ABSTRACT
In this Extra View, we extend our recent work on the protein LIN-28 and its role in adult stem cell divisions. LIN-28 is an mRNA- and microRNA-binding protein that is conserved from worms to humans. When expressed ectopically, it promotes the reprogramming of differentiated vertebrate cells into pluripotent stem cells as well as the regeneration of vertebrate tissues after injury. However, its endogenous function in stem cell populations is less clear. We recently reported that LIN-28 is specifically expressed in progenitor cells in the adult Drosophila intestine and enhances insulin signaling within this population. Loss of lin-28 alters the division patterns of these progenitor cells, limiting the growth of the intestinal epithelium that is ordinarily caused by feeding. Thus, LIN-28 is part of an uncharacterized circuit used to remodel a tissue in response to environmental cues like nutrition. Here, we extend this analysis by reporting that the levels of LIN-28 in progenitor cells are sensitive to nutrient availability. In addition, we speculate about the role of LIN-28 in the translational control of target mRNAs such as Insulin Receptor (InR) and how such translational control may be an important mechanism that underlies the stem cell dynamics needed for tissue homeostasis and growth.
Main Text
The plasticity of adult tissues, their growth, remodeling and regeneration, is fueled by the behavior of adult stem cells. To generate the supply of cells needed for tissue growth, for example, stem cells can increase their division rates and alter their division patterns. After a symmetric renewal division, both daughter cells retain stem cell fate whereas only one cell does after an asymmetric renewal division. The net balance between symmetric and asymmetric divisions in a population of stem cells drives the behavior of cycling tissues: more symmetric division expands the stem cell population, leading to tissue growth, while more asymmetric division maintains the stem cell population, leading to tissue homeostasis.Citation1-3 The cellular factors that allow stem cells to switch between symmetric and asymmetric renewal are critical for tissue growth during development and after injury, but are almost completely unknown.Citation3-6
The fly intestine is an excellent model to study how nutrients are translated into systemic signals that then impact the stem cell dynamics underlying the remodeling and regeneration of particular tissues.Citation7,8 It bears functional homology to the mammalian intestine and contains a population of ISCs whose number shrink and expand in response to food consumption.Citation9-11 Young adults eclose with a founding population of ISCs; this population expands after feeding, enabling the growth of the intestine needed for enhanced nutrient absorption. After a few days of growth, the intestine reaches homeostasis and contains a steady population of ISCs, but this population will shrink if food is withdrawn.Citation9,10 These ISC population dynamics are due to the divisions patterns of individual ISCs, which can symmetrically renew to generate 2 ISCs, asymmetrically renew to generate one ISC and one transient progenitor cell known as an enteroblast (EB), or symmetrically divide to generate 2 EBs.Citation10,12 EB daughters will then directly differentiate into either the absorptive enterocytes (EC) or hormone-producing enteroendocrine cells (EE) that populate the intestine.Citation13,14 How the initial asymmetry between an ISC and EB is triggered is unclear but may involve segregation of aPKC.Citation15 Then, Delta-Notch signaling between the 2 cells ensures that they will adopt divergent fates.Citation13,14,16 Feeding during early adulthood triggers elevated insulin peptide Ilp-3, which is released from the visceral muscle surrounding the midgut,Citation10 suggesting that ISCs drive tissue growth by sensing nutritional cues and dividing symmetrically instead of asymmetrically. However, the responsible mechanism and whether it also underlies injury induced regeneration and/or tissue homeostasis are major open questions.
As entry point into these questions, we reported that the LIN-28 RNA-binding is an ISC/EB-specific modulator of insulin signaling that promotes ISC symmetric renewal and consequently expansion of the ISC population.Citation17 Analyzing a null lin-28 mutant strain, we found that lin-28Δ1 mutants eclosed with a normal number of ISCs but that this number did not increase even after feeding and remained constant through the first 3 weeks of adulthood. To specifically assess ISC dynamics in response to food consumption, we compared ISC numbers in animals that had been starved or fed for 3 hours; in control animals, feeding led to a rapid increase in ISC division rate and symmetric renewal and a consequent doubling of ISC number. In contrast, these food-triggered changes in ISC behavior were absent in lin-28Δ1 mutants. These results indicated that LIN-28 was not required for stem cell identity per se; instead, LIN-28 poises ISCs to divide symmetrically in response to nutrient availability.
LIN-28 expression is nutrient sensitive
These observations suggested that LIN-28 activity is sensitive to nutrients and might be dampened once the intestine reaches homeostasis after a few days of feeding. To investigate this possibility, we evaluated LIN-28 expression levels and subcellular distribution in 5-day old starved and fed animals using a previously described lin-28 rescuing transgene that encodes a functional version of LIN-28 tagged with Venus.Citation17 While the general distribution of LIN-28::Venus throughout the cytoplasm of progenitor cells appeared similar under these conditions, the levels of LIN-28::Venus appeared elevated in starved intestines (). To quantify this difference, we first measured the average area of intestinal progenitors and found that those in fed animals were larger (41.53 ± 13.77 µm2 ; n = 40) than those in starved animals (30.06 ± 8.86 µm2 ; n = 40)(). We then quantified LIN-28::Venus signal intensity and normalized it to cell area to calculate the corrected total cell fluorescence (CTCF) of LIN-28::Venus. The CTCF of LIN-28::Venus in intestinal progenitors under starved conditions was 3657.9 ± 987.7 gray µm2 (n=20), significantly higher than the CTCF of 1935 ± 844.5 gray µm2 under fed conditions (n = 20) (). These results indicated that LIN-28 levels were elevated in progenitor cells in tissue poised for growth relative to tissue at homeostasis.
Figure 1. Lin-28 levels and subcellular distribution are nutrient sensitive. (A) LIN-28::Venus expression in the intestinal progenitor cells of fed (left) and starved (right) animals. Insets are magnified views. LIN-28::Venus was detected with anti-GFP antibodies. (B) LIN-28 expression in the intestinal progenitor cells of fed (left) and starved (right) animals. LIN-28::Venus was detected with anti-LIN-28 antibodies. (C) Scatter dot plot showing the area of Lin-28::Venus expressing progenitor cells in fed vs. starved animals. Mean and standard deviation (SD) are shown. (D) Scatter dot plot of the Corrected Total Cell Fluorescence (CTCF) of Lin-28::Venus expressing cells in fed vs. starved animals. Mean and SD are shown. (E) Scatter dot plot of the CTCF readings from Lin-28 expressing cells in fed vs. starved animals. Mean and SD are shown.
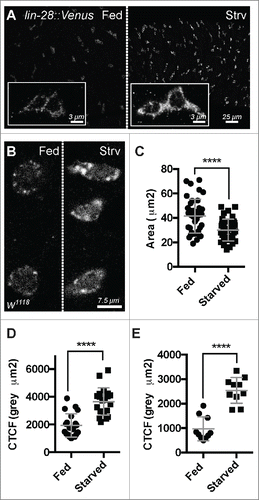
To confirm these results, we also analyzed the level and subcellular distribution of native LIN-28 in fed and starved intestines using anti-LIN-28 antisera (). We found that native LIN-28 protein behaved similarly to LIN-28::VENUS, exhibiting a higher CTCF in nutrient-deprived progenitor cells (2543 ± 527.5 gray µm2 ; n = 10) compared to nutrient-supplied progenitor cells (971 ± 489.9 gray µm2; n = 10) (). In addition, we noted that nutrient-deprivation altered the subcellular distribution of LIN-28. LIN-28 was detected in cytoplasmic puncta, and these puncta appeared substantially larger in progenitors from the intestines of starved animals relative to fed animals (). We suspect the same is true for LIN-28::Venus but that the higher level of LIN-28::Venus signal relative to LIN-28 signal made this difference more difficult to detect.
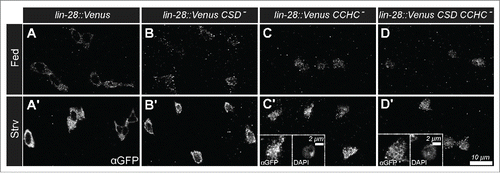
To extend these observations, we also analyzed the nutrient dependent expression and subcellular distribution of variants of LIN-28::Venus with defective RNA-binding activity. LIN-28 encodes 2 RNA binding domains, a N-terminal cold shock domain (CSD) and a C-terminal CysCysHisCys-type zinc finger domain (CCHC). We had previously generated UAS-LIN-28 transgenic stocks that contained point mutation designed to disrupt the activity of these domains, and found that CSD activity was required for an increase in progenitor cell number but that CCHC activity was not.Citation17 To investigate the requirements of these domains in nutrient dependent LIN-28 expression and distribution, we prepared transgenes containing the same mutations in LIN-28::Venus and crossed these into a lin-28Δ1 mutant background. Then, we analyzed LIN-28::Venus expression in the intestines of these strains under fed and starved conditions. We found LIN-28::Venus variants that contained mutations in either the CSD domain or the CCHC domain or both domains displayed elevated levels in progenitor cells under starvation conditions, similar to a wildtype version of the protein (). In addition, we noted that the CCHC mutations and, to a lesser extent, the CSD domain mutations altered the subcellular distribution of LIN-28 under both nutrient-deprived and -supplied conditions. Wildtype LIN-28 is not ordinarily detected in the nucleus of progenitor cells, but the mutant versions were detected in cell nuclei (). This nuclear accumulation was particularly apparent under starved conditions, since LIN-28::Venus levels were elevated (’, D’ insets). These results indicated that the nutrient-dependent change in LIN-28::Venus levels was not due to the RNA-binding activity of LIN-28 that might, for example, stabilize the protein, but pointed instead to transcriptional or some other form of post-translational control. Future work will be focused on molecularly characterizing this nutrient-dependent pathway.
Figure 2. Nutrient-dependent increase in Lin-28::Venus does not require RNA-binding Activity. (A-D) Lin-28::Venus, Lin-28::VenusCSD-, Lin-28::VenusCCHC-, and Lin-28::VenusCSD- CCHC- expression in intestinal progenitor cells from fed (A-D) and starved (A'-D’) animals, respectively. Insets in C' and D’ are magnified views of a starved intestinal progenitor cell stained for Lin-28::Venus (left) and DAPI (right).
The role of LIN-28 in translational control
In our previous work, we also investigated the molecular mechanism by which LIN-28 promoted stem cell expansion. In this context, we unexpectedly found that LIN-28 functioned independently of its established microRNA target, let-7.Citation18,19 Extensive analysis of our own publishedCitation20-22 and unpublished let-7 reagents indicates that let-7 was not involved: we found no evidence for let-7 expression, mutant phenotypes, or genetic interaction with lin-28 in the adult intestinal epithelium. Because LIN-28 is known to physically interact with and regulate the expression of mRNAs, we deep sequenced RNAs from immunopurified LIN-28 isolated from embryos. Because loss of lin-28 was associated with reduced insulin signaling, we searched the top mRNAs for known components of the insulin-signaling pathway. The only mRNA identified was Insulin Receptor (InR), and its physical interaction with LIN-28 was confirmed by qRT-PCR analysis of LIN-28 IPs from both embryos and adults.Citation17
We found 2 pieces of evidence that InR is a target of LIN-28. First, overexpression of LIN-28 in progenitor cells resulted in elevated InR levels, indicating that LIN-28 promotes InR expression. Secondly, driving InR expression completely rescued the stem cell defects displayed by lin-28Δ1 mutant ISC lineages, including the symmetric renewal and amplification of ISCs. Importantly, this rescuing activity was relatively specific, since forcing expression of components of other signaling pathways, including the Yorkie, Epidermal Growth Factor and Wingless pathways, failed to rescue. This key finding indicated that reduced InR is responsible for lin-28Δ1 mutant stem cell phenotypes and suggested that a major function of the LIN-28 is to promote InR translation. InR mRNA is unusual since, unlike most mRNAs, its translation increases during serum deprivation of Drosophila tissue culture cells, likely due to a verified, conserved internal ribosome entry site (IRES) located in the InR 5′UTR whose activity is stimulated by 4E-BP.Citation23-25 The in vivo function of this IRES, or even its specific location in the InR 5′UTR, has not been characterized but our data suggests the possibility that LIN-28 stimulates expression of InR IRES-containing mRNAs. Despite a surge of recent genome-wide analyses from multiple systems indicating a larger than anticipated diversity in the recruitment of cellular mRNAs to ribosomes,Citation26,27 how cellular IRESs work remains enigmatic and controversial.Citation28-30 Two possible ways that trans-acting factors might promote internal ribosome entry under stress conditions include unwinding of highly structured 5′UTRs or alleviating translational repression at short upstream ORFs (uORFs).Citation31,32 Thus, our finding that ISC symmetric renewal likely relies on the post-translational control of InR has identified a context in which the elements and mechanisms required for this control can be rigorously assessed.
Concluding remarks and perspectives
Based on the analyses described above, our current view is that LIN-28 is an essential component of an intestinal progenitor-specific ribonucleoprotein (RNP) complex that modulates stem cell division type to adjust tissue growth to dietary conditions. This RNP complex protects a set of key mRNAs, including InR, from the widespread translational repression that occurs in response to nutrient deprivation,Citation33 possibly by promoting or licensing their translation via IRESs. Recent analysis of larval tissue found no evidence of enhancement of InR translation in total larval extract,Citation34 so such translational enhancement may only occur in stages and cells where the developmentally-regulated LIN-28 protein is known to be highly expressed, like embryos and ISCs.Citation17,35 Translation of InR then poises ISCs to divide symmetrically in response to insulin released upon food consumption. After feeding and the ensuing tissue growth, LIN-28 protein levels are downregulated leading to dampened InR translation and a switch to homeostatic ISC division patterns. Importantly, elimination of the RNP complex leads to constant basal levels of stem cell InR that are not responsive to dietary conditions. In this view, the RNP regulates the expression of a stem cell survival factor that is expressed in restricting amounts: basal levels of InR limit stem cell numbers, which LIN-28 can expand by boosting InR translation.
Whether this regulation extends to all ISCs along the entire stretch of the Drosophila intestine remains to be determined. We speculate the existence of at least 2 classes of ISCs: a founding population of ISCs and a secondary actively cycling population of ISCs derived from the former, analogous to the often quiescent +4 cells that give rise of the proliferative and multipotent Lgr5(+) cells of vertebrate intestinal crypt.Citation36 Could the founding population be the stem cells present at the time of eclosion? Our extended data presented here arguably represent the early growth phases of intestinal epithelia, and therefore reflective of the translational control in the founding population of progenitor cells. Distinguishing these 2 populations of ISCs, however, remains a challenge.
Methods
Strain construction
Mutant versions of the LIN-28::Venus rescuing transgene were generated using the Multi Lightning Site Directed Mutagenesis Kit (Agilent). The CSD mutant contains W45A, F54A, and V65A point mutations while the CCHC mutant contains H136A and H158 point mutations. These mutations were modeled on those reported by Balzer and Moss.Citation37 Resulting transgenes were integrated into the attP2 landing site and recombined onto lin-28Δ1 mutant chromosomes by standard methods.
Fly husbandry
Flies for nutrient-dependent analyses were collected within one day of eclosion and separated into 2 groups. One set (fed) were reared on standard media supplemented with yeast paste (1 part yeast: 2 parts water) for 4 d while the second set (starved) were reared in empty glass vials containing Kimwipe paper soaked in a 1% sucrose solution.
Immunostaining and antibodies
Intestines from fed and starved adult flies were dissected in 1X PBS and processed in the same vial. Antibody staining was performed as previously described.Citation17 The following antibodies were used: rabbit anti-GFP (1: 4000, (Invitrogen A11122), and rat anti-Lin-28 (1:200).
Image collection, processing, measurement and statistical analyses
Confocal images of intestinal epithelium were collected using Leica SP5 under identical conditions. Simultaneous level processing of resulting micrographs was done using Adobe Illustrator and Adobe Photoshop CS6. FIJI was used to obtain greyscale LUT, measure cell area and integrated density value (Area of selected cell × Mean Gray Value). The following calculation was carried out to obtain the background Corrected Total Cell Fluorescence = Integrated Density - (Area of Selected cell × Mean fluorescence of background reading) as described in reference 38.Citation38 Graphpad Prism6 was used to perform unpaired parametric 2-tailed t-test with Welch's correction, for area and fluorescence readings, comparing between fed and starved animals. **** denotes p < 0.0001.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Adam Mercer and Ching-Huan Chen for help with plasmid and strain construction and the Developmental Studies Hybridoma Bank for reagents.
Funding
This work was funded by the National Institutes of Health (Award R21OD019916) and the Indiana University Faculty Research Support Program.
References
- Klein AM, Simons BD. Universal patterns of stem cell fate in cycling adult tissues. Development 2011; 138:3103-11; PMID:21750026; http://dx.doi.org/10.1242/dev.060103
- Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a pattern of neutral drift. Science 2010; 330:822-5; PMID:20929733; http://dx.doi.org/10.1126/science.1196236
- Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell 2011; 145:851-62; PMID:21663791; http://dx.doi.org/10.1016/j.cell.2011.05.033
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 2006; 441:1068-74; PMID:16810241; http://dx.doi.org/10.1038/nature04956
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 2009; 10:207-17; PMID:19209183; http://dx.doi.org/10.1038/nrm2636
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005; 122:289-301; PMID:16051152; http://dx.doi.org/10.1016/j.cell.2005.05.010
- Miguel-Aliaga I. Nerveless and gutsy: intestinal nutrient sensing from invertebrates to humans. Semin Cell Dev Biol 2012; 23:614-20; PMID:22248674; http://dx.doi.org/10.1016/j.semcdb.2012.01.002
- Luhur A, Chawla G, Sokol NS. MicroRNAs as components of systemic signaling pathways in Drosophila melanogaster. Curr Topics Dev Biol 2013; 105:97-123; PMID:23962840; http://dx.doi.org/10.1016/B978-0-12-396968-2.00004-X
- Lucchetta EM, Ohlstein B. The Drosophila midgut: a model for stem cell driven tissue regeneration. Wiley interdisciplinary reviews Developmental biology 2012; 1:781-8.
- O'Brien LE, Soliman SS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell 2011; 147:603-14; PMID:22036568; http://dx.doi.org/10.1016/j.cell.2011.08.048
- McLeod CJ, Wang L, Wong C, Jones DL. Stem cell dynamics in response to nutrient availability. Curr Biol 2010; 20:2100-5; PMID:21055942; http://dx.doi.org/10.1016/j.cub.2010.10.038
- de Navascues J, Perdigoto CN, Bian Y, Schneider MH, Bardin AJ, Martinez-Arias A, Simons BD. Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J 2012; 31:2473-85; PMID:22522699; http://dx.doi.org/10.1038/emboj.2012.106
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 2006; 439:475-9; PMID:16340959; http://dx.doi.org/10.1038/nature04371
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 2006; 439:470-4; PMID:16340960; http://dx.doi.org/10.1038/nature04333
- Goulas S, Conder R, Knoblich JA. The Par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell stem cell 2012; 11:529-40; PMID:23040479; http://dx.doi.org/10.1016/j.stem.2012.06.017
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 2007; 315:988-92; PMID:17303754; http://dx.doi.org/10.1126/science.1136606
- Chen CH, Luhur A, Sokol N. Lin-28 promotes symmetric stem cell renewal and drives adaptive growth in the adult Drosophila intestine. Development 2015; 142:3478-87; PMID:26487778; http://dx.doi.org/10.1242/dev.127951
- Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell 2008; 32:276-84; PMID:18951094; http://dx.doi.org/10.1016/j.molcel.2008.09.014
- Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science 2008; 320:97-100; PMID:18292307; http://dx.doi.org/10.1126/science.1154040
- Chawla G, Sokol NS. Hormonal activation of let-7-C microRNAs via EcR is required for adult Drosophila melanogaster morphology and function. Development 2012; 139:1788-97; PMID:22510985; http://dx.doi.org/10.1242/dev.077743
- Sokol NS, Xu P, Jan YN, Ambros V. Drosophila let-7 microRNA is required for remodeling of the neuromusculature during metamorphosis. Gen Dev 2008; 22:1591-6; PMID:18559475; http://dx.doi.org/10.1101/gad.1671708
- Wu YC, Chen CH, Mercer A, Sokol NS. Let-7-complex microRNAs regulate the temporal identity of Drosophila mushroom body neurons via chinmo. Dev Cell 2012; 23:202-9; PMID:22814608; http://dx.doi.org/10.1016/j.devcel.2012.05.013
- Marr MT, 2nd, D'Alessio JA, Puig O, Tjian R. IRES-mediated functional coupling of transcription and translation amplifies insulin receptor feedback. Gen Dev 2007; 21:175-83; PMID:17234883; http://dx.doi.org/10.1101/gad.1506407
- Olson CM, Donovan MR, Spellberg MJ, Marr MT, 2nd. The insulin receptor cellular IRES confers resistance to eIF4A inhibition. eLife 2013; 2:e00542; PMID:23878722
- Spriggs KA, Cobbold LC, Ridley SH, Coldwell M, Bottley A, Bushell M, Willis AE, Siddle K. The human insulin receptor mRNA contains a functional internal ribosome entry segment. Nucleic Acids Res 2009; 37:5881-93; PMID:19654240; http://dx.doi.org/10.1093/nar/gkp623
- Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 2012; 485:55-61; PMID:22367541; http://dx.doi.org/10.1038/nature10912
- Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 2012; 485:109-13; PMID:22552098; http://dx.doi.org/10.1038/nature11083
- Gilbert WV. Alternative ways to think about cellular internal ribosome entry. J Biol Chem 2010; 285:29033-8; PMID:20576611; http://dx.doi.org/10.1074/jbc.R110.150532
- Jackson RJ. The current status of vertebrate cellular mRNA IRESs. Cold Spring Harb Perspect Biol 2013; 5:pii: a011569; PMID:23378589; http://dx.doi.org/10.1101/cshperspect.a011569
- Shatsky IN, Dmitriev SE, Andreev DE, Terenin IM. Transcriptome-wide studies uncover the diversity of modes of mRNA recruitment to eukaryotic ribosomes. Critical Rev Biochem Mol Biol 2014; 49:164-77; PMID:24520918; http://dx.doi.org/10.3109/10409238.2014.887051
- Ruggero D. Translational control in cancer etiology. Cold Spring Harb Perspect Biol 2013; 5:pii: a012336; PMID:22767671; http://dx.doi.org/10.1101/cshperspect.a012336
- Somers J, Poyry T, Willis AE. A perspective on mammalian upstream open reading frame function. Int J Biochem Cell Biol 2013; 45:1690-700; PMID:23624144; http://dx.doi.org/10.1016/j.biocel.2013.04.020
- Proud CG. Regulation of mammalian translation factors by nutrients. Eur J Biochem / FEBS 2002; 269:5338-49; PMID:12423332; http://dx.doi.org/10.1046/j.1432-1033.2002.03292.x
- Nagarajan S, Grewal SS. An investigation of nutrient-dependent mRNA translation in Drosophila larvae. Biology open 2014; 3:1020-31; PMID:25305039; http://dx.doi.org/10.1242/bio.20149407
- Moss EG, Tang L. Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev Biol 2003; 258:432-42; PMID:12798299; http://dx.doi.org/10.1016/S0012-1606(03)00126-X
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science 2010; 327:542-5; PMID:20110496; http://dx.doi.org/10.1126/science.1180794
- Balzer E, Moss EG. Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules. RNA Biol 2007; 4:16-25; PMID:17617744; http://dx.doi.org/10.4161/rna.4.1.4364
- Burgess A, Vigneron S, Brioudes E, Labbe JC, Lorca T, Castro A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci U S A 2010; 107:12564-9; PMID:20538976; http://dx.doi.org/10.1073/pnas.0914191107
