ABSTRACT
Collagen is the most abundant component of tumor extracellular matrix (ECM). ECM collagens are known to directly interact with the tumor cells via cell surface receptor and play crucial role in tumor cell survival and promote tumor progression. Collagen receptor DDR1 is a member of receptor tyrosine kinase (RTK) family with a unique motif in the extracellular domain resembling Dictyostelium discoideum protein discoidin-I. DDR1 displays delayed and sustained activation upon interaction with collagen and recent findings have demonstrated that DDR1-collagen signaling play important role in cancer progression. In this review, we discuss the current knowledge on the role of DDR1 in cancer metastasis and possibility of a potential therapeutic approach of DDR1 targeted therapy in cancer.
Introduction
Metastasis is the major cause of cancer related deaths [Citation1] in the United States. It is a complex phenomenon involving dissemination of cancer cells from the primary site to secondary organs. In order to reach secondary organs, cancer cells need to acquire ability to invade the basement membrane, survive the dissemination process through the bloodstream and eventually extravasate into the target organ [Citation2]. Often times they remain dormant for a certain period of time before outgrowing and colonizing the organ. The exact mechanisms of dormancy and reactivation is an active area of study and recent work has indicated the role of discoidin domain receptor 1 (DDR1) in the metastatic reactivation of cancer cells [Citation3].
Genomic location of DDR1 and mechanism of activation
DDR belongs to the family of receptor tyrosine kinases (RTK) and consists of two members DDR1 and DDR2. DDR1 gene is located in the p arm of human chromosome 6 (6p21.3) while its homolog DDR2 is located in the q arm of chromosome 1 (1q23.3). Structurally, they are composed of an outer extracellular domain, which interacts with the ligand, transmembrane region, and intracellular cytoplasmic domain which transmits the signal via kinase domain. There are at least five isoforms of DDR1 reported, DDR1a, DDR1b, and DDR1c, which has functionally active kinase domain while DDR1d and DDR1e, either lack kinase domain or has inactive kinase domain due to premature truncation. There is an additional sixth isoform of DDR1, which is found in rat testis and lack extracellular domain [Citation4]. DDR1 and DDR2 belong to the receptor tyrosine kinase (RTK) family and are non-integrin collagen receptors and are activated by native triple helical collagen but not by heat denatured collagens. However, there are differences between DDR1 and DDR2 in specificities towards different collagens. DDR2 preferentially binds to collagen I, III and X but not collagen II and IV while DDR1 can bind to I, II, III, IV and with lower affinity to X [Citation5,Citation6].
One of the notable features of DDR is their ability to dimerize in the absence of ligand [Citation7–Citation9]. Contacts between extracellular domain, cytoplasmic domain and transmembrane region are involved in the dimerization process. However, interaction between the extracellular and cytoplasmic region is dispensable for dimerization. But a mutation in the leucine zipper motif of the transmembrane region is sufficient to disrupt the dimerization highlighting the importance of transmembrane region in ligand independent dimerization of DDR1 [Citation9]. Unlike most of the RTK’s wherein ligand induces dimerization, DDR’s are activated by forming lateral clusters in the presence of collagen thereby phosphorylating the DDR dimers leading to activation [Citation7]. In the absence of ligand DDR1 dimers are kept in an inactivated state via N-glycosylation at highly conserved N211 residue as found by mutational study [Citation10].
Collagen interacts with DDR via discoidin domain. Initial mutagenesis studies indicated that collagen-binding regions are located in three surface exposed loops present within the discoidin domain of the protein [Citation6,Citation11–Citation14]. Further insights by NMR study on DDR structure suggested that the binding region is formed by interlope trench consisting of charged residues surrounded by hydrophobic one [Citation15]. Finally, crystal structure of DDR2 with ‘GVMGFO’ motif narrowed it down to critically important tryptophan residue and a salt bridge. Not all amino acids surrounding the GVMGFO are conserved in DDR1 in line with different collagen specificity found between the two proteins [Citation16]. Binding of collagen to extracellular membrane auto phosphorylation albeit at a slower rate compared to majority of tyrosine kinases [Citation5,Citation17].
Phenotypic function of DDR1
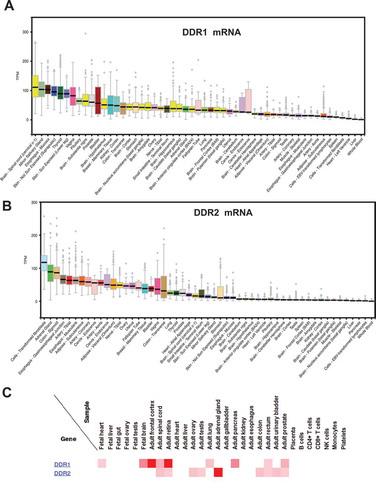
Loss of DDR1 in mice was found to have substantial effect on mammary gland development and bone disease. DDR1 null mice were found to be smaller in size and the majority of the females were unable to bear offspring due to improper blastocyst implantation into the uterine wall. In some female mice where implantation did occur, they were found to be incapable of lactation indicating defects in mammary ducts [Citation18]. Additionally, high incidence of osteoarthritis in the temporomandibular joint was also found in DDR1 deficient mice [Citation19]. The protein and mRNA expression of DDR1 and DDR2 across different human tissues analyzed by using human proteome map (http://www.humanproteomemap.org/) [Citation20] and GTEx portal (www.gtexportal.org) respectively is shown in . Apart from these organs DDR1 was also found to have an additional role in normal and diseased cells.
Figure 1. (a & b) DDR1 and DDR2 mRNA expression in human tissue analyzed by GTEx portal (www.gtexportal.org). (c) Distribution of DDR1 and DDR2 expressions in benign human tissue analyzed by human proteome map (http://www.humanproteomemap.org/).
Role of DDR1 on prosurvival and chemoresistance
DDR1 plays a crucial role in cell survival and resistance to genotoxic stress. Previous studies have indicated that DDR1 is regulated transcriptionally by p53 and DDR1 relays feedforward loop increasing p53 levels and some of its target genes. Subsequently, on genotypic stress increased apoptosis was observed in cells with loss of DDR1 activity highlighting the role of DDR1 in cell survival [Citation21]. Further studies have shown functionally relevant interaction between DDR1 and Notch 1. On DDR1 activation via collagen, DDR1 was found to interact with Notch that led to downstream upregulation of Notch target genes. Notch inhibition led to increase apoptosis as been noted previously with DDR1 loss of function. Inhibition of DDR1 led to decrease levels of nuclear Notch 1 and tumor growth invivo indicating the role of DDR1 in tumor growth via Notch1 [Citation22]. DDR1 is also found to activate COX2 and NF-κB and mediates chemoresistance via these signaling pathways [Citation23]. Epistasis study revealed that NF-κB was upstream of COX2 activity and when NF-κB was inhibited via I-κB superrepressor mutant, loss of COX2 activity was found and increase chemosensitivity was noted indicating potential for targeting cancer cells in combination with either NF-κB or COX2 inhibitor [Citation23].
Self-renewal, differentiation and DDR1
The role of DDR1 in self renewal and differentiation is cell context dependent. In mouse embryonic stem cells (mESC), presence of Collagen I leads to maintenance of self-renewal state of mESC. The phenotypic effect is attributed to DDR1 mediated activation of Bmi1 via ERK signaling in concordance with β1 integrin signaling. Bmi1 activation seems essential to maintain the undifferentiated state [Citation24]. However, collagen mediated DDR1 activation is also involved in axon extension of granule neurons. Loss of DDR1 activity was found to inhibit neurite outgrowth in immature granule cells hinting its role in neuronal differentiation [Citation25]. Additional studies in myoblast cell line C2C12 has also led to identification of DDR1 role in cellular differentiation and myofiber formation [Citation26]. Self-renewal and cellular differentiation potential are the major properties of embryonic stem cells [Citation27]. Similarly, cancer stem cells (CSC) also exhibit self-renewal and differentiation potential which is often associated with cancer progression and therapy resistance [Citation28,Citation29]. Although previous finding showed that collagen-DDR1 interaction is crucial for stem cell like feature (mammosphere formation) of breast cancer cells [Citation3], however, the role of DDR1 in maintaining cancer stem like cells or tumor heterogeneity has largely remained unexplored.
DDR1 and cell adhesion and migration
One of the major functions of DDR1 is cell adhesion. In normal and malignant cells DDR1 has been shown to have some effect on cell adhesion as well as cell-cell adhesion. DDR1 was found to be necessary for localization of melanocytes in the basal layer of human epidermis wherein it is in contact with Collagen IV [Citation30]. In pituitary glioma cells, however, increased cell adhesion was found with Collagen I [Citation31]. Surprisingly, in cell – cell adhesion the role of DRR1 was found to be independent of collagen interactions. DDR1 was found to interact with E-Cadherin in normal as well as malignant cell line. This complex with E-cadherin abrogated DDR1’s ability to activate via Collagen. Additionally, DDR1 able to interact with Par3/Par6 proteins and reduce actomyosin contractility via RhoE recruitment. Neither kinase inactive (K618A) nor collagen binding mutant (R105A) had any effect on this function of DDR1 indicating that DDR1 functions independent of Collagen interaction to regulate cell-cell adhesion [Citation32]. This reduction in actomyosin contractility leads to collective invasion of cancer cells [Citation32]. In addition, DDR1 has also been shown to remodel collagen through mechano-transduction forces in an integrin independent fashion by clustering and interacting with non-muscle myosin IIA [Citation33] and this may associate with cell motility. Taken together these studies highlight the role of DDR1 in cell migration which further associate with tumor metastasis.
DDR1 in tumor progression and metastasis
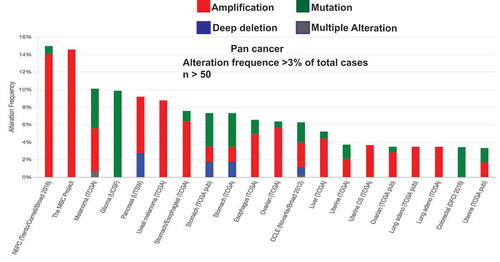
Current advancement of transcriptomic and genomic sequencing demonstrates the importance of receptor tyrosine kinases (RTK) in cancer progression [Citation34–Citation40]. Activation of RTKs remains a crucial rate limiting step over numerous downstream signaling pathways and biological events [Citation41–Citation45]. DDR1 is an RTK activated by collagens and plays a crucial role in various physiological as well as pathophysiological processes [Citation46–Citation49]. DDR1 interacts with ECM collagen that induces DDR1 tyrosine phosphorylation [Citation3,Citation7] . However surprisingly, compare to other RTKs, DDR 1 exhibit significantly slow kinetics in ligand (collagen) induced phosphorylation, what take place several hours after initial stimulation and remain detectable even after a day [Citation7] [Citation17]. Upon interaction with collagen DDR1 forms cluster [Citation7]. However, collagen-I induced DDR1 cluster remains for a significantly longer time compared to cluster induced by collagen-IV (3 hours vs 10 minutes) [Citation3]. DDR1 amplification is commonly observed in various cancers [Citation50,Citation51] including ~ 14% of metastatic breast cancer (mbcproject.org) and aggressive neuroendocrine prostate cancer (NEPC [Citation52]; ) indicating that the DDR1 signaling pathway is significantly deregulated in aggressive cancers.
DDR1 is activated by collagens [Citation11]. Collagens account for ~ 30% of body proteins and are a major part of the ECM (extracellular matrix) [Citation53–Citation56]. Collagen from ECM is known to regulate multiple signaling pathways that control cancer cell behavior by regulating mortality and invasiveness of cells [Citation10,Citation57–Citation59]. In tumor micro environmental ECM, type I collagen exhibit in high density and are known to be associated with tumor aggressiveness [Citation60–Citation63]. Tumor cell surface integrins are known to play important role in tumor-ECM collagen interaction [Citation64–Citation67]. However recent studies have demonstrated that tumor cell surface DDR also play crucial role in collagen induced signaling pathway in tumor cells () [Citation6,Citation11]. Collagen DDR1 interaction induces phosphorylation and kinase activation of DDR1 that leads to activation of multiple downstream signaling pathways (Summarized in ). Collagen DDR1 mediated Src kinase activation regulates proliferation and cell migration [Citation68]. DDR1 also induces activation of proline-rich tyrosine kinase 2 (Pyk2) that ultimately induces N cadherin expression and regulates epithelial to mesenchymal transition (EMT) of cancer cells [Citation69]. Collagen DDR1 interaction also plays an important role in chemo-resistance and cell survival via NFκB-COX2 mediated pathway [Citation23] (). Interestingly previous studies also showed that collagen DDR1 axis can induce matrix metalloproteinase (MMP) expression and activation [Citation70,Citation71]. MMPs plays a crucial role in degradation of extracellular matrix and tumor invasion [Citation72]. Therefore interaction of collagen and DDR1 can regulate MMP mediated ECM degradation resulting in invasiveness and tumor progression ().
Figure 3. (a) Schematic Representation of DDR1-collagen signaling pathway in cancer. Collagen DDR1 interaction induces canonical and non-canonical signaling pathway which is summarize in the figure. Canonically interaction between collagen and DDR1 induces tyrosine phosphorylation of DDR1 and induces DDR1 kinase activation. DDR1 kinase activation induces Src, Notch and IKK signaling pathway. DDR1 also regulates Pyk2 mediate RAP1 activation that leads to epithelial to mesenchymal transition (EMT). Collagen also induces DDR1 and TM4SF1 interaction which is a kinase independent (Non-canonical; inside box) function of DDR1. DDR1-TM4SF1 interaction regulates survival and reactivation of breast cancer cells in metastatic site [Citation3].(b) Schematic Representation of DDR1-collagen signaling on induction/activation of MMP that regulates degradation of matrix and invasiveness of cancer cells.
![Figure 3. (a) Schematic Representation of DDR1-collagen signaling pathway in cancer. Collagen DDR1 interaction induces canonical and non-canonical signaling pathway which is summarize in the figure. Canonically interaction between collagen and DDR1 induces tyrosine phosphorylation of DDR1 and induces DDR1 kinase activation. DDR1 kinase activation induces Src, Notch and IKK signaling pathway. DDR1 also regulates Pyk2 mediate RAP1 activation that leads to epithelial to mesenchymal transition (EMT). Collagen also induces DDR1 and TM4SF1 interaction which is a kinase independent (Non-canonical; inside box) function of DDR1. DDR1-TM4SF1 interaction regulates survival and reactivation of breast cancer cells in metastatic site [Citation3].(b) Schematic Representation of DDR1-collagen signaling on induction/activation of MMP that regulates degradation of matrix and invasiveness of cancer cells.](/cms/asset/1b3db697-87a3-4f6d-9aa6-b7ed01f8f954/kcam_a_1520556_f0003_oc.jpg)
Collagen-DDR1 signaling pathway in reactivation and metastasis
Primary tumor cells disseminated from the site of origin with subsequent seeding and regrowth to the distant site is known as cancer metastasis [Citation2,Citation73,Citation74]. Process of metastasis is a complex series of events and least understood aspect of cancer biology [Citation75]. Metastasis is associated with poor prognosis and accounting for the majority of the cancer related deaths [Citation76–Citation78]. In many tumor type after colonization at the metastatic site cancer cells undergo a prolonged dormancy, however, duration between metastatic dormancy varies in different cancer type [Citation2,Citation79]. Cancer cells adopt various strategies to survive at the distant metastatic site and then proliferate and regrow and give rise to metastatic lesion [Citation2,Citation80,Citation81]. Recent evidence suggest that interplay between metastatic initiating cells and the microenvironment of the metastatic site plays a crucial role in reactivation-metastasis cascade of cancer [Citation2,Citation73,Citation76,Citation78].
Earlier studies have shown that DDR1 is a crucial component in bone metastasis of lung cancer [Citation82]. Interestingly the authors showed that shRNA mediated knockdown of DDR1 neither show any significant differences in 2D cell growth or in subcutaneous lung tumor growth. However, intracardiac injection of DDR1 knockdown lung cancer cells fail to induce significant bone metastasis compare to control cells indicating that bone microenvironment might play an essential role in DDR1 mediated bone metastasis of lung cancer [Citation82]. It was also shown that DDR1 directly interacted with Notch [Citation22]. Upon Collagen-mediated DDR1 kinases activation, Notch was activated and regulates downstream signaling pathway [Citation22]. Previous studies showed the important implication of Notch signaling pathway in reactivation of breast cancer cells at metastatic site [Citation83]. Taken together these studies indicate that DDR1-collagen signaling may play a crucial role in reactivation of breast cancer cells in metastatic site possibly through activation of notch ().
Earlier studies also demonstrated that collagen-DDR1 can induce epithelial to mesenchymal (EMT) transition in cancer cells [Citation84]. Previously, Shintani et al demonstrated that collagen up regulated mesenchymal protein N-cadherin expression in pancreatic cancer cells leading to tumor growth, invasion and metastasis. Interestingly the study also showed that DDR1 knockdown pancreatic cancer cells failed to upregulate collagen induced N-cadherin expression [Citation69]. EMT transition is known to be one of the major step of cancer metastasis indicating that cell surface DDR1 can interact with microenvironmental collagen, leading to EMT and metastasis [Citation69,Citation85].
Previously Juin et al showed that DDR is crucial for linear invadosome formation and invasion of tumor cells in collagen reach environment and DDR1-kinase activity is not required for this process [Citation86]. The authors showed that collagen mediated activation of Rho-GTPase Cdc42 is DDR1 dependent which plays an important role in invadosome formation. Furthermore, the data also demonstrated the essential role of Tuba, a Cdc412 specific guanine nucleotide-exchange factor(GEF) in collagen-DDR1-Cdc42 mediated invadosome formation [Citation86]. Moreover recently Yang et al also showed that DDR1-TM4SF1 interaction promotes invadopodia formation, which regulates cell migration and metastasis in pancreatic cancer [Citation87].
Gao et al have demonstrated that in metastatic breast cancer cell, DDR1 interacts with cell surface Transmembrane 4 L Six Family Member 1 (TM4SF1) and regulates tumor dormancy and reactivation [Citation3]. The interaction between DDR1 and TM4SF1 is collagen dependent and control Protein Kinase C Alpha (PKCa) mediated downstream JAK-STAT signaling pathway [Citation3] (). Interestingly the data also showed that TM4SF1 failed to interact with DDR2. Importantly DDR1 kinase inhibitor failed to inhibit downstream PKCa-JAK-STAT signaling pathway indicating that a non-canonical DDR1 kinase activity independent mechanism regulates breast cancer metastasis [Citation3]. Similarly knocking down of DDR1 but not kinase inhibitor significantly downregulated collagen induced mammosphere formation. Furthermore, DDR1 positive solitary breast tumor cells in mice lung underwent proliferation when they came in contact with collagen I from lung microenvironment. However, in the absence of of environmental collagen I the cells remain quiescent inside lung. This study demonstrated that stromal collagen I at metastatic site controls DDR1 mediated tumor metastasis [Citation3].
Implication of DDR1 targeted therapy in cancer
Emerging evidence suggests crucial role of DDR1 in tumor progression and metastasis in various solid tumors [Citation3,Citation22,Citation87]. Knockdown of DDR1 significantly inhibited invitro tumor sphere formation as well as multi organ metastasis of breast cancer [Citation3]. RNAi mediated knockdown of DDR also inhibited migration of pancreatic cancer cells [Citation88]. Moreover, DDR1 knockout mice exhibited significantly less Kras driven lung tumor growth in mice [Citation89]. Therefore, targeting DDR1 could be a promising therapeutic approach for aggressive metastatic cancer.
Tyrosine kinase inhibitor dasatinib, imatinib nilotinib and ponatinib have been identified to inhibit DDR1 and DDR2 kinase activity [Citation90]. Combine inhibition of DDR1 and Notch by dasatinib and demcizumab significantly inhibited PDX lung adenocarcinoma growth in mice [Citation89].Two clinical trials of dasatinib was conducted in patients with advance cancer harboring DDR2 mutation (NCTO1514864). However due to lack of efficacy this trial had been terminated. Feedback activation of alternative signaling pathways often impedes the efficacy of DDR pathway inhibition [Citation91]. Very recently, Jetany et al demonstrated that inhibition of DDR signaling by Nilotinib could inhibit metastasis of colorectal cancer [Citation92]. Nilotinib is currently under a phase 2 clinical trial for patients whose tumor harbor the mutation of DDR1 or DDR2 (NCT02020001). Gao et el have identified a series of 3-(2-(pyrazolo[1,5-a]pyrimidin-6-yl) ethynyl)benzamides as selective and orally bioavailable DDR1 inhibitor with very low IC50 values. These small molecule inhibitors successfully inhibit invasion, proliferation and colony formation of transformed cells [Citation93]. Furthermore, recently Aguilera et al showed that DDR1 inhibitor in combination with conventional chemotherapy significantly reduces pancreatic tumor growth in preclinical pancreatic cancer PDX model [Citation88]. Taken together the data indicates that targeting DDR1 can be a promising therapeutic strategy for various type of aggressive cancer.
Summary
The present review demonstrates the important role of DDR1 signaling in cancer metastasis. Considering the complex and crucial role of DDR1 in various cancers, in-depth study is required to understand the molecular mechanism by which DDR1 regulates the tumor metastasis. Understanding the biology and molecular consequence underlying DDR1 signaling will be helpful in designing DDR1 targeted therapy for advanced metastatic cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18(1–2):43–73.
- Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155(4):750–764.
- Gao H, Chakraborty G, Zhang Z, et al. Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell. 2016;166(1):47–62.
- Mullenbach E, Walter L, Dressel R. A novel discoidin domain receptor 1 (Ddr1) transcript is expressed in postmeiotic germ cells of the rat testis depending on the major histocompatibility complex haplotype. Gene. 2006;372:53–61.
- Vogel W, Gish GD, Alves F, et al. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1(1):13–23.
- Leitinger B, Kwan AP. The discoidin domain receptor DDR2 is a receptor for type X collagen. Matrix Biol. 2006;25(6):355–364.
- Juskaite V, Corcoran DS, Leitinger B. Collagen induces activation of DDR1 through lateral dimer association and phosphorylation between dimers. Elife. 2017;6.
- Mihai C, Chotani M, Elton TS, et al. Mapping of DDR1 distribution and oligomerization on the cell surface by FRET microscopy. J Mol Biol. 2009;385(2):432–445.
- Noordeen NA, Carafoli F, Hohenester E, et al. A transmembrane leucine zipper is required for activation of the dimeric receptor tyrosine kinase DDR1. J Biol Chem. 2006;281(32):22744–22751.
- Fu HL, Valiathan RR, Payne L, et al. Glycosylation at Asn211 regulates the activation state of the discoidin domain receptor 1 (DDR1). J Biol Chem. 2014;289(13):9275–9287.
- Leitinger B. Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2. Identification of collagen binding sites in DDR2. J Biol Chem. 2003;278(19):16761–16769.
- Leitinger B, Steplewski A, Fertala A. The D2 period of collagen II contains a specific binding site for the human discoidin domain receptor, DDR2. J Mol Biol. 2004;344(4):993–1003.
- Yeh YC, Lin HH, Tang MJ. A tale of two collagen receptors, integrin beta1 and discoidin domain receptor 1, in epithelial cell differentiation. Am J Physiol Cell Physiol. 2012;303(12):C1207–C1217.
- Yoshimura T, Matsuyama W, Kamohara H. Discoidin domain receptor 1: a new class of receptor regulating leukocyte-collagen interaction. Immunol Res. 2005;31(3):219–230.
- Ichikawa O, Osawa M, Nishida N, et al. Structural basis of the collagen-binding mode of discoidin domain receptor 2. EMBO J. 2007;26(18):4168–4176.
- Carafoli F, Mayer MC, Shiraishi K, et al. Structure of the discoidin domain receptor 1 extracellular region bound to an inhibitory Fab fragment reveals features important for signaling. Structure. 2012;20(4):688–697.
- Shrivastava A, Radziejewski C, Campbell E, et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1(1):25–34.
- Vogel WF, Aszodi A, Alves F, et al. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21(8):2906–2917.
- Schminke B, Muhammad H, Bode C, et al. A discoidin domain receptor 1 knock-out mouse as a novel model for osteoarthritis of the temporomandibular joint. Cell Mol Life Sci. 2014;71(6):1081–1096.
- Kim MS, Pinto SM, Getnet D, et al. A draft map of the human proteome. Nature. 2014;509(7502):575–581.
- Ongusaha PP, Kim JI, Fang L, et al. p53 induction and activation of DDR1 kinase counteract p53-mediated apoptosis and influence p53 regulation through a positive feedback loop. EMBO J. 2003;22(6):1289–1301.
- Kim HG, Hwang SY, Aaronson SA, et al. DDR1 receptor tyrosine kinase promotes prosurvival pathway through Notch1 activation. J Biol Chem. 2011;286(20):17672–17681.
- Das S, Ongusaha PP, Yang YS, et al. Discoidin domain receptor 1 receptor tyrosine kinase induces cyclooxygenase-2 and promotes chemoresistance through nuclear factor-kappaB pathway activation. Cancer Res. 2006;66(16):8123–8130.
- Suh HN, Han HJ. Collagen I regulates the self-renewal of mouse embryonic stem cells through alpha2beta1 integrin- and DDR1-dependent Bmi-1. J Cell Physiol. 2011;226(12):3422–3432.
- Bhatt RS, Tomoda T, Fang Y, et al. Discoidin domain receptor 1 functions in axon extension of cerebellar granule neurons. Genes Dev. 2000;14(17):2216–2228.
- Vogel W, Brakebusch C, Fassler R, et al. Discoidin domain receptor 1 is activated independently of beta(1) integrin. J Biol Chem. 2000;275(8):5779–5784.
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111.
- Wu XZ. Origin of cancer stem cells: the role of self-renewal and differentiation. Ann Surg Oncol. 2008;15(2):407–414.
- Bai X, Ni J, Beretov J, et al. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat Rev. 2018;69:152–163.
- Fukunaga-Kalabis M, Martinez G, Liu ZJ, et al. CCN3 controls 3D spatial localization of melanocytes in the human skin through DDR1. J Cell Biol. 2006;175(4):563–569.
- Ram R, Lorente G, Nikolich K, et al. Discoidin domain receptor-1a (DDR1a) promotes glioma cell invasion and adhesion in association with matrix metalloproteinase-2. J Neurooncol. 2006;76(3):239–248.
- Hidalgo-Carcedo C, Hooper S, Chaudhry SI, et al. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol. 2011;13(1):49–58.
- Coelho NM, Arora PD, van Putten S, et al. Discoidin domain receptor 1 mediates myosin-dependent collagen contraction. Cell Rep. 2017;18(7):1774–1790.
- Zwick E, Bange J, Ullrich A. Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr Relat Cancer. 2001;8(3):161–173.
- Stahtea XN, Kousidou O, Roussidis AE, et al. Small tyrosine kinase inhibitors as key molecules in the expression of metalloproteinases by solid tumors. Connect Tissue Res. 2008;49(3):211–214.
- Sharma PS, Sharma R, Tyagi T. Receptor tyrosine kinase inhibitors as potent weapons in war against cancers. Curr Pharm Des. 2009;15(7):758–776.
- Sastry SK, Elferink LA. Checks and balances: interplay of RTKs and PTPs in cancer progression. Biochem Pharmacol. 2011;82(5):435–440.
- Takeuchi K, Ito F. Receptor tyrosine kinases and targeted cancer therapeutics. Biol Pharm Bull. 2011;34(12):1774–1780.
- McDonell LM, Kernohan KD, Boycott KM, et al. Receptor tyrosine kinase mutations in developmental syndromes and cancer: two sides of the same coin. Hum Mol Genet. 2015;24(R1):R60–R66.
- Butti R, Das S, Gunasekaran VP, et al. Receptor tyrosine kinases (RTKs) in breast cancer: signaling, therapeutic implications and challenges. Mol Cancer. 2018;17(1):34.
- Neben CL, Lo M, Jura N, et al. Feedback regulation of RTK signaling in development. Dev Biol. 2017.
- Fantauzzo KA, Soriano P. Receptor tyrosine kinase signaling: regulating neural crest development one phosphate at a time. Curr Top Dev Biol. 2015;111:135–182.
- Piiper A, Zeuzem S. Receptor tyrosine kinases are signaling intermediates of G protein-coupled receptors. Curr Pharm Des. 2004;10(28):3539–3545.
- Regad T. Targeting RTK signaling pathways in cancer. Cancers (Basel). 2015;7(3):1758–1784.
- Schlessinger J. Receptor tyrosine kinases: legacy of the first two decades. Cold Spring Harb Perspect Biol. 2014;6:3.
- Canning P, Tan L, Chu K, et al. Structural mechanisms determining inhibition of the collagen receptor DDR1 by selective and multi-targeted type II kinase inhibitors. J Mol Biol. 2014;426(13):2457–2470.
- Krohn JB, Hutcheson JD, Martinez-Martinez E, et al. Discoidin domain receptor-1 regulates calcific extracellular vesicle release in vascular smooth muscle cell fibrocalcific response via transforming growth factor-beta signaling. Arterioscler Thromb Vasc Biol. 2016;36(3):525–533.
- Toy KA, Valiathan RR, Nunez F, et al. Tyrosine kinase discoidin domain receptors DDR1 and DDR2 are coordinately deregulated in triple-negative breast cancer. Breast Cancer Res Treat. 2015;150(1):9–18.
- Valiathan RR, Marco M, Leitinger B, et al. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. 2012;31(1–2):295–321.
- Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404.
- Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1.
- Beltran H, Prandi D, Mosquera JM, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22(3):298–305.
- An B, Lin YS, Brodsky B. Collagen interactions: drug design and delivery. Adv Drug Deliv Rev. 2016;97:69–84.
- Bella J, Hulmes DJ. Fibrillar Collagens. Subcell Biochem. 2017;82:457–490.
- Posey KL, Coustry F, Hecht JT. Cartilage oligomeric matrix protein: cOMPopathies and beyond. Matrix Biol. 2018.
- Theocharis AD, Skandalis SS, Gialeli C, et al. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27.
- Peng DH, Ungewiss C, Tong P, et al. ZEB1 induces LOXL2-mediated collagen stabilization and deposition in the extracellular matrix to drive lung cancer invasion and metastasis. Oncogene. 2017;36(14):1925–1938.
- Sapudom J, Rubner S, Martin S, et al. The phenotype of cancer cell invasion controlled by fibril diameter and pore size of 3D collagen networks. Biomaterials. 2015;52:367–375.
- He X, Lee B, Jiang Y. Cell-ECM Interactions in Tumor Invasion. Adv Exp Med Biol. 2016;936:73–91.
- Jung HY, Fattet L, Yang J. Molecular pathways: linking tumor microenvironment to epithelial-mesenchymal transition in metastasis. Clin Cancer Res. 2015;21(5):962–968.
- Kharaishvili G, Simkova D, Bouchalova K, et al. The role of cancer-associated fibroblasts, solid stress and other microenvironmental factors in tumor progression and therapy resistance. Cancer Cell Int. 2014;14:41.
- Shen K, Luk S, Hicks DF, et al. Resolving cancer-stroma interfacial signalling and interventions with micropatterned tumour-stromal assays. Nat Commun. 2014;5:5662.
- Vennin C, Chin VT, Warren SC, et al. Transient tissue priming via ROCK inhibition uncouples pancreatic cancer progression, sensitivity to chemotherapy, and metastasis. Sci Transl Med. 2017;9:384.
- Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339(1):269–280.
- Duan W, Ma J, Ma Q, et al. The activation of beta1-integrin by type i collagen coupling with the hedgehog pathway promotes the epithelial-mesenchymal transition in pancreatic cancer. Curr Cancer Drug Targets. 2014;14(5):446–457.
- Heino J, Kapyla J. Cellular receptors of extracellular matrix molecules. Curr Pharm Des. 2009;15(12):1309–1317.
- Zheng X, Liu W, Xiang J, et al. Collagen I promotes hepatocellular carcinoma cell proliferation by regulating integrin beta1/FAK signaling pathway in nonalcoholic fatty liver. Oncotarget. 2017;8(56):95586–95595.
- Dejmek J, Dib K, Jonsson M, et al. Wnt-5a and G-protein signaling are required for collagen-induced DDR1 receptor activation and normal mammary cell adhesion. Int J Cancer. 2003;103(3):344–351.
- Shintani Y, Fukumoto Y, Chaika N, et al. Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol. 2008;180(6):1277–1289.
- Hou G, Vogel WF, Bendeck MP. Tyrosine kinase activity of discoidin domain receptor 1 is necessary for smooth muscle cell migration and matrix metalloproteinase expression. Circ Res. 2002;90(11):1147–1149.
- Ferri N, Carragher NO, Raines EW. Role of discoidin domain receptors 1 and 2 in human smooth muscle cell-mediated collagen remodeling: potential implications in atherosclerosis and lymphangioleiomyomatosis. Am J Pathol. 2004;164(5):1575–1585.
- Lu P, Takai K, Weaver VM, et al. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:12.
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–284.
- Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9(4):302–312.
- Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168(4):670–691.
- Wick MR. Metastases of malignant neoplasms: historical, biological, & clinical considerations. Semin Diagn Pathol. 2018;35(2):112–122.
- Obenauf AC, Massague J. Surviving at a distance: organ-specific metastasis. Trends Cancer. 2015;1(1):76–91.
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695.
- Gomis RR, Gawrzak S. Tumor cell dormancy. Mol Oncol. 2016 Oct 7; pii: S1574-7891(16)30109-0.6.
- Weinberg RA. The many faces of tumor dormancy. APMIS. 2008;116(7–8):548–551.
- Oskarsson T, Batlle E, Massague J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell. 2014;14(3):306–321.
- Valencia K, Ormazabal C, Zandueta C, et al. Inhibition of collagen receptor discoidin domain receptor-1 (DDR1) reduces cell survival, homing, and colonization in lung cancer bone metastasis. Clin Cancer Res. 2012;18(4):969–980.
- Abravanel DL, Belka GK, Pan TC, et al. Notch promotes recurrence of dormant tumor cells following HER2/neu-targeted therapy. J Clin Invest. 2015;125(6):2484–2496.
- Koh M, Woo Y, Valiathan RR, et al. Discoidin domain receptor 1 is a novel transcriptional target of ZEB1 in breast epithelial cells undergoing H-Ras-induced epithelial to mesenchymal transition. Int J Cancer. 2015;136(6):E508–E520.
- Chaffer CL, San Juan BP, Lim E, et al. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35(4):645–654.
- Juin A, Di Martino J, Leitinger B, et al. Discoidin domain receptor 1 controls linear invadosome formation via a Cdc42-Tuba pathway. J Cell Biol. 2014;207(4):517–533.
- Yang JC, Zhang Y, He SJ, et al. TM4SF1 promotes metastasis of pancreatic cancer via regulating the expression of DDR1. Sci Rep. 2017;7:45895.
- Aguilera KY, Huang H, Du W, et al. Inhibition of discoidin domain receptor 1 reduces collagen-mediated tumorigenicity in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2017;16(11):2473–2485.
- Ambrogio C, Gomez-Lopez G, Falcone M, et al. Combined inhibition of DDR1 and Notch signaling is a therapeutic strategy for KRAS-driven lung adenocarcinoma. Nat Med. 2016;22(3):270–277.
- Kothiwale S, Borza CM, Lowe EW Jr., et al. Discoidin domain receptor 1 (DDR1) kinase as target for structure-based drug discovery. Drug Discov Today. 2015;20(2):255–261.
- Beauchamp EM, Woods BA, Dulak AM, et al. Acquired resistance to dasatinib in lung cancer cell lines conferred by DDR2 gatekeeper mutation and NF1 loss. Mol Cancer Ther. 2014;13(2):475–482.
- Jeitany M, Leroy C, Tosti P, et al. Inhibition of DDR1-BCR signalling by nilotinib as a new therapeutic strategy for metastatic colorectal cancer. EMBO Mol Med. 2018.
- Gao M, Duan L, Luo J, et al. Discovery and optimization of 3-(2-(Pyrazolo[1,5-a]pyrimidin-6-yl)ethynyl)benzamides as novel selective and orally bioavailable discoidin domain receptor 1 (DDR1) inhibitors. J Med Chem. 2013;56(8):3281–3295.