a dose-dependent manner ( and ) while isoquercitrin had no significant inhition on NBT-II cell proliferation under stimulation with or without HGF (Supplemental figure 1). We also tested the ability of isoquercitrin to inhibit HGF/SF-mediated invasion of NBT-II cells across Matrigel-coated 8-μm pore filters. Isoquercitrin down-regulated HGF/SF-mediated invasion of NBT-II cells at concentration as low as 1 μg/ml in dose-dependent manner (). Moreover, the mechanism was explored by analyzing the association between isoquercitrin and EMT. The epithelial marker E-cadherin and mesenchymal marker Vimentin and N-cadherin were selected to test the role of isoquercitrin in HGF-induced EMT. Our results showed that the protein expression of E-cadherin was reduced and that of N-cadherin was upregulated in HGF induced NBT-II cells compared with control cells. Notably, the reduction of E-cadherin and the upregulation of Vimentin and N-cadherin were decreased when treating NBT-II cells with isoquercitrin before the induction of HGF (). The immunostaining assay further demonstrated that the expression and accumulation of E-cadherin on the cell membrane were reverted back with the effect of isoquercitrin (Supplemental figure 2A and 2B). These data indicate that isoquercitrin is a potent inhibitor of HGF/SF-mediated cell motility and invasion by blocking the initiation of EMT in vitro.
Figure 3. Inhibition of HGF/SF-induced cell migration A. NBT-II cells were seeded at a density of 20,000 cells/well in 24-well plates and cultured for 48 h. Confluent cells were scratched with a 1-ml micropipette tip, and fresh media containing the indicated concentrations of isoquercitrin were added 2 h before the treatment with or without HGF (100 units/ml). After 24 h, cells were photographed under a phase-contrast microscope. The migratory capability was quantified as described in Materials and Methods and showed in B. C.Transwell filters were coated with Matrigel (20 μg/filter) before the application of cells at a density of 20,000 cells/filter. Isoquercitrin was added at the indicated concentrations in both the upper and lower chambers 2 h before the addition of HGF (100 units/ml) to both chambers. After 48 h, filters were processed as described in Materials and methods. Invading cells were counted under light microscope. Cell proliferation rate under the same conditions was measured and used to correct the invading cell number as follows: corrected cell number = number of cells counted/relative ratio of cell population compared to that without isoquercitrin. D. Western blotting analysis of epithelial marker E-cadherin and mesenchymal marker Vimentin and N-cadherin in indicated cells. Densitometry was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Vim, Vimentin; E-cad, E-cadherin; N-cad, N-cadherin; Ctr, Control; H+D, HGF+DMSO; H+I,HGF+isoquercitrin. Data are means ± SD from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; N.S, No significance; paired T test; Ctr, control; H+D, HGF+DMSO; H+I,HGF+isoquercitrin; Vim, Vimentin.
Isoquercitrin inhibites metastasis of HGF autocrine NBT-II cells in vivo
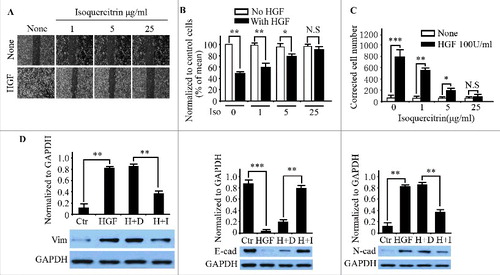
To assess whether HGF/SF-Met signaling was also abolished by isoquercitrin in vivo, we established a stable clone with autocrine HGF/SF-Met signaling by transfecting with HGF cDNA into NBT-II cells (C2 cells hereafter). HGF-SF mRNA was detected in C2 cells but not in parental NBT-II cells (). As expected, the conditioned medium of C2 cells induced more NBT-II cell scattering than low concentration HGF/SF (data not shown) while isoquercitrin had no significant inhition on C2 cell proliferation (Supplemental figure 2C). The results showed that C2 cells had higher invasion through Matrigel-coated filter than HGF-treated parental NBT-II cells (), indicative of their highly invasive and metastatic properties. Similarly, invasion of C2 cells through Matrigel-coated membrane was also dose-dependently inhibited by isoquercitrin after correcting for cell number (), showing that isoquercitrin also works in cells with Met signaling activated in autocrine manner. Furthermore, syngeneic nude mice were used for in vivo spontaneous metastasis assays. As shown in , and supplemental figure 3, isoquercitrin significantly blocked metastasis in the orthotopically injected C2 cells.
Figure 4. Isoquercitrin inhibited metastasis of HGF autocrine NBT-II cells in vivo A. Transwell filters were coated with Matrigel (20 μg/filter) before the application of cells at a density of 20,000 cells/filter. HGF/SF (100 units/ml) was applied to NBT-II cells or C2 cells in both the upper and lower chambers. After 48 h, invading cells were counted under light microscope. The corrected cell number = number of invading cells/relative ratio of cell population compared to that without HGF/SF. Inset figure shows the expression of HGF/SF mRNA by RT-PCR. B. Transwell filters were coated with Matrigel (20 μg/filter) before the application of C2 cells at a density of 20,000 cells/filter. Isoquercitrin was added at the indicated concentrations to both upper and lower chambers. After 48h, invading cells were counted under light microscope. Corrected cell number = number of cells counted/relative ratio of cell population compared to that without isoquercitrin. C. NBT-II cells or C2 cells were orthotopically injected into nude mice as described in Materials and methods. The bioluminescence of metastatic lesions were detected under Xenogen machine and quantified in D. Ctr, Control; Iso, Isoquercitrin; *P < 0.05; **P < 0.01; ***P < 0.001; paired T test.
Taken together, our data demonstrate that isoquercitrin inhibits HGF-induced scattering through its effect on Met and that this inhibition is applicable to autocrine activation of HGF/SF-mediated cancer cell migration and invasion both in vitro and in vivo.
Discussion
Activation of HGF/SF-Met signaling has been observed in a variety of human cancers. Various approaches to inhibit HGF/SF-Met signaling in experimental systems have included neutralizing anti-HGF/SF monoclonal antibodies [Citation25], ribozyme against HGF/SF or Met [Citation26,Citation27], and HGF/SF variants that inhibit the binding of HGF/SF to Met [Citation28,Citation29]. In addition, there have been attempts to inhibit the tyrosine kinase activity of Met with small molecule inhibitors selected from chemical libraries [Citation30–Citation32]. However, small molecule inhibitors from chemical libraries have their own limitations in that there are possible unpredictable toxicity problems [Citation13]. As one of the possible ways to by-pass the toxicity problems, we focused on extracts from edible plants and the known small molecules found in them. Through the use of cell-based searching assay, we found that isoquercitrin, a kind of flavonoid in the extracts of the root tuber from T.hemsleyanum, had a significant inhibitory effect on HGF/ SF-Met signaling.
Isoquercitrin (quercetin3-O-b-D-glucopyranoside) is a natural flavonoid glucoside that is distributed in medicinal and dietary plants, such as vegetables, herbs, and flowers [Citation33]. Isoquercitrin has been found to have a wide range of biological properties, such as anti-inflammatory effects [Citation34]; antioxidant activity, including decreasing ROS levels and reducing lipid peroxidation both in vivo and in vitro [Citation35]; neuroprotection; and promotion of neurite elongation [Citation36]. In particular, recent studies reported that isoquercitrin had antitumor effect in pancreatic cancer [Citation37], colon cancer [Citation38] and liver cancer [Citation39] through mitogen-activated protein kinase signaling [Citation37], Wnt/ β-catenin signaling [Citation38] or AMP–activated protein kinase (AMPK) signal [Citation40] pathway. The objective of the present study was to investigate the effect of isoquercitrin on HGF/ SF-Met signaling and to further understand the biological characteristics of the participation of isoquercitrin in the progression of cancer.
In this report, we took advantage of HGF-induced Met phosphorylation to dissect the action of isoquercitrin on HGF/SFMet signaling. The phosphorylation of c-Met was blocked by isoquercitrin in a dose-dependent manner (), suggesting that isoquercitrin is a possible inhibitor on HGF/SFMet signaling. We additionally showed the effect of isoquercitrin on HGF/SF-induced tumor metastasis in vivo. Our data strengthen the possibility of using isoquercitrin to treat human cancers associated with dysregulated HGF/SF-Met signaling in either a paracrine or an autocrine manner. However, we do not know the precise mode of action of isoquercitrin. Whether isoquercitrin inhibits ligand-receptor binding or induces a conformational change of the receptor after ligand binding? which portion of Met is the working target of isoquercitrin? The questions about the effect of isoquercitrin on different modes of activation of HGF/SFMet signaling, as well as the mechanistic insight that isoquercitrin works on either an extracellular or transmembrane part of Met, were addressed here.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Supplementry_material.zip
Download Zip (15.7 MB)Acknowledgment
This work was supported by the National Natural Science Foundation of China (No. 81572879).
Additional information
Funding
References
- Naldini L, Vigna E, Ferracini R, et al. The tyrosine kinase encoded by the MET proto-oncogene is activated by autophosphorylation. Mol Cell Biol. 1991;11(4):1793–1803. doi:10.1128/MCB.11.4.1793.
- Park WS, Dong SM, Kim SY, et al. Somatic mutations in the kinase domain of the Met/hepatocyte growth factor receptor gene in childhood hepatocellular carcinomas. Cancer Res. 1999;59(2):307–310.
- Bottaro DP, Rubin JS, Faletto DL, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251(4995):802–804. doi:10.1126/science.1846706.
- Nakamura T, Teramoto H, Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci U S A. 1986;83(17):6489–6493. doi:10.1073/pnas.83.17.6489.
- Stoker M, Gherardi E, Perryman M, et al. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327(6119):239–242. doi:10.1038/327239a0.
- Bussolino F, Di Renzo MF, Ziche M, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119(3):629–641. doi:10.1083/jcb.119.3.629.
- Tsarfaty I, Resau JH, Rulong S, et al. The met proto-oncogene receptor and lumen formation. Science. 1992;257(5074):1258–1261. doi:10.1126/science.1387731.
- Jeffers M, Rong S, Vande Woude GF. Hepatocyte growth factor/scatter factor-Met signaling in tumorigenicity and invasion/metastasis. J Mol Med (Berl). 1996;74(9):505–513. doi:10.1007/BF00204976.
- Birchmeier W, Brinkmann V, Niemann C, et al. Role of HGF/SF and c-Met in morphogenesis and metastasis of epithelial cells. Ciba Found Symp. 1997;212:230–240. discussion 240-236.
- Schmidt L, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16(1):68–73. doi:10.1038/ng0597-68.
- Lee JH, Han SU, Cho H, et al. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene. 2000;19(43):4947–4953. doi:10.1038/sj.onc.1203874.
- Jiang WG, Martin TA, Parr C, et al. Hepatocyte growth factor, its receptor, and their potential value in cancer therapies. Crit Rev Oncol Hematol. 2005;53(1):35–69. doi:10.1016/j.critrevonc.2004.09.004.
- Michieli P, Mazzone M, Basilico C, et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell. 2004;6(1):61–73. doi:10.1016/j.ccr.2004.05.032.
- Ono T, Tsuji T, Sakai M, et al. Induction of hepatocyte growth factor production in human dermal fibroblasts and their proliferation by the extract of bitter melon pulp. Cytokine. 2009;46(1):119–126. doi:10.1016/j.cyto.2008.12.016.
- Jiang WG, Ye L, Ruge F, et al. YangZheng XiaoJi exerts anti-tumour growth effects by antagonising the effects of HGF and its receptor, cMET, in human lung cancer cells. J Transl Med. 2015;13:280. doi:10.1186/s12967-015-0639-1.
- Xiong Y, Wu X, Rao L. Tetrastigma hemsleyanum (Sanyeqing) root tuber extracts induces apoptosis in human cervical carcinoma HeLa cells. J Ethnopharmacol. 2015;165:46–53. doi:10.1016/j.jep.2015.02.030.
- Peng X, Zhuang DD, Guo QS. Induction of S phase arrest and apoptosis by ethyl acetate extract from Tetrastigma hemsleyanum in human hepatoma HepG2 cells. Tumour Biol. 2015;36(4):2541–2550. doi:10.1007/s13277-014-2869-x.
- Feng Z, Hao W, Lin X, et al. Antitumor activity of total flavonoids from Tetrastigma hemsleyanum Diels et Gilg is associated with the inhibition of regulatory T cells in mice. Onco Targets Ther. 2014;7:947–956.
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi:10.1038/nrc822.
- Zhou W, Thiery JP. Loss of Git2 induces epithelial-mesenchymal transition by miR146a-Cnot6L-controlled expression of Zeb1. J Cell Sci. 2013;126(Pt 12):2740–2746. doi:10.1242/jcs.126367.
- Zhou W, Ye XL, Xu J, et al. The lncRNA H19 mediates breast cancer cell plasticity during EMT and MET plasticity by differentially sponging miR-200b/c and let-7b. Sci Signal. 2017;10(483): doi:10.1126/scisignal.aak9557.
- Jo E, Park SJ, Choi YS, et al. Kaempferol Suppresses Transforming Growth Factor-beta1-Induced Epithelial-to-Mesenchymal Transition and Migration of A549 Lung Cancer Cells by Inhibiting Akt1-Mediated Phosphorylation of Smad3 at Threonine-179. Neoplasia. 2015;17(7):525–537. doi:10.1016/j.neo.2015.06.004.
- Bhat FA, Sharmila G, Balakrishnan S, et al. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. 2014;25(11):1132–1139. doi:10.1016/j.jnutbio.2014.06.008.
- Zhu Y, Wu J, Li S, et al. Apigenin inhibits migration and invasion via modulation of epithelial mesenchymal transition in prostate cancer. Mol Med Rep. 2015;11(2):1004–1008. doi:10.3892/mmr.2014.2801.
- Cao B, Su Y, Oskarsson M, et al. Neutralizing monoclonal antibodies to hepatocyte growth factor/scatter factor (HGF/SF) display antitumor activity in animal models. Proc Natl Acad Sci U S A. 2001;98(13):7443–7448. doi:10.1073/pnas.131200498.
- Abounader R, Ranganathan S, Lal B, et al. Reversion of human glioblastoma malignancy by U1 small nuclear RNA/ribozyme targeting of scatter factor/hepatocyte growth factor and c-met expression. J Natl Cancer Inst. 1999;91(18):1548–1556. doi:10.1093/jnci/91.18.1548.
- Abounader R, Lal B, Luddy C, et al. In vivo targeting of SF/HGF and c-met expression via U1snRNA/ribozymes inhibits glioma growth and angiogenesis and promotes apoptosis. FASEB J. 2002;16(1):108–110. doi:10.1096/fj.01-0421fje.
- Guerin C, Luddy C, Abounader R, et al. Glioma inhibition by HGF/NK2, an antagonist of scatter factor/hepatocyte growth factor. Biochem Biophys Res Commun. 2000;273(1):287–293. doi:10.1006/bbrc.2000.2935.
- Brockmann MA, Papadimitriou A, Brandt M, et al. Inhibition of intracerebral glioblastoma growth by local treatment with the scatter factor/hepatocyte growth factor-antagonist NK4. Clin Cancer Res. 2003;9(12):4578–4585.
- Christensen JG, Schreck R, Burrows J, et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63(21):7345–7355.
- Sattler M, Pride YB, Ma P, et al. A novel small molecule met inhibitor induces apoptosis in cells transformed by the oncogenic TPR-MET tyrosine kinase. Cancer Res. 2003;63(17):5462–5469.
- Wang SY, Chen B, Zhan YQ, et al. SU5416 is a potent inhibitor of hepatocyte growth factor receptor (c-Met) and blocks HGF-induced invasiveness of human HepG2 hepatoma cells. J Hepatol. 2004;41(2):267–273. doi:10.1016/j.jhep.2004.04.013.
- Razavi SM, Zahri S, Zarrini G, et al. Biological activity of quercetin-3-O-glucoside, a known plant flavonoid. Bioorg Khim. 2009;35(3):414–416.
- Rogerio AP, Kanashiro A, Fontanari C, et al. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res. 2007;56(10):402–408. doi:10.1007/s00011-007-7005-6.
- Li R, Yuan C, Dong C, et al. In vivo antioxidative effect of isoquercitrin on cadmium-induced oxidative damage to mouse liver and kidney. Naunyn Schmiedebergs Arch Pharmacol. 2011;383(5):437–445. doi:10.1007/s00210-011-0613-2.
- Jung SH, Kim BJ, Lee EH, et al. Isoquercitrin is the most effective antioxidant in the plant Thuja orientalis and able to counteract oxidative-induced damage to a transformed cell line (RGC-5 cells). Neurochem Int. 2010;57(7):713–721. doi:10.1016/j.neuint.2010.08.005.
- Chen Q, Li P, Li P, et al. Isoquercitrin inhibits the progression of pancreatic cancer in vivo and in vitro by regulating opioid receptors and the mitogen-activated protein kinase signalling pathway. Oncol Rep. 2015;33(2):840–848. doi:10.3892/or.2014.3626.
- Amado NG, Predes D, Fonseca BF, et al. Isoquercitrin suppresses colon cancer cell growth in vitro by targeting the Wnt/beta-catenin signaling pathway. J Biol Chem. 2014;289(51):35456–35467. doi:10.1074/jbc.M114.621599.
- Huang G, Tang B, Tang K, et al. Isoquercitrin inhibits the progression of liver cancer in vivo and in vitro via the MAPK signalling pathway. Oncol Rep. 2014;31(5):2377–2384. doi:10.3892/or.2014.3099.
- Zhou J, Yoshitomi H, Liu T, et al. Isoquercitrin activates the AMP-activated protein kinase (AMPK) signal pathway in rat H4IIE cells. BMC Complement Altern Med. 2014;14:42. doi:10.1186/1472-6882-14-42.
