ABSTRACT
The genus Zaprionus consists of approximately 60 species of drosophilids that are native to the Afrotropical region. The phylogenetic position of Zaprionus within the Drosophilidae family is still unresolved. In the present study, ultrastructural features of spermatozoa of 6 species of Zaprionus as well as the species Drosophila willistoni and Scaptodrosophila latifasciaeformis were analyzed. The ultrastructure revealed that the species have the same flagellar ultrastructure. Two mitochondrial derivatives, one larger than the other, close to the axoneme were present, primarily in D. willistoni (subgenus Sophophora). Except for Z. davidi and Z. tuberculatus, the analyzed species had paracrystalline material in both mitochondrial derivatives. Moreover, the testes showed 64 spermatozoa per bundle in all of the species. In the cluster analysis, 6 Zaprionus species were grouped closely, but there were some incongruent positions in the cladogram. The results indicated that sperm ultrastructure is an important tool for elucidating the phylogeny and taxonomy of insects.
Introduction
Approximately 150,000 species of flies are described in the world and more than 24,000 species are described in the Neotropics.Citation1 The genus Zaprionus (Diptera, Drosophilidae) consists of approximately 60 species of which about 10 are grouped in the subgenus Anaprionus and 50 in the subgenus Zaprionus.Citation2,3 Phylogenetic relationships within groups and subgroups of drosophilids as well as the phylogenetic position of Zaprionus within the Drosophilidae family are still uncertain.Citation3-8 Although most phylogenetic analyses associate the genus Zaprionus to the subgenus Drosophila, new comparative analyses are needed to test the robustness of this association.Citation3,7,9
Ultrastructural sperm analyses are important tools for study of the taxonomy and phylogeny of insects.Citation10-16 Mojica et al. characterized the primary evolutionary radiation that occurred in the Drosophila tripunctata group based on the ultrastructure of the mitochondrial derivatives and the number of sperm per cysts. The authors highlighted the need for new ultrastructural studies of the gametes of these insects to provide additional clarification of their evolutionary relationships.Citation17
Ultrastructural analyses of sperm in Zaprionus are restricted to the species Zaprionus indianus and Zaprionus sepsoides.Citation18 The authors described important characteristics of these drosophilid gametes, such as the presence of granules in the peritoneal sheath, the presence of 2 mitochondrial derivatives of different sizes, the presence or absence of paracrystalline material in the derivatives, the arrangement of the axoneme, and the number of sperm per cyst.
This study aimed to characterize the ultrastructure of sperm of 6 other species of Zaprionus (Z. africanus, Z. camerounensis, Z. davidi, Z. gabonicus, Z. megalorchis and Z. tuberculatus) and the species Drosophila willistoni (subgenus Sophophora). Scaptodrosophila latifasciaeformis (subgenus Scaptodrosophila) was used as an outgroup (). In addition, data from the literature for other species of Zaprionus and Drosophila were used to help understand the relationships between Zaprionus and Drosophila.
Results and discussion
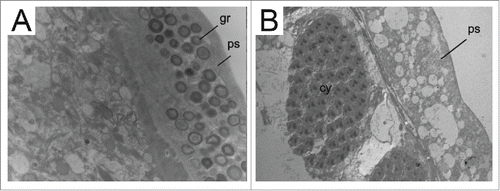
In this paper, intraspecific variation in spermatogenesis was not observed. All species showed globular granules in the cytoplasm of the coating layer of the testicular envelope, called the peritoneal sheath (, Supplementary Figs. 1–6), except for Z. davidi (, Supplementary Fig. 7) and D. willistoni (Supplementary Fig. 8). These pigmented granules are responsible for the color of the peritoneal coating sheath of the testes and for the formation of a physical barrier that can protect the testes and store nutrients.Citation19 Rego et al. detected the presence of glycogen in the composition of these granules. Cruz-Landim has also observed glycogen in the testicles of bees.Citation18,20
Figure 1. TEM micrographs of Zaprionus testes. A. Z. africanus; B. Z. davidi. Note peritoneal sheath (ps) filled with granules (gr) of different sizes and electron densities in Z. africanus and their absence in Z. davidi. Scale: Figure A: 11000 x; Figure B: 10000 x.
The color of the peritoneal sheath of the testes is critically important for taxonomy. In the genus Drosophila, the color is diagnostic to the species level.Citation21 The peritoneal sheaths of the Zaprionus species analyzed in this study and those of D. willistoni and S. latifasciaeformis showed yellowing. Yellowing has also been observed in the sheaths of Z. indianus, Z. sepsoides and Z. spinipilus that were analyzed previously.Citation18,22,23 However, Z. vittiger was polymorphic for sheath color, which may be yellow or brownish purple.Citation23
The sperm of Pterygota (a primitive group of Insecta) has 2 mitochondrial derivatives that flank the axial filament.Citation24 In drosophilids, the mitochondrial derivatives are of different sizes ().Citation10,17,25-28 Mojica et al. used the size of these mitochondrial derivatives as an evolutionary tool to understand the radiation of the genus Drosophila.Citation17 They observed that the 2 derivatives differed in size and that this size difference was greater in Sophophora than in Drosophila. In the present study, although measurements were not taken, the simple observation of prints showed that our results are consistent with those of Mojica et al.: the difference in the size of these mitochondrial derivatives was greater in D. willistoni (which belongs to the Sophophora subgenus) () than in the other species analyzed ( and ). The exception was Z. davidi, which was similar to D. willistoni. Mojica et al. suggested that the relative size of the mitochondrial derivatives may have changed as Drosophila species have evolved.
Figure 2. Ultrastructure of transverse sections of the spermatozoal tail of Z. gabonicus (A) showing the paracrystalline material (p) on both mitochondrial derivatives; the axonemes in D. willistoni (B) and Z. davidi (C) have the arrangement of 9 + 9 + 2 microtubules; the cysts containing 64 spermatozoa in Z. camerounensis (D) and Z. tuberculatus (E). Scale: Figures A, B, C: 84000 x; Figures D, E: 10000 x.
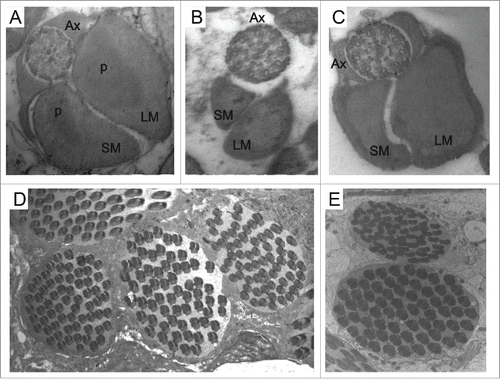
Figure 3. Ultrastructure of transverse section of the spermatozoal tail of S. latifasciaeformis (A), Z. tuberculatus (B) and Z. megalorchis (C) Note the presence of the axoneme (Ax) and 2 mitochondrial derivatives of different sizes: larger mitocondrial derivative (LM), smaller mitochondrial derivative (SM); the accumulation of paracrystalline material (p) is visible in S. latifasciaeformis and Z megalorchis. Scale: Figures A, B, C: 84000 x.
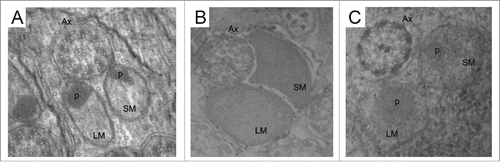
Table 1. Studied species and their geographical origin.
Table 2. Ultrastructural parameters of sperm used for comparisons of species.
Except for Z. davidi and Z. tuberculatus (), the analyzed species have paracrystalline material on both mitochondrial derivatives (). This same characteristic has been observed in Z. indianus, Z. sepsoides and D. hydei.Citation17,18 However, in most species of the genus Drosophila (), the paracrystalline material is present only in the larger mitochondrial derivative.Citation17,29-32
The structure of the sperm axoneme is of great importance for phylogenetic studies in insects.Citation10,14,27 Most species of insects present the ‘9 + 9 + 2’ arrangement, consisting of one pair of central microtubules and 9 double peripheral microtubules, surrounded by 9 additional accessory microtubules,Citation10,27 although some species have a peculiar number, such as 9 + 9 + 3 in Neuroptera,Citation33 9 + 9 + 1 in Culicidae (Diptera),Citation34 9 + 7 in Tricoptera,Citation25 and 9 + 0 in Ephemeroptera.Citation25 Moreover, as the majority of species of the suborder Brachyocera,Citation27 all drosophilids species analyzed had an axoneme structure of the 9 + 9 + 2 configuration, which is the typical arrangement of 9 + 2 internal microtubules surrounded by 9 additional accessories microtubules () ().
Spermiogenesis in all analyzed species occurs within cysts where the sperm are organized and exist at the same developmental stage (). This phenomenon is referred to as cystic spermatogenesis and is characterized by synchronized cell division within a given cyst.Citation35 So far, studies have indicated that all insects have cystic spermatogenesis.Citation36 In Triatominae, the cysts develop independently; that is, a cyst does not influence the developmental stage of neighboring cysts.Citation37 In drosophilids, as described for Plalycentropus (Trichoptera: Limnephilidae),Citation25 we suggest that neighboring cysts are also synchronized for cystic spermatogenesis ( and E).
For all of the species analyzed, we observed the presence of 64 cells inside a cyst ( and E) (). The number of cyst cells varies in some species of Drosophila. In D. dunni, this number varies from 44 to 56 sperm per cyst; in D. cardini, it varies from 36 to 40 sperm per cyst; in D. melanogaster, this fixed number is 64; and in D. subobscura, this number can reach up to 128 sperm per cyst.Citation17,26,28,29
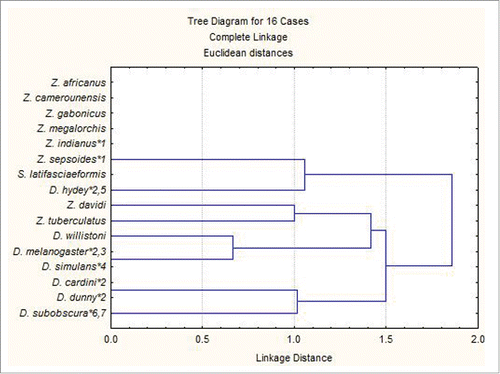
In the cluster analysis, on the basis of the analyzed characteristics, 5 species of Zaprionus as well as 6 species of Drosophila were grouped, suggesting their relatedness within each genus (). Although an increase in the number of characteristics and species is necessary to validate these results, they are already indicative that the ultrastructure of sperm is a promising tool for phylogenetic and taxonomic studies of insect groups.
Materials and methods
The species and strains of Zaprionus and other species used in this study and their geographic location are shown in . The testes of 24 3-day-old adults of each species were processed according to the methods of Cotta-Pereira et al. with modifications.Citation38 Ultrathin sections of 70 nm, contrasted with uranyl acetate and lead citrate, were examined with a transmission electron microscope.
The five ultrastructural parameters of sperm used by Rego et al. were used in this study for comparison between species ().Citation18
A cluster analysis was conducted using the Euclidean distance and joining method (Statistica; Statsoft Inc.) with the data from from which a presence-absence matrix of the characteristics was generated.Citation39
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KFLY_A_1142636_Supplemental.zip
Download Zip (30.5 MB)Acknowledgments
We thank Dr. Jean David (Center National de la Recherche Scientifique (CNRS) / GIF-SUR-Yvete / FRANCE) for providing some of the lines of Zaprionus, Rosana Silistino Souza (Academic Support Assistant II) for technical assistance in sample preparation and Luis Fernando Segala (Assistant Professor) for help with statistical analyses.
Funding
This paper was supported by Fundacao de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP) (Process number 2010/01193–9) and Coordenadoria de Aperfei, coamento do Pessoal do Ensino Superior (CAPES).
References
- Amorim DD, Silva VC, Balbi MI, Costa C, Vanin SA, Lobo JM, Melic A. Estado do conhecimento dos Diptera neotropicais. Proyecto de Red Iberoamericana de Biogeografia y Entomología Sistemática (C. Costa, SA Vanin, JM Lobo, A. Melic, orgs.). Sociedad Entomológica Aragonesa y CYTED, Zaragoza 2002; 2:29-36
- Okada T, Carson HL. The genera Phorticella Duda and Zaprionus Coquillett (Diptera, Drosophilidae) of the Oriental region and New Guinea. Kontyu 1983; 51:539-53
- Yassin A, Araripe LO, Capy P, Da Lage JL, Klaczko LB, Maisonhaute C, Ogereau D, David JR. Grafting the molecular phylogenetic tree with morphological branches to reconstruct the evolutionary history of the genus Zaprionus (Diptera: Drosophilidae) Mol Phylogenet Evol 2008; 47:903-15; PMID:18462955; http://dx.doi.org/10.1016/j.ympev.2008.01.036
- Throckmorton LH. The problem of phylogeny in the genus Drosophila. Univ Texas Publ 1962; 6205:207-343
- Throckmorton LH. The phylogeny, ecology and geography of Drosophila. In: King RC, guest editor. Handbook of Genetics New York: Plenum Press; 1975; pp. 421-469
- De Setta N, Van Sluys MA, Capy P, Carareto CM. Multiple invasions of Gypsy and Micropia retroelements in genus Zaprionus and melanogaster subgroup of the genus drosophila. BMC Evol Biol 2009; 9:e279; http://dx.doi.org/10.1186/1471-2148-9-279
- Yassin A, David JR. Revision of the Afrotropical species of Zaprionus (Diptera, Drosophilidae), with descriptions of two new species and notes on internal reproductive structures and immature stages. ZooKeys 2010; 51(51):33-72; PMID:21594121; http://dx.doi.org/10.3897/zookeys.51.380
- Commar LS, Galego LG, Ceron CR, Carareto CM. Taxonomic and evolutionary analysis of Zaprionus indianus and its colonization of Palearctic and Neotropical regions. Genet Mol Biol 2012; 35(2):395-406; PMID:22888286; http://dx.doi.org/10.1590/S1415-47572012000300003
- Yassin A, Borai F, Capy P, David JR, Elias E, Riad SA, Shalaby HG, Serour S, Abou-Youssef AY. Evolutionary genetics of Zaprionus. II. Mitochondrial DNA and chromosomal variation of the invasive drosophilid Zaprionus indianus in Egypt. Mitochondrial DNA 2009; 20(2-3):34-40; PMID:19444699; http://dx.doi.org/10.1080/19401730902890042
- Jamieson BGM, Dallai R, Afzelius BA. Insects: their spermatozoa and phylogeny Enfield, NH: Science and Publishing House; 1999
- Dallai R, Lombardo BM, Lupetti P. Sperm ultrastructure in Chironomoidea (Insecta, Diptera). Tiss Cell 2007; 39(3):179-94; http://dx.doi.org/10.1016/j.tice.2007.03.003
- Araújo VA, Moreira J, Lino-Neto J. Structure and ultrastructure of the spermatozoa of Trypoxylon (Trypargilum) albitarse Fabricius. (Hymenoptera: Apoidea: Crabonidae 1804). Micron 2009; 40:719-23; PMID:19556139; http://dx.doi.org/10.1016/j.micron.2009.05.003
- Mancini K, Lino-Neto J, Dolder H, Dallai R. Sperm structure of European hornet Vespa crabo (Linnaeus, 1758) (Hymenoptera: Vespidae). Arthropod Struct Dev 2009; 38(1):54-9; PMID:18675936; http://dx.doi.org/10.1016/j.asd.2008.07.001
- Gomes LF, Badke JP, Zama U, Dolder H, Lino-Neto J. Morphology of the male reproductive system and spermatozoa in Centris Fabricius, 1804 (Hymenoptera: Apidae, Centridini). Micron 2012; 43(6):695-704; PMID:22377697; http://dx.doi.org/10.1016/j.micron.2012.01.013
- Araújo VA, Lino-Neto J, Ramalho FS, Zanuncio JC, Serrão JE. Ultrastructure and heteromorphism of spermatozoa in five species of bugs (Pentatomidae: Heteroptera). Micron 2011; 42:560-567; PMID:21376606; http://dx.doi.org/10.1016/j.micron.2011.02.001
- Name KPO, Barros-Cordeiro KB, Filho G, Wolff M, Pujol-Luz JR, Báo SN. Morphological and cytochemical aspects of spermatozoa in the genus cochliomyia (Diptera: Calliphoridae). J Electron Microsc (Tokyo) 2012; 61(6):415-22; PMID:22997238; http://dx.doi.org/10.1093/jmicro/dfs061
- Mojica JM, File-Emperador S, Bruck DL. Sperm bundle and spermatozoon ultrastructure in two species of the cardini group of Drosophila. Invertebr Repr Dev 2000; 37(2):147-55; http://dx.doi.org/10.1080/07924259.2000.9652413
- Rego LNAA, Silistino-Souza R, Azeredo-Oliveira MTVd, Madi-Ravazzi L. Spermatogenesis of Zaprionus indianus and Zaprionus sepsoides (Diptera: Drosophilidae): cytochemical, structural and ultrastructural characterization. Gen Mol Biol 2013; 36(1):50-60; http://dx.doi.org/10.1590/S1415-47572013000100008
- Báo SN, Dolder H. Testicular organization in adult ceratitis capitata (Diptera: Tephritidae): RA mutant and wild-type lineages. Rev Bras Biol 1991; 51:313-9
- Cruz-Landim C. Organization of the cysts in bee (Hymenoptera, Apidae) testis: number of spermatozoa per cyst. Iheringia Sér Zool 2001; 91:183-9; http://dx.doi.org/10.1590/S0073-47212001000200025
- Markow TA, O'Grady PM. Evolutionary genetics of reproductive behavior in Drosophila: connecting the dots. Annu Rev Genet 2005; 39(1):263-91; PMID:16285861; http://dx.doi.org/10.1146/annurev.genet.39.073003.112454
- Araripe LO, Klaczko LB, Moreteau B, David JR. Male sterility thresholds in a tropical cosmopolitan drosophilid, Zaprionus indianus. J Therm Biol 2004; 29(2):73-80; http://dx.doi.org/10.1016/j.jtherbio.2003.11.006
- Yassin A, Amabis JM, Da Lage JL, Debiais-Thibaud M, Davi JR. On the relationship between Zaprionus spinipilus Chassagnard & McEvey and Z. Vittiger Coquillett, the type species of the genus Zaprionus (Diptera: Drosophilidae). Ann Soc Entomol Fr 2010; 46:471-6
- Chapman RF. The insects: structure and function Cambridge: Cambridge University Press; 2012
- Phillips DM. Insect sperm: their structure and morphogenesis. J Cell Biol 1970; 44(2):243-77; PMID:4903810; http://dx.doi.org/10.1083/jcb.44.2.243
- Lindsley DL, Tokuyasu KT. Spermatogenesis. In: Genetics and Biology of Drosophila London: Academy Press 1980; pp. 225-294
- Dallai R, Bellon PL, Vecchia SL, Afzelius BA. The dipteran sperm tail: ultrastructural characteristics and phylogenetic considerations. Zoo Scripta 1993; 22(2):193-202; http://dx.doi.org/10.1111/j.1463-6409.1993.tb00351.x
- Fuller MT. Spermatogenesis. In: Bate M, Martinez-Arias A, guest editors The development of Drosophila melanogaster Cold Spring Harbor, NY: Cold Spring Harbor Press; 1993; pp. 71-147
- Hauschteck-Jungen E, Maurer B. Sperm dysfunction in sex-ratio males of Drosophila subobscura. Genetica 1976; 46(4):459-77; http://dx.doi.org/10.1007/BF00128092
- Ramamurthy G, Alfert M, Stern C. Ultrastructural studies on spermatogenesis in a sex-ratio mutant strain of Drosophila simulans. Am J Anat 1980; 15:205-19; http://dx.doi.org/10.1002/aja.1001570208
- Pasini ME, Redi CA, Caviglia O, Perotti ME. Ultrastructural and cytochemical analysis of sperm dimorphism in Drosophila subobscura. Tiss Cell 1996; 28(2):165-75; http://dx.doi.org/10.1016/S0040-8166(96)80005-X
- Noguchi T, Miller KG. A role for actin dynamics in individualization during spermatogenesis in Drosophila melanogaster. Development 2003; 130:1805-1816; PMID:12642486; http://dx.doi.org/10.1242/dev.00406
- Zizzari ZV, Lupetti P, Mencarelli C, Dallai R. Sperm ultrastructure and spermiogenesis of Coniopterygidae (Neuroptera, Insecta). Arthropod Struct Dev 2008; 37(5):410-7; PMID:18534907; http://dx.doi.org/10.1016/j.asd.2008.03.001
- Justine J, Mattei X. Ultrastructure of the spermatozoon of the mosquito Toxorhynchites (Diptera, Culicidae). Zool Scripta 1988; 17(3):289-91; http://dx.doi.org/10.1111/j.1463-6409.1988.tb00103.x
- Smith EA. Spermatogenesis of the dragon-fly Sympetrum semicinctum (say) with remarks upon Libellula basalis. Biol Bull 1916; 31(4):269-302; http://dx.doi.org/10.2307/1536236
- Dumser JB. The regulation of spermatogenesis in insects. Annu Rev Entomol 1980; 25(1):341-69; http://dx.doi.org/10.1146/annurev.en.25.010180.002013
- Alevi KCC, Castro NFC, Oliveira J, Rosa JA, Azeredo-Oliveira MTV. Cystic spermatogenesis in three species of the prolixus complex (Hemiptera: Triatominae). Ital J Zool 2015; 82:172-8
- Cotta-Pereira G, Rodrigo FG, David-Ferreira JF. The use of tannic acid-glutaraldehyde in the study of elastic and elastic-related fibers. Stain Technol 1976; 51(1):7-11; PMID:59416; http://dx.doi.org/10.3109/10520297609116662
- Statsoft Inc. Statistica, version 7; data analysis software system]; 2004. Available from: www.statsoft.com