ABSTRACT
The mammalian Sterile 20-like kinase 1/2 (MST1/2) belongs to the serine/threonine (GC) protein kinase superfamily. Collective studies confirm the vital role MST1/2 in inflammation and immunity. MST1/2 is closely related to the progress of inflammation. Generally, MST1/2 aggravates the inflammatory injury through MST1-JNK, MST1-mROS, MST1-Foxo3, and NF-κB pathways, as well as several regulatory factors such as tumor necrosis factor-α (TNF-α), mitochondrial extension factor 1 (MIEF1), and lipopolysaccharide (LPS). Moreover, MST1/2 is also involved in the regulation of immunity to balance immune activation and tolerance by regulating MST1/2-Rac, MST1-Akt1/c-myc, MST1-Foxos, MST1-STAT, Btk pathways, and lymphocyte function-related antigen 1 (LFA-1), which subsequently prevents immunodeficiency syndrome and autoimmune diseases. This article reviews the effects of MST1/2 on inflammation and immunity.
Introduction
The mammalian Sterile 20-like (MST) kinases belong to the serine/threonine (GC) protein kinase superfamily [Citation1]. MST kinases are evolutionarily conserved homologue of yeast Sterile 20 (STE20) kinase 1 [Citation2–4]. In mammals, there are five kinds of MST kinases, including MST1 (also known as STK4), MST2 (STK3), MST3 (STK24), MST4 (STK26), and YSKI (STK25 or SOK1) [Citation1]. In addition, MST kinases play an important role in the mammalian cells proliferation, migration and apoptosis, immune regulation, and inflammatory response [Citation1,Citation2].
As a class II GC protein kinase [Citation5], MST1/2 is the main component of the mammalian Hippo signaling pathway [Citation2] which regulates the proliferation and survival of cells [Citation6–9]. Increasing studies suggest that MST1/2 is widely involved in the development or prognosis of diseases that relate to inflammatory [Citation10–12] and immune [Citation13,Citation14]. Accordingly, the effects of MST1/2 on inflammation and immunity are reviewed in this paper.
Structure and function of MST1/2
The structure of MST1/2
MST1 is composed of 487 amino acids [Citation9] and contains the N-terminal catalytic domain of class STE20 which is followed by a non-catalytic tail containing, successively, an autoinhibitory segment, and a coiled-coil domain [Citation15] mediating dimerization [Citation16]. MST2 is composed of 491 amino acids which is the accessory nucleotide of MST1 [Citation16]. Accordingly, MST2 is closely related to MST1.
There is a unique helical Salvador-Rassf-Hippo (SARAH) domain near the carboxyl terminal of MST1/2 [Citation5]. In the non-phosphorylated state, SARAH domain stably interacts with other peptide substrates which contain SARAH domain for homodimerization and heterodimerization [Citation5] to regulate the signal transduction of MST1/2 [Citation17].
The function of MST1/2
The role of MST1/2 in inflammatory response
It is reported that current researches on MST1/2 involving in the inflammatory response are mainly focused on myocardial cell injury [Citation18]. Overexpression of MST1 promotes excessive inflammatory reaction of the myocardial cells [Citation19] to promote the myocardial cell necrosis [Citation18,Citation20], which induces myocardial fibrosis and cardiac hypertrophy [Citation21]. MST1-mROS signaling pathway is activated by suppressor of ras val-2 (SRV2) [Citation22] which is up-regulated after hypoxia treatment [Citation18], thus aggravating the inflammation of myocardial cells [Citation18] to promote the death of the myocardial cells [Citation23]. MST1 catalyzes the myocardial inflammation in diabetic mice, the activation of MST1 is partially inhibited by chronic exercise training to relieve response of inflammatory [Citation24]. In addition, with MST1 is deficient, the levels of inflammatory factors decreases in the myocardial cells treated by hypoxia in vitro, mitochondrial homeostasis is maintained, mitochondrial fission is inhibited, thus inhibiting the inflammatory reaction of the myocardial cells, and the survival of the myocardial cells being promoted [Citation19]. The reperfusion therapy of XMU-MP-1 inhibits the phosphorylation of MST1 [Citation25], protecting the myocardial cells [Citation12,Citation24,Citation25].
Furthermore, MST1 aggravates the secondary brain injury (SBI) following the intracerebral hemorrhage (ICH) [Citation12]. XMU-MP-1 reperfusion therapy effectively inhibits the activation of downstream protein P-LATS1 and P-YAP of MST1 to reduce the neuronal cell death and the inflammatory reaction in rats with ICH to alleviate SBI [Citation12].
MST1 aggravates the poor prognosis of spinal cord injury [Citation26,Citation27]. On the one hand, MST1 damages the mitochondrial function by attenuating the phosphorylation of adenosine phosphorylation-activated protein kinase α (AMPKα) to promote NF-κB-related inflammatory reaction in the injured spinal cord [Citation26]. On the other hand, MST1 damages the mitochondrial function to reduce the formation of ATP by promoting the release of cytochrome c and the activation of caspase-3, which strengthens the mitochondrial-mediated apoptosis pathway and promotes the activation of microglia and glial cells to enhance the local inflammatory response after the spinal cord injury [Citation27]. The miR-139-5p inhibits the expression of MST1 and accelerates the functional recovery of the injured spinal cord to promote the survival of neurons [Citation26].
The lipopolysaccharide (LPS)-mediated MST1-JNK signaling pathway increases the levels of SRV2 which induces mitochondrial fission to enhance the inflammatory response of mouse microglia cells (BV-2) and inhibition of MST1-JNK signaling pathway by Sirtuin 3 (Sirt3) to protect BV-2 microglia from inflammation-mediated cell damage [Citation22] ().
Figure 1. MST1-JNK pathway.
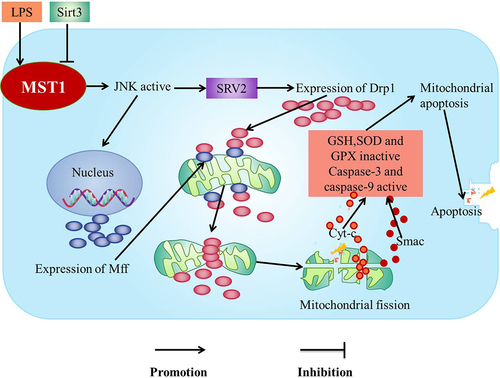
In addition, MST1 promotes tumor necrosis factor-α (TNF-α) inducing the injury of the nasal epithelium through increasing the inflammation [Citation28]. The deletion of MST1 alleviates the inflammatory injury of the nasal epithelium [Citation29].
MST1/2 inhibits inflammatory injury. However, the role of current MST1 in the inflammation remains contradictory. Some authors have suggested that MST1/2 may be a factor in alleviating the inflammatory damage. It is showed that MST1/2 inhibits YAP activity of the renal tubules to inhibit the chronic kidney disease (CKD) [Citation30].
The role of MST1/2 in immune
MST1/2 is involved in the regulation of innate immunity. MST1/2 plays an important role in regulating the innate immune. It is reported that MST1/2 is involved in macrophage phagocytosis [Citation31]. CaMKII-MST1/2-Rac signaling pathway is activated by toll-like receptor 4 (TLR4), which enhances the bactericidal activity of the macrophages to involve in the innate immunity [Citation32]. Thus, the inhibition of MST1/2 results in reduced ability of the macrophages to phagocytize Escherichia coli and Lactobacillus monocytogenes [Citation29]. Accordingly, MST1 may involve in innate immune by enhancing the bactericidal activity of the macrophages.
In addition, it is reported that MST1/2 is expressed in eosinophils and associates with the apoptosis of the eosinophils [Citation33]. However, no research has shown whether MST1 is involved in the regulation of the eosinophils. Furthermore, though MST1/2 is not expressed in neutrophils, the absence of MST1 inhibits the neutrophils penetrating the perivascular basement membrane and extravasating into the inflammatory tissues [Citation34]. Moreover, meanwhile, the function of MST1 is inhibited resulting that the loss of the mitochondrial membrane potential of the neutrophils increases subsequently the susceptibility to apoptosis increases [Citation13]. Accordingly, MST1 may involve in innate immune by promoting the exudation and the survival of the neutrophils.
MST1/2 is a selective driver of the CD8a+ antigen-presenting dendritic cells (DCS) and maintains the bioenergetic activities and the mitochondrial dynamics of CD8a+ DCs [Citation35]. In addition, MST1/2 is closely related to the expression of IL-6 [Citation36] and IL-12 in CD8α+DCs [Citation35]. Moreover, CD4(+) Foxp3(+) Regulatory T cells (Treg cells) dynamic mobile contact with antigen-pulsed DCs that bearing stably associates naïve T cells in depends on MST1 [Citation37]. Meanwhile, MST1 deficiency decreases DCs in the marginal area of the spleen and impairs the antigen presentation function of the CD8α+DCs [Citation38]. Accordingly, MST1/2 may be involved in innate immune by regulating the function of DCs.
MST1/2 is involved in the regulation of adaptive immunity. It is confirmed that MST1/2 is involved in the regulation of the adaptive immune [Citation39]. MST1 deficiency has impaired the adaptive immunity of individuals, such as the insufficient vaccination responses, the decrease of T and B lymphocytes [Citation40], which leads to combined immunodeficiency [Citation41].
MST1/2 is involved in the regulation of T cells. MST1/2 is a key factor of T cells [Citation42] and involved in the regulation of T cells adhesion, migration, homing, and survival [Citation31] to maintain the homeostasis of T cells [Citation2]. Over-expression of MST1 leads to the increase of the proliferation and the migration of T cells [Citation43]. In addition, MST1/2 is recruited by the constitutive interaction between Rap1 and Rassf5B (known as Nore1B/RAPL) which is Rap1-GTP binding protein to control the adhesion and the migration of T cells [Citation44]. Moreover, MST1 mediates the migration of the mature thymocytes from the thymus to the periphery [Citation45] and is closely related to the selection and the antigen self-recognition of thymocytes [Citation38,Citation46]. In MST1 −/− mice, the selection and the migration of thymocytes are impaired, and the antigen recognition efficiency is down-regulated [Citation38].
MST1 maintains the homeostasis of the peripheral naïve T cells [Citation47] and promotes the recognition of the antigens by naïve T cells [Citation48]. However, MST1 inhibits the activation and the proliferation of naïve T cells through Rassf5-MST1 complex [Citation47]. In addition, MST1-Foxos signaling pathway maintains the peripheral homeostasis of naïve T cells [Citation49].
MST1/2 plays an important role in promoting the maturation and the function of Treg cells [Citation31]. It is reported MST1/2 is involved in Treg cells [Citation37] to maintain the balance between the activation and the tolerance of the immune [Citation48], which affects the autoimmune inflammation and the normal immune function [Citation14,Citation29]. In addition, MST1 improves the differentiation and the function of Treg cells [Citation14] by regulating the activities of Forkhead box o1/3 (Foxo1/3) [Citation2], Sirt1 [Citation50], TEAD1 [Citation51], and Akt [Citation14], meanwhile maintains the immune tolerance [Citation29] by regulating the activation of STAT5 [Citation52,Citation53]. MST1 directly or indirectly enhances the stability of Foxo1/3 by phosphorylating Foxo1/3 or inhibiting activity of Akt mediated by antigen-specific T cell antibody (TCR) in the peripheral T cells, thereby increasing the expression of Foxp3 in the mice to regulate Treg cells [Citation14]. In addition, MST1 increases the acetylation of Foxp3 directly or by inhibiting the activity of the human recombinant protein 1 (Sirt1) to increase the stability of Foxp3 [Citation50], which improves the activity of Foxp3 to regulate Treg cells [Citation29,Citation52] ().
Figure 2. MST1/2 regulates Treg cells.
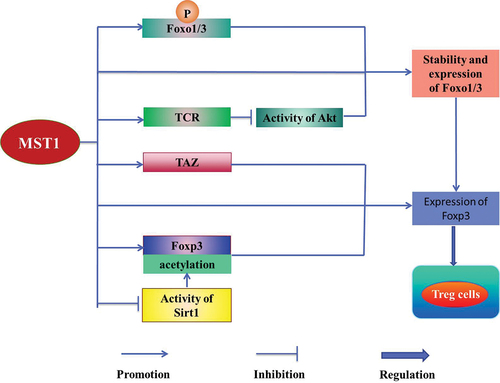
Meanwhile, MST1/2 directly or through MST1/2-TAZ axis promotes the expression of Foxp3 to regulate the reciprocal differentiation of Treg cells and Th17 cells [Citation29] (). Foxp3+ Treg cells and Th17 cells, as a pair of balancers [Citation29], play an important role in maintaining the homeostasis of cells [Citation2]. It is reported that Foxp3+ Treg cells attenuate the proliferation of T cells and the secretion of pro-inflammatory cytokines, mediating the immune tolerance [Citation29] and the immunosuppression [Citation54]. In contrast, the pro-inflammatory role of the Th17 cells protects the hosts against the infection [Citation2]. MST1/2-TAZ signaling inhibits the development of Th17 cells, but improves the differentiation of Treg cells [Citation55]. Accordingly, MST1/2 plays a key role in maintaining the homeostasis of T cells [Citation2,Citation29].
The homeostasis and the function of T cells are frequently impaired with the absence of MST1 resulting that the hosts present susceptibility to the immunodeficiency syndrome, which causes various diseases because of recurrent bacterial and viral infections [Citation13,Citation41,Citation56], such as lung infections, candidiasis, and non-degenerative skin warts [Citation57]. Moreover, the deficiency of MST1 causes hypergammaglobulinemia with the production of autoantibody [Citation42], which leads to the autoimmune diseases such as Sjogren’s syndrome and colitis [Citation14]; meanwhile, the hypergammaglobulinemia is associated with increased levels of IgG, IgA, and IgE [Citation41]. It is reported that the hypergammaglobulinemia is likely attributed to the inhibition of MST1-Foxo1 signaling pathway which decreases the expression of Foxp3 to impair the function of the Treg cells, ultimately leads to the inability to maintain the dominant tolerance [Citation41]. Accordingly, MST1 is an important regulator of Treg cells and is critical for preventing the autoimmune diseases and maintaining the immune homeostasis [Citation55]. The deficiency of MST1 causes the immunodeficiency syndrome [Citation14] or the autoimmune diseases. Accordingly, MST1/2 may be involved in the adaptive immune by regulating the function of T cells.
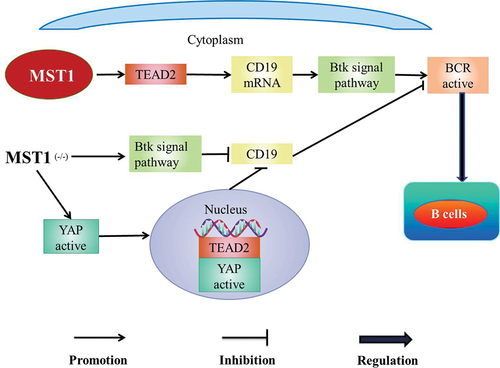
MST1/2 is involved in the regulation of B cells. MST1 promotes the diffusion and the transportation of B cells [Citation58]. In addition, MST1 co-promotes the development and the activation of B cells with B cell antigen receptor (BCR) [Citation59]. MST1 positively regulates BCR signal by activating Bruton tyrosine kinase (Btk) signal pathway, which leads to the decrease of B cells in the marginal zone (MZ) and the increase of B cells in the germinal center (GC) [Citation60] (). Meanwhile, mouse MST1/Wiskott-Aldrich syndrome protein (WASP) is knocked out (DKO), early activation events of B cells such as BCR signaling, BCR clustering, and B-cells spreading are severely impaired and the development of B cells is seriously hindered in bone marrow [Citation59]. MST1/2 promotes the maturation of B cells in the white pulp of the spleen and then B cells gain the ability of recycling to the lymph nodes or the bone marrow [Citation58]. Meanwhile, MST1/2 promotes the effective transportation of follicular B cells to the splenic red pulp to produce marginal B cells [Citation58]. The number of B cells decreases [Citation38] and the development of B cells defects in mice with MST1 (-/-) [Citation61] besides the germinal center is dysregulated in the spleen margin area, which eventually leads to primary immunodeficiency [Citation62]. Accordingly, MST1/2 may be involved in the adaptive immune by regulating B cells.
Figure 3. MST1-Btk pathway.
MST1/2 is involved in regulation of lymphocytes. MST1/2 regulates the survival [Citation48], polarity, adhesion [Citation63], and transportation [Citation58] of lymphocytes through the non-canonical Hippo pathway [Citation48]. It is confirmed that MST1 is the most abundant in the lymphoid organs including the thymus, the spleen, and the lymph nodes [Citation2]. MST1 promotes the transportation of lymphocytes to the secondary lymphoid organs [Citation58]. Meanwhile, MST1 is lacking in the mouse lymphocytes, the spleen may become swollen [Citation14] and the peripheral lymphoid tissue atrophy [Citation38]. In addition, MST1 is a key effector of Rassf5 which mediates effective transport of the immune cells [Citation63]. The levels of Rassf5 decreased significantly [Citation47] and lymphocytes present profound transport defect in MST1 (-/-) lymphocytes [Citation64]. Moreover, MST1 promotes the adhesion of lymphocytes by transmitting Rap1-RAPL signals [Citation63]. It is reported MST1 (-/-) lymphocytes are incapable to adhere firmly to the high endothelial venules through alpha4 integrin, which results the significant reduction of time that lymphocytes stop on the endothelial cells and significantly decrease of the homing ability of lymphocytes [Citation38]. Meanwhile, MST1/2 promotes the survival of lymphocytes [Citation63]. Dysfunction of MST1 leads to the decrease of the mitochondrial membrane potential and the increase of susceptibility to apoptosis of lymphocytes [Citation13]. Besides, MST1/2(-/-) leads to severe lymphopenia [Citation38,Citation65]. Accordingly, MST1/2 may be involved in the immune by regulating the function of lymphocytes.
MST1/2 and signaling pathway
Hippo signaling pathway is evolutionarily conserved [Citation66], which is involved in the regulation of proliferation and apoptosis of cells [Citation66,Citation67]. The major role of Hippo signaling pathway may be regulating the immunity in the mammals [Citation68], which is essential for maintaining the homeostasis of the immune system [Citation29]. MST1/2 is the core kinase of Hippo signaling pathway and cross-talks with the various signaling pathways [Citation29] such as MST1-JNK signaling pathway [Citation22], MST1-NF-κB signaling pathway [Citation69], and MST1/2-Rac signaling pathway [Citation32] in the various immune cells [Citation29]. In addition, MST1/2 is involved in the regulation of the immunity [Citation70] and the inflammation in Hippo pathway [Citation12].
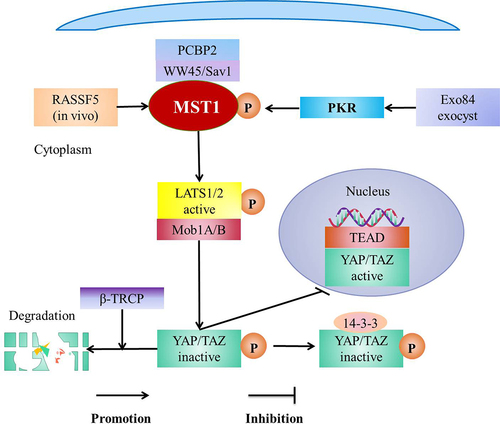
Hippo pathway is composed of a kinase cascade [Citation66], including MST1/2 and its scaffold protein WW domain protein 1 (WW45)/Salvador1 (SAV1), large tumor suppressor kinases 1 and 2 (LATS1/2, Warts homologs) and its scaffold-protein MPs 1 binding compounds l A and B (Mob1A/B), and downstream effector YAP/TAZ [Citation19,Citation30]. The core components of Hippo pathway are MST1/2, LATS1/2 and their respective adaptor proteins [Citation19]. In addition, the main effect factors of Hippo pathway are YAP and TAZ [Citation30]. The MST1/2-WW45/SAV1 complex phosphorylates and activates LATS1/2-Mob1A/B complex which then phosphorylates YAP/TAZ and then the phosphorylated YAP/TAZ is degraded by β-TRCP or isolated in the cytoplasm by 14-3-3 [Citation29]. MST1/2 activates Hippo signaling pathway [Citation71] to decrease the accumulation of nuclear YAP and inhibit the retention of TAZ in the mesothelial cells [Citation72]. Meanwhile, MST1/2 is inactivated to close Hippo pathway, which leads to the accumulation of YAP/TAZ in the nucleus and then YAP/TAZ binds to TEAD family transcription factors [Citation73], subsequently initiates the expression of downstream genes [Citation29,Citation66] (). In the mammals, MST1/2 regulates LATS1/2 and NDR1/2 by scaffolding WW45/Sav1 [Citation19]. In addition, NDR1/2 is the same kinase family as LATS [Citation45] and is a branch of LATS1/2 [Citation2].
Figure 4. Hippo pathway and regulation of Hippo pathway.
The signaling pathways of MST1/2 related to inflammation
MST1-JNK signaling pathway
The activation of MST1-JNK signaling pathway aggravates the inflammation in the myocardial cells [Citation21], liver cells [Citation74], and the neurons [Citation22]. It is reported that the activity of MST1 is up-regulated after LPS treatment [Citation75] to promote the activation of JNK pathway [Citation21], leading to the up-regulation of SRV2 [Citation22], which subsequently increases the levels of fission-promoting factor mitochondrial Kinetic associated protein (Drp1) [Citation21]. Furthermore, the phosphorylated JNK is transported to the nucleus to increase the expression of the drp1 receptor (Mff) [Citation22,Citation76]. Drp1 migrates from the cytoplasm to the mitochondrial surface and binds with Mff on the mitochondrial outer membrane to recruit Drp1 [Citation21]. Drp1 subsequently forms a GPT-dependent contraction ring which divides the mitochondria into several fragments to promote the mitochondrial fission, the mitochondrial potential collapsing, and the mitochondrial permeability transition pore (mPTPs) opening [Citation21]. The mitochondrial pro-apoptotic mediators (such as cyt-c [Citation77] and Smac) leak into the cytoplasm after mPTPs being opened [Citation77] to active the apoptotic executive caspase-3, caspase-9 and rapidly down-regulate the activities of antioxidants such as SOD, GSH, and GPX [Citation78], which leads to the mitochondrial apoptosis [Citation76], thus the apoptosis of the myocardial cells and BV-2 cells occurring [Citation21,Citation22] ().
Sirt3 is a key factor for the survival of the microglia. It is reported that Sirt3 inhibits the expression of SRV2, which maintains the antioxidant levels to inhibit the cleavage and the apoptosis of the mitochondria through MST1-JNK signaling pathway, which is effective in preventing microglia death induced by the neuroinflammation [Citation22] ().
MST1-JNK signaling pathway is regulated by the various factors, such as small G protein [Citation79], nuclear receptor-associated protein 1 (NURR1) [Citation80], and hydrogen sulfide (H2S) [Citation81]. These factors activate MST1-JNK signaling pathway; meanwhile, melatonin/irisin co-treatment [Citation75], Suramin [Citation74], and Tat-SynGAP (670-685aa) inhibit the activation of this pathway [Citation79]. It is suggested that small G protein is involved in the regulation of the tissue damage via promoting the activation of MST1-JNK signaling pathway [Citation79]. In addition, NURR1 promotes the activation of MST1-JNK-mPTP pathway to decrease the mitochondrial membrane potential, which promotes the opening of mPTPs, and increases the oxidative stress, which results in the myocardial cell necrosis [Citation80]. Exposure of H2S causes outbreak of reactive oxygen species (ROS) to activate JNK-MST1-Foxo1 pathway to aggravate the inflammatory damage [Citation81]. Meanwhile, Tat-SynGAP inhibits MST1-JNK signaling pathway by inhibiting the cleavage of caspase-3 and the phosphorylation of MST1 or JNK, and promoting the expression of angiogenesis-related molecules VEGF, Ang-l to reduce the apoptosis of the neuronal and the volume of the cerebral infarction to maintain the stability of the vascular and the integrity of BBB [Citation79]. Moreover, the Melatonin/irisin co-treatment significantly inhibits MST1-JNK pathway to prevent the death of the myocardial cells [Citation75]. It is reported that the expression of MST1 aggravates LPS-mediated hepatocytes death [Citation74], meanwhile the loss of MST1 weakens LPS-mediated mitochondrial damage [Citation74,Citation82]. In addition, Suramin reduces the expression of MST1 by inhibiting JNK-MST1 signaling pathway to inhibit the mitochondrial oxidative stress and block the caspase-9-mediated mitochondrial apoptosis to exert anti-apoptotic or anti-inflammatory effects and maintain the viability of the liver cells [Citation74]. Accordingly, the activation of MST1-JNK pathway aggravates the inflammatory injury [Citation22] and the inflammatory injury is significantly improved after this pathway being inhibited [Citation75,Citation79] ().
Figure 5. The regulation of MST1-JNK pathway.
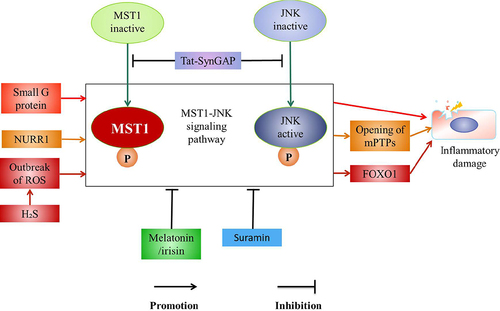
MST1-NF-κB signaling pathway
The nuclear factor-κB (NF-κB) pathway plays an important role in the inflammation [Citation69]. MST1 presents a negative feedback regulation effect on NF-κB pathway induced by LPS [Citation10]. It is reported that MST1 negatively regulates NF-κB signaling pathway mediated by TLR4 in the process of the macrophage activation to reduce the production of the pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α [Citation2,Citation10]. In addition, leonurine (LNR) inhibits NF-κB signaling pathway by up-regulating the expression of MST1 to inhibit LPS-induced trophoblastic inflammation response [Citation83]. Paradoxically, MST1 promotes the inflammatory response and the mitochondrial dysfunction directly or by activating NF-κB signaling pathway in the process of aneurysm formation, finally to aggravate the inflammatory damage [Citation84]. It seems that the effect of MST1 on NF-κB signaling pathway is bidirectional.
MST1-mROS signaling pathway
MST1-mROS signaling pathway is related to the inflammation and the death of the myocardial cells [Citation18]. With I/R and hypoxia-reoxygenation (HR) injury, the transcription of Interleukin-2 (IL-2), IL-8, and TNF-α rapidly increase [Citation18]. In addition, GSH, GPX, and SOD are rapidly downregulated [Citation18]. These alterations are abolished by SRV2 deletion [Citation18]. The levels of SRV2 in the myocardial cells increase after I/R and HR injury to active MST1-mROS signaling pathway through up-regulating MST1 and mROS, which aggravates the inflammation of the cardiomyocytes and promotes the death of the cardiomyocytes [Citation18].
MST1-Foxo3 signaling pathway
It is suggested that LPS increases the expression of MST1 and p-Foxo3 protein to active MST1-Foxo3 signaling pathway, which causes the inflammatory reaction, leading to the apoptosis of hippocampal neurons [Citation85]. Tanshinol (TSL) inhibits the expression of MST1 and p-Foxo3 to improve working memory by significantly inhibiting the levels of IL-1β, IL-6, and TNF-α in the plasma and hippocampus [Citation85]. Accordingly, the activation of MST1-Foxo3 pathway aggravates the inflammatory injury and the inflammatory injury is significantly improved after this pathway being inhibited.
Signaling pathways of MST1/2 related to immunity
MST1/2-rac signaling pathway
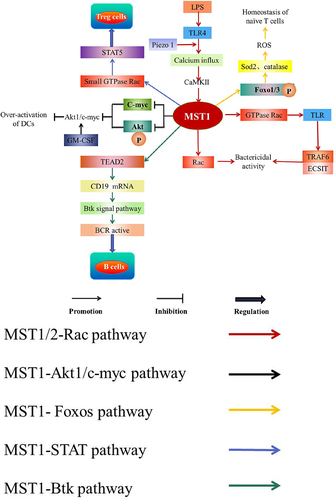
TLR4 augments the bactericidal activity of the macrophages through the mechanical sensor Piezo 1; meanwhile, LPS stimulates TLR4 to induce calcium influx mediated by Piezo 1 to active CaMKII-MST1/2-Rac pathway to ingest and kill the pathogens, thus driving the innate immune response of the microbial infection [Citation32]. In addition, MST1/2 promotes TLR to trigger the assembly of TRAF6-ECSIT (pathway mediator) complex by activating GTPase Rac, leading to the recruitment of mitochondria to phagosomes and increase of mROS, which finally kills the intracellular bacteria [Citation86] (). Accordingly, MST1 is involved in TLR-MST1/2-Rac signal axis [Citation86] and CaMKII-MST1/2-Rac axis [Citation32] to augment the bactericidal activity.
Figure 6. Pathways of MST1 related to immunity.
MST1-Akt1/c-myc pathway
MST1 inhibits over-activation of DCs [Citation87]. It is reported that MST1 inhibits the phosphorylation of Akt1 and c-myc protein levels to down-regulate Akt1/c-myc axis response to granulocyte-monocyte colony-stimulating factor (GM-CSF) to inhibit the over-activation of DCs [Citation87] (). In addition, the absence of MST1 in DCs leads to increase the expression of the cell surface molecules such as B7 and MHC class II and the pro-inflammatory cytokines such as IL-23, TNF-α, and IL-12p40, indicating that MST1-deficiency may induce the hyperactivation of DCs [Citation87].
MST1-Foxos signaling pathway
MST1-Foxos signaling pathway is essential for naïve T cells to survive [Citation49]. MST1 phosphorylates and activates Foxo1/3 in the peripheral blood T cells of the mice, which up-regulates the targets of Foxos such as superoxide dismutase 2 (Sod2) and catalase to maintain the levels of ROS and protect naïve T cells from oxidative stress, thereby maintaining the peripheral homeostasis of naïve T cells [Citation2,Citation49] (). Meanwhile, the inactivation of MST1 inhibits the phosphorylation of Foxos to inhibit the downstream signaling pathway of MST1-Foxos [Citation88], which finally affects the peripheral homeostasis of naïve T cells [Citation49].
MST1-STAT signaling pathway
MST1 is a signal-dependent amplifier of IL-2-STAT5 activity in Treg cells [Citation53]. IL-2 signaling is considered a major regulator for controlling the homeostasis and function of Treg cells [Citation89,Citation90]. MST1/2 in Treg cells activates the small GTPase Rac to active STAT5 under the action of IL-2, which maintains the subpopulation of highly inhibited phosphorylated-STAT5+Treg cells and the pool of stable Treg cells [Citation53] (). Recently, studies discover a highly suppressive p-STAT5+ Treg cell subpopulation is crucial for the suppression of autoreactive T cells and incipient autoimmunity [Citation91].
MST1-Btk signaling pathway
MST1 up-regulates the messenger RNA levels of CD19 by regulating TEAD2, which mediates Btk signaling pathway activating BCR signaling to regulate MZ and GC B cells [Citation61] (). With MST1 deficient, the expression of CD19 which maintains the normal function of B cells is decreased through Btk signaling pathway [Citation61]. In addition, the deficiency of MST1 leads to the increase of YAP expression and the collection of YAP in the nucleus [Citation61]. Moreover, YAP binds and activates TEAD2 to inhibit the expression of CD19, which inhibits the activity of BCR signal and weakens the function of MST1 to regulate B cells [Citation61] ().
MST1/2 and upstream regulatory factors
MST1/2 is activated undergo homodimerization or inactivation undergo heterodimerization through SARAH domain contained in non-catalytic polypeptides Rassf1–6 or Sav1/WW45 [Citation5].
There are a variety of factors that regulates the activity of MST1, such as Poly(RC) binding protein 2 (PCBP2) [Citation71], Rassf5 (in vivo) [Citation92], and immune kinase protein kinase R (PKR) [Citation93]. PCBP2 specifically interacts with scaffold protein SAV1 to prevent proteolytic cleavage of MST1 [Citation71]. PKR assembles and phosphorylates MST1 by Exo84 exocyst under the viral infection to activate MST1 [Citation93]. In addition, Rassf is a key regulator of MST1/2 [Citation94], it belongs to RAS-association (RA) domain family [Citation95]. It is reported that Rassf1–6 binds MST1 and regulates the activity of MST1 [Citation95]. Rassf5 activates MST1/2 in the vivo [Citation92] (). In vivo, membrane-anchored Ras dimer (or nanoclusters) promotes the heterodimerization of SARAH domain [Citation96], meanwhile the homodimerized domain of MST1/2 is exposed and trans-autophosphorylated to be activated [Citation96,Citation97], which is consistent with the fact that the expression of MST1 in Treg cells decreases after Rassf5C being hypermethylated [Citation42]. However, the membrane is lack and the homodimerization and the trans-autophosphorylation of MST1/2 domain is absent in vitro, thus Rassf5 inhibiting MST1/2 [Citation97].
MST1/2 regulatory factors related to inflammation
MST1 and TNF-α
The expression of MST1 increases rapidly under the influence of TNF-α in the vitro [Citation98]. In the presence of TNF-α, knocking out MST1 weakens the mitochondrial dysfunction, reduces the cell oxidative stress, increases the cell vitality, and significantly inhibits TNF-α-induced inflammatory neuronal death [Citation98]. However, in a TNF-α-induced inflammatory environment, the up-regulation of MST1 activates JNK pathway, leading to the mitochondrial homeostasis imbalance, the cell viability decrease, and the neuron death [Citation98]. Accordingly, the simultaneous action of MST1 and TNF-α aggravates the inflammation, TNF-α cannot aggravate the injury with MST1 deficient [Citation98].
MST1 and MIEF1
The inflammatory injury increases the levels of MST1 and the mitochondrial elongation factor 1 (MIEF1) to active MST1-MIEF1 pathway leading to the activation of ROS-related oxidative damage, the dysfunction of the mitochondria, and the initiation of the caspase-involved cell death [Citation99]. Melatonin effectively inhibits MST1-MIEF1 axis and maintains the function of the mitochondrial to increase the survival rates of cells [Citation99]. Accordingly, the activation of MST1-MEF1 axis damages the mitochondrial function and aggravates the inflammatory injury.
MST1/2 regulatory factors related to immunity
MST1 and LFA-1
MST1 plays an important role in the recognition of lymphocyte function-related antigen 1 (LFA-1, also named to αLβ2 integrin) and its ligand intercellular adhesion molecule-1 (ICAM-1) in the medulla [Citation100]. LFA-1 and ICAM-1 mediate the antigen-specific adhesion between T cells and antigen-presenting cells (APC) during the formation of the immune synapse (IS) [Citation100]. The interaction between T cells and APC is impaired with MST1 is deficient [Citation100]. In addition, MST1 regulates spatial distribution of LFA-1 and promotes the transmission of LFA-1 to the leading edge of lymphocytes [Citation101] after chemokines stimulating T lymphocytes [Citation63].
Conclusion
MST1/2 plays an important role in the inflammation and the immunity. MST1/2 directly or indirectly aggravates the inflammatory injury and inhibits MST1/2 improve the prognosis of the inflammatory injury. MST1/2 may be considered a potential therapeutic target for the inflammatory injury of the body. However, MST1/2 may be a factor to alleviate the inflammatory damage in some cases. The reason for the difference of MST1/2 effect on the inflammation is still unclear. Meanwhile, MST1/2 is involved in regulating the innate and adaptive immunity and balancing the immune activation and tolerance to prevent the autoimmune inflammation and maintain the immune function. Patients with MST1/2 deficiency present susceptibility to the autoimmune diseases and the immunodeficiency syndrome.
MST1/2 is widely involved in the regulation of the inflammatory signaling pathways. The activation of MST1-JNK, MST1-mROS, and MST1-Foxo3 signaling pathways aggravates the inflammatory injury. MST1 negatively regulates NF-κB signaling pathway to reduce the inflammatory injury in the process of the macrophage activation. Paradoxically, MST1 activates NF-κB signaling pathway to aggravate the inflammatory injury in the process of aneurysm formation. The reason why MST1 shows two opposite effects on NF-κB signaling pathway is not yet clear.
MST1 maintains normal immune function via regulating immune-related signaling pathways to maintain the immune tolerance and prevent the autoimmune diseases. MST1 activates MST1/2-Rac axis to maintain effective bactericidal activity. In addition, MST1 inhibits over-activation of DCs through inhibiting Akt1/c-myc pathway. Moreover, MST1 activates Foxo1/3 pathway to maintain the peripheral homeostasis of naïve T cells and activates Bkt signal pathway to promote the development and activation of B cells.
Multiple factors are involved in the regulation of MST1/2 activity. PCBP2, Rassf5, and PKR activate MST1. In addition, TNF-α, MIEF1, and LPS promote MST1-mediated inflammation. Meanwhile, MST1 promotes antigen-specific adhesion by regulating LFA-1 and ICAM-1 in the immune response.
Taken together, MST1 is a therapeutic target for the inflammation and the immunity.
Ethics approval
This is a review. The First Affiliated Hospital of Kunming Medical University Research Ethics Committee has confirmed that no ethical approval is required.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data are presented in this review.
Additional information
Funding
References
- Chu P, He L, Xiong L, et al. Molecular cloning, expression analysis and localization pattern of the MST family in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunology. 2018;76:316–323. doi: 10.1016/j.fsi.2018.03.021
- Shi Z, Zhou Z. MST kinases in innate immune signaling. Cell Stress. 2017;21(1):4–13. doi: 10.15698/cst2018.01.119
- Creasy CL, Chernoff J. Cloning and characterization of a human protein kinase with homology to Ste20. J Biol Chem. 1995;27037:21695–21700. doi: 10.1074/jbc.270.37.21695
- Manning G, Whyte DB, Martinez R, et al. The protein kinase complement of the human genome. Science. 2002;2985600:1912–1934. doi: 10.1126/science.1075762
- Araiza-Olivera D, Chernoff J. Hras helps hippo heterodimerize to evade tumor suppression. Small GTPases. 2018;94:327–331. doi: 10.1080/21541248.2016.1228794
- Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;185:311–321. doi: 10.1016/j.cub.2008.02.006
- Abukar Y, Ramchandra R, Hood SG, et al. Increased cardiac sympathetic nerve activity in ovine heart failure is reduced by lesion of the area postrema, but not lamina terminalis. Basic Res Cardiol. 2018;1135:35. doi: 10.1007/s00395-018-0695-9
- Gorick CM, Sheybani ND, Curley CT, et al. Listening in on the microbubble crowd: advanced acoustic monitoring for improved control of Blood-brain barrier opening with focused ultrasound. Theranostics. 2018;811:2988–2991. doi: 10.7150/thno.26025
- Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;115:220–230. doi: 10.1016/S0962-8924(01)01980-8
- Roh KH, Lee Y, Yoon JH, et al. TRAF6-mediated ubiquitination of MST1/STK4 attenuates the TLR4-NF-κB signaling pathway in macrophages. Cell Mol Life Sci. 2021;785:2315–2328. doi: 10.1007/s00018-020-03650-4
- Shang X, Zhang Y, Xu J, et al. SRV2 promotes mitochondrial fission and Mst1-Drp1 signaling in LPS-induced septic cardiomyopathy. Aging. 2020;122:1417–1432. doi: 10.18632/aging.102691
- Zhang P, Wang T, Zhang D, et al. Exploration of MST1-mediated secondary brain injury induced by Intracerebral Hemorrhage in rats via Hippo signaling pathway. Transl Stroke Res. 2019;10:729–743. doi: 10.1007/s12975-019-00702-1
- Abdollahpour H, Appaswamy G, Kotlarz D, et al. The phenotype of human STK4 deficiency. Blood. 2012;11915:3450–3457. doi: 10.1182/blood-2011-09-378158
- Du X, Shi H, Li J, et al.Mst1/Mst2 regulate development and function of regulatory T cells through modulation of Foxo1/Foxo3 stability in autoimmune disease. J Immunology (Baltimore, Md1950). 2014;1924:1525–1535. doi:10.4049/jimmunol.1301060.
- Creasy CL, Ambrose DM, Chernoff J. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J Biol Chem. 1996;27135:21049–21053. doi: 10.1074/jbc.271.35.21049
- Praskova M, Khoklatchev A, Ortiz-Vega S, et al. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J. 2004;381Pt(2):453–462. doi: 10.1042/BJ20040025
- Koehler TJ, Tran T, Weingartner KA, et al. Kinetic Regulation of the Mammalian Sterile 20-like Kinase 2 (MST2). Biochemistry. 2022;61(16):1683–1693. doi: 10.1021/acs.biochem.2c00022
- Zhang J, Zhang F. Suppressor of ras val-2 promotes inflammation-mediated oxidative stress and cell apoptosis in cardiomyocytes through activating Mst1-mROS signaling pathway. J Recept Signal Transduct Res. 2020;403:224–230. doi: 10.1080/10799893.2020.1726953
- Tian Y, Song H, Jin D, et al. MST1-Hippo pathway regulates inflammation response following myocardial infarction through inhibiting HO-1 signaling pathway. J Recept Signal Transduct Res. 2020;403:231–236. doi: 10.1080/10799893.2020.1726954
- Randriamboavonjy V, Kyselova A, Elgheznawy A, et al. Calpain 1 cleaves and inactivates prostacyclin synthase in mesenteric arteries from diabetic mice. Basic Res Cardiol. 2017;1121:10. doi: 10.1007/s00395-016-0596-8
- Wang X, Song Q. Mst1 regulates post-infarction cardiac injury through the JNK-Drp1-mitochondrial fission pathway. Cell Mol Biol Lett. 2018;23:21. doi: 10.1186/s11658-018-0085-1
- Zhou D, Jiang Y. Sirtuin 3 attenuates neuroinflammation-induced apoptosis in BV-2 microglia. Aging. 2019;1120:9075–9089. doi: 10.18632/aging.102375
- Shang X, Li J, Yu R, et al. Sepsis-related myocardial injury is associated with Mst1 upregulation, mitochondrial dysfunction and the Drp1/F-actin signaling pathway. J Mol Hist. 2019;50:91–103. doi: 10.1007/s10735-018-09809-5
- Zhao HX, Zhang Z, Zhou HL, et al. Exercise training suppresses Mst1 activation and attenuates myocardial dysfunction in mice with type 1 diabetes. Can J Physiol Pharmacol. 2020;9811:777–784. doi: 10.1139/cjpp-2020-0205
- Liu Y, Chu G, Shen W, et al. XMU-MP-1 protects heart from ischemia/reperfusion injury in mice through modulating Mst1/AMPK pathway. Eur J Pharmacol. 2022;919:174801. doi: 10.1016/j.ejphar.2022.174801
- Wang P, Zhang Y, Xia Y, et al. MicroRNA-139-5p promotes functional recovery and reduces pain hypersensitivity in mice with spinal cord injury by targeting mammalian Sterile 20-like kinase 1. Neurochem Res. 2021;462:349–357. doi: 10.1007/s11064-020-03170-4
- Wang PF, Xu DY, Zhang Y, et al. Deletion of mammalian sterile 20-like kinase 1 attenuates neuronal loss and improves locomotor function in a mouse model of spinal cord trauma. Mol Cell Biochem. 2017;4311-2:11–20. doi: 10.1007/s11010-017-2969-1
- Song H, Wang M, Xin T. Mst1 contributes to nasal epithelium inflammation via augmenting oxidative stress and mitochondrial dysfunction in a manner dependent on Nrf2 inhibition. J Cell Physiol. 2019;23412:23774–23784. doi: 10.1002/jcp.28945
- Chen L. Non-canonical Hippo signaling regulates immune responses. Adv Immunol. 2019;144:87–119.
- Xu C, Wang L, Zhang Y, et al. Tubule-Specific Mst1/2 Deficiency Induces CKD via YAP and Non-YAP Mechanisms. J Am Soc Nephrol. 2020;315:946–961. doi: 10.1681/ASN.2019101052
- Zhou X, Li WY, Wang HY. The roles and mechanisms of MST1/2 in the innate immune response. Yi Chuan. 2017;397:642–649.
- Geng J, Shi Y, Zhang J, et al. TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection. Nat Commun. 2021;121:3519. doi: 10.1038/s41467-021-23683-y
- De Souza PM, Kankaanranta H, Michael A, et al. Caspase-catalyzed cleavage and activation of Mst1 correlates with eosinophil but not neutrophil apoptosis. Blood. 2002;999:3432–3438. doi: 10.1182/blood.V99.9.3432
- Kurz AR, Pruenster M, Rohwedder I, et al. MST1-dependent vesicle trafficking regulates neutrophil transmigration through the vascular basement membrane. J Clin Investig. 2016;12611:4125–4139. doi: 10.1172/JCI87043
- Du X, Wen J, Wang Y, et al. Hippo/Mst signalling couples metabolic state and immune function of CD8α(+) dendritic cells. Nature. 2018;5587708:141–145. doi: 10.1038/s41586-018-0177-0
- Li C, Bi Y, Li Y, et al. Dendritic cell MST1 inhibits Th17 differentiation. Nat Commun. 2017;8(1):14275. doi: 10.1038/ncomms14275
- Tomiyama T, Ueda Y, Katakai T, et al. Antigen-specific suppression and immunological synapse formation by regulatory T cells require the Mst1 kinase. PLoS One. 2013;8:e73874. doi: 10.1371/journal.pone.0073874
- Katagiri K, Katakai T, Ebisuno Y, et al. Mst1 controls lymphocyte trafficking and interstitial motility within lymph nodes. EMBO J. 2009;289:1319–1331. doi: 10.1038/emboj.2009.82
- Hong L, Li X, Zhou D, et al. Role of Hippo signaling in regulating immunity. Cell Mol Immunol. 2018;1512:1003–1009. doi: 10.1038/s41423-018-0007-1
- E JS, Al-Mousawi A, Assing K, et al. STK4 deficiency impairs innate immunity and Interferon production through Negative regulation of TBK1-IRF3 signaling. J Clin Immunol. 2021;411:109–124. doi: 10.1007/s10875-020-00891-7
- Park E, Kim MS, Song JH, et al. MST1 deficiency promotes B cell responses by CD4(+) T cell-derived IL-4, resulting in hypergammaglobulinemia. Biochem Biophys Res Commun. 2017;4891:56–62. doi: 10.1016/j.bbrc.2017.05.094
- Fukuhara T, Tomiyama T, Yasuda K, et al. Hypermethylation of MST1 in IgG4-related autoimmune pancreatitis and rheumatoid arthritis. Biochem Biophys Res Commun. 2015;4634:968–974. doi: 10.1016/j.bbrc.2015.06.043
- Tang H, Guo Z, Tang X, et al. MST1 modulates Th17 activation in psoriasis via regulating TLR4-NF-κB pathway. Hum Cell. 2021;341:28–36. doi: 10.1007/s13577-020-00423-w
- Galan JA, Avruch J. MST1/MST2 Protein Kinases: Regulation and Physiologic Roles. Biochemistry. 2016;5539:5507–5519. doi: 10.1021/acs.biochem.6b00763
- Tang F, Gill J, Ficht X, et al. The kinases NDR1/2 act downstream of the Hippo homolog MST1 to mediate both egress of thymocytes from the thymus and lymphocyte motility. Sci Signaling. 2015;8397:ra100. doi: 10.1126/scisignal.aab2425
- Ueda Y, Katagiri K, Tomiyama T, et al. Mst1 regulates integrin-dependent thymocyte trafficking and antigen recognition in the thymus. Nat Commun. 2012;3(1):1098. doi: 10.1038/ncomms2105
- Zhou D, Medoff BD, Chen L, et al. The Nore1B/Mst1 complex restrains antigen receptor-induced proliferation of naïve T cells. Proc Natl Acad Sci, USA. 2008;10551:20321–20326. doi: 10.1073/pnas.0810773105
- Ueda Y, Kondo N, Kinashi T. MST1/2 balance immune activation and tolerance by orchestrating adhesion, transcription, and Organelle Dynamics in lymphocytes. Front Immunol. 2020;11:733. doi: 10.3389/fimmu.2020.00733
- Choi J, Oh S, Lee D, et al. Mst1-FoxO signaling protects naïve T lymphocytes from cellular oxidative stress in mice. PLoS One. 2009;4:e8011. doi: 10.1371/journal.pone.0008011
- Li J, Du X, Shi H, et al. Mammalian Sterile 20-like kinase 1 (Mst1) enhances the stability of Forkhead box P3 (Foxp3) and the function of regulatory T cells by modulating Foxp3 acetylation. J Biol Chem. 2015;29052:30762–30770. doi: 10.1074/jbc.M115.668442
- Braga TL, Pinto SR, Dos Reis SRR, et al. Octreotide nanoparticles showed affinity for in vivo MIA paca-2 inducted pancreas ductal adenocarcinoma mimicking pancreatic polypeptide-secreting tumor of the distal pancreas (PPoma). Pharm Res. 2019;3610:143. doi: 10.1007/s11095-019-2678-4
- van Loosdregt J, Coffer PJ. Post-translational modification networks regulating FOXP3 function. Trends Immunol. 2014;358:368–378. doi: 10.1016/j.it.2014.06.005
- Shi H, Liu C, Tan H, et al. Hippo kinases Mst1 and Mst2 sense and Amplify IL-2R-STAT5 signaling in regulatory T cells to establish stable regulatory activity. Immunity. 2018;495:899–914.e896. doi: 10.1016/j.immuni.2018.10.010
- Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236(1):219–242. doi: 10.1111/j.1600-065X.2010.00923.x
- Geng J, Yu S, Zhao H, et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of T(H)17 cells and T(reg) cells. Nat Immunol. 2017;187:800–812. doi: 10.1038/ni.3748
- Nehme NT, Schmid JP, Debeurme F, et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood. 2012;11915:3458–3468. doi: 10.1182/blood-2011-09-378364
- Crequer A, Picard C, Patin E, et al. Inherited MST1 deficiency underlies susceptibility to EV-HPV infections. PLoS One. 2012;7:e44010. doi: 10.1371/journal.pone.0044010
- Alsufyani F, Mattoo H, Zhou D, et al. The Mst1 kinase is required for follicular B cell homing and B-1 B cell development. Front Immunol. 2018;9:2393. doi: 10.3389/fimmu.2018.02393
- Huang L, Sun X, Yang D, et al. WASP and Mst1 coregulate B-cell development and B-cell receptor signaling. Blood Adv. 2020;43:573–585. doi: 10.1182/bloodadvances.2018027870
- Yang L, Li N, Yang D, et al. CCL2 regulation of MST1-mTOR-STAT1 signaling axis controls BCR signaling and B-cell differentiation. Cell Death Diff. 2021;289:2616–2633. doi: 10.1038/s41418-021-00775-2
- Bai X, Huang L, Niu L, et al. Mst1 positively regulates B-cell receptor signaling via CD19 transcriptional levels. Blood Adv. 2016;13:219–230. doi: 10.1182/bloodadvances.2016000588
- Bagherzadeh Yazdchi S, Witalis M, Meli AP, et al. Hippo pathway kinase Mst1 is required for long-lived humoral immunity. J Immunol (Baltimore, Md : 1950). 2019;2021:69–78. doi: 10.4049/jimmunol.1701407.
- Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol. 2006;7:919–928. doi: 10.1038/ni1374
- Dang TS, Willet JD, Griffin HR, et al. Defective leukocyte adhesion and chemotaxis contributes to Combined immunodeficiency in Humans with autosomal recessive MST1 deficiency. J Clin Immunol. 2016;36:117–122. doi: 10.1007/s10875-016-0232-2
- Dong Y, Du X, Ye J, et al. A cell-intrinsic role for Mst1 in regulating thymocyte egress. J Immunol (Baltimore, Md. : 1950). 2009;1836:3865–3872. doi: 10.4049/jimmunol.0900678.
- Lu J, Hu Z, Deng Y, et al. MEKK2 and MEKK3 orchestrate multiple signals to regulate Hippo pathway. J Biol Chem. 2021;296:100400. doi: 10.1016/j.jbc.2021.100400
- Yuan L, Li ZW. Research advances in the role of the Hippo signaling pathway in pathogenesis of liver cancer]. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese J Hepat. 2017;2510:797–800.
- Bouchard A, Witalis M, Chang J, et al. Hippo signal transduction Mechanisms in T cell immunity. Immune Netw. 2020;205:e36. doi: 10.4110/in.2020.20.e36
- Lee IY, Lim JM, Cho H, et al. MST1 negatively regulates TNFα-induced NF-κB signaling through modulating LUBAC activity. Molecular Cell. 2019;736:1138–1149.e1136. doi: 10.1016/j.molcel.2019.01.022
- Wang Y, Jia A, Cao Y, et al. Hippo kinases MST1/2 regulate immune cell Functions in cancer, infection, and autoimmune diseases. Crit Rev Eukaryot Gene Expr. 2020;305:427–442. doi: 10.1615/CritRevEukaryotGeneExpr.2020035775
- Li F, Bullough KZ, Vashisht AA, et al. Poly(rc)-binding protein 2 regulates Hippo signaling to control growth in breast epithelial cells. Mol Cell Biol. 2016;3616:2121–2131. doi: 10.1128/MCB.00104-16
- Maille E, Brosseau S, Hanoux V, et al. MST1/Hippo promoter gene methylation predicts poor survival in patients with malignant pleural mesothelioma in the IFCT-GFPC-0701 MAPS phase 3 trial. Br J Cancer. 2019;1204:387–397. doi: 10.1038/s41416-019-0379-8
- Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;2214:1962–1971. doi: 10.1101/gad.1664408
- Wang A, Wang J, Wu J, et al. Suramin protects hepatocytes from LPS-induced apoptosis by regulating mitochondrial stress and inactivating the JNK-Mst1 signaling pathway. J Physiol Sci. 2019;693:489–502. doi: 10.1007/s12576-019-00666-9
- Ouyang H, Li Q, Zhong J, et al. Combination of melatonin and irisin ameliorates lipopolysaccharide-induced cardiac dysfunction through suppressing the Mst1-JNK pathways. J Cell Physiol. 2020;23510:6647–6659. doi: 10.1002/jcp.29561
- Jin Q, Li R, Hu N, et al. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 2018;14:576–587. doi: 10.1016/j.redox.2017.11.004
- Zhou H, Hu S, Jin Q, et al. Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-Mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP Opening. J Am Heart Assoc. 2017;63. doi: 10.1161/JAHA.116.005328
- Zhao Z, Lu C, Li T, et al. The protective effect of melatonin on brain ischemia and reperfusion in rats and humans: in vivo assessment and a randomized controlled trial. J Pineal Res. 2018;654:e12521. doi: 10.1111/jpi.12521
- Yang H, Liu Z, Liu X, et al. Tat-SynGAP improves angiogenesis and post-stroke recovery by inhibiting MST1/JNK signaling. Brain Res Bull. 2022;180:38–45. doi: 10.1016/j.brainresbull.2021.12.013
- Ma G, Liu Y. NURR1 inhibition reduces hypoxia-mediated cardiomyocyte necrosis via blocking Mst1-JNK-mPTP pathway. J Recept Signal Transduct Res. 2019;394:350–358. doi: 10.1080/10799893.2019.1690514
- Chi Q, Hu X, Liu Z, et al. H(2)S exposure induces cell death in the broiler thymus via the ROS-initiated JNK/MST1/FOXO1 pathway. Ecotoxicol Environ Saf. 2021;222:112488. doi: 10.1016/j.ecoenv.2021.112488
- Shang X, Lin K, Zhang Y, et al. Mst1 deletion reduces septic cardiomyopathy via activating Parkin-related mitophagy. J Cell Physiol. 2020;2351:317–327. doi: 10.1002/jcp.28971
- Zong F, Zhao Y. Alkaloid leonurine exerts anti-inflammatory effects via modulating MST1 expression in trophoblast cells. Immun Inflamm Dis. 2021;94:1439–1446. doi: 10.1002/iid3.493
- Lu K, Zhao J, Liu W. Macrophage stimulating 1-induced inflammation response promotes aortic aneurysm formation through triggering endothelial cells death and activating the NF-κB signaling pathway. J Recept Signal Transduct Res. 2020;404:374–382. doi: 10.1080/10799893.2020.1738484
- Yang Y, Wang L, Wu Y, et al. Tanshinol suppresses inflammatory factors in a rat model of vascular dementia and protects LPS-treated neurons via the MST1-FOXO3 signaling pathway. Brain Res. 2016;1646:304–314. doi: 10.1016/j.brainres.2016.06.017
- Geng J, Sun X, Wang P, et al. Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat Immunol. 2015;1611:1142–1152. doi: 10.1038/ni.3268
- Cho KM, Kim MS, Jung HJ, et al. Mst1-deficiency induces hyperactivation of monocyte-Derived dendritic cells via Akt1/c-myc pathway. Front Immunol. 2019;10:2142. doi: 10.3389/fimmu.2019.02142
- Bitra A, Sistla S, Mariam J, et al. Rassf proteins as modulators of Mst1 kinase activity. Sci Rep. 2017;7(1):45020. doi: 10.1038/srep45020
- Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;381:13–25. doi: 10.1016/j.immuni.2013.01.004
- Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;332:153–165. doi: 10.1016/j.immuni.2010.08.004
- Liu Z, Gerner MY, Van Panhuys N, et al. Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature. 2015;5287581:225–230. doi: 10.1038/nature16169
- Liu G, Shi Z, Jiao S, et al. Structure of MST2 SARAH domain provides insights into its interaction with RAPL. J Struct Biol. 2014;1853:366–374. doi: 10.1016/j.jsb.2014.01.008
- Zaman A, Wu X, Lemoff A, et al. Exocyst protein subnetworks integrate Hippo and mTOR signaling to promote virus detection and cancer. Cell Rep. 2021;365:109491. doi: 10.1016/j.celrep.2021.109491
- Ni L, Li S, Yu J, et al. Structural basis for autoactivation of human Mst2 kinase and its regulation by RASSF5. Structure (London, England: 1993). 2013;2110:1757–1768. doi: 10.1016/j.str.2013.07.008.
- Dhanaraman T, Singh S, Killoran RC, et al. RASSF effectors couple diverse RAS subfamily GTPases to the Hippo pathway. Sci Signal. 2020;13. doi: 10.1126/scisignal.abb4778
- Liao TJ, Jang H, Tsai CJ, et al. The dynamic mechanism of RASSF5 and MST kinase activation by Ras. Phys Chem Chem Phys. 2017;199:6470–6480. doi: 10.1039/C6CP08596B
- Liao TJ, Tsai CJ, Jang H, et al. RASSF5: An MST activator and tumor suppressor in vivo but opposite in vitro. Curr Opin Struct Biol. 2016;41:217–224. doi: 10.1016/j.sbi.2016.09.001
- Geng C, Wei J, Wu C. Mammalian STE20-like kinase 1 knockdown attenuates TNFα-mediated neurodegenerative disease by repressing the JNK pathway and mitochondrial stress. Neurochem Res. 2019;447:1653–1664. doi: 10.1007/s11064-019-02791-8
- Lu K, Liu X, Guo W. Melatonin attenuates inflammation-related venous endothelial cells apoptosis through modulating the MST1-MIEF1 pathway. J Cell Physiol. 2019;23412:23675–23684. doi: 10.1002/jcp.28935
- Kondo N, Ueda Y, Kita T, et al. NDR1-dependent regulation of Kindlin-3 controls High-Affinity LFA-1 binding and immune synapse organization. Mol Cell Biol. 2017;378. doi: 10.1128/MCB.00424-16
- Nishikimi A, Ishihara S, Ozawa M, et al. Rab13 acts downstream of the kinase Mst1 to deliver the integrin LFA-1 to the cell surface for lymphocyte trafficking. Sci Signaling. 2014;7336:ra72. doi: 10.1126/scisignal.2005199
