ABSTRACT
From Drosophila to man, multinucleated muscle cells form through cell-cell fusion. Using Drosophila as a model system, researchers first identified, and then demonstrated, the importance of actin cytoskeletal rearrangements at the site of fusion. These actin rearrangements at the fusion site are regulated by SCAR and WASp mediated Arp2/3 activation, which nucleates branched actin networks. Loss of SCAR, WASp or both leads to defects in myoblast fusion. Recently, we have found that the actin regulator Diaphanous (Dia) also plays a role both in organizing actin and in regulating Arp2/3 activity at the fusion site. In this Extra View article, we provide additional data showing that the Abi-SCAR complex accumulates at the fusion site and that excessive SCAR activity impairs myoblast fusion. Using constitutively active Dia constructs, we provide additional evidence that Dia functions upstream of SCAR activity to regulate actin dynamics at the fusion site and to localize the Abi-SCAR complex.
Introduction
Cell-cell fusion is a crucial step in muscle development and repair.Citation1-5 During Drosophila muscle development, fusion occurs between the founder cells (FCs)/myotubes and the fusion competent myoblasts (FCMs). The FCs express transcription factors that determine the identity of individual muscles. When a naïve FCM fuses with a FC, the newly added myonucleus is reprogrammed to adopt the founder cell nucleus identity, and another round of fusion is initiated.Citation6 During fusion, formation of an actin-rich focus occurs at the fusion site;Citation7,8 this actin focus provides an invasive force from the FCM into the FC/myotube, promoting fusion.Citation9 Actin polymerization at the fusion site is regulated by Arp2/3, a branched actin nucleating complex.Citation7,8,10,11 The activity of Arp2/3 is controlled by the nucleation promoting factors WASp and SCAR (also known as WAVE). WASp and SCAR share a C-terminal VCA domain that activates Arp2/3 by both promoting conformational changes in Arp2/3 and presenting G-actin to Arp2/3.Citation12,13 The importance of WASp and SCAR during myoblast fusion has been demonstrated in previous studies from our lab and others in multiple systems.Citation8,9,11,14-22 Loss or reduction of WASp and/or SCAR activity results in a fusion block: FCMs make contact with FC/myotubes, but are unable to form or expand fusion pores, preventing myoblast fusion. As both WASp and SCAR are required for Arp2/3 activation, and therefore the branched actin network formation at the fusion site, loss of both WASp and SCAR activity results in no actin focus formation.Citation9,23 Interestingly, loss of WASp function alone results in a fusion block with normal-sized actin foci, while loss of SCAR function alone results in a fusion block with enlarged actin foci.Citation8,9
In our recent PLoS Genetics paper, “The formin Diaphanous regulates myoblast fusion through actin polymerization and Arp2/3 regulation,“ we reported that Diaphanous (Dia) plays a critical role in myoblast fusion.Citation24 Dia, a member of the Formin family of actin polymerization factors, controls actin rearrangements at the fusion site by a) nucleating and elongating linear actin filaments and b) localizing WASp and SCAR. Although we have demonstrated the importance of Dia in localizing Arp2/3 regulators, how Dia interacts with SCAR and WASp and directs their localization to the fusion site remained unclear. In this Extra View article, we concentrate on the activity of the SCAR complex and investigate how Dia regulates SCAR at the fusion site.
The activity of SCAR is controlled by the SCAR regulatory complex (WRC). The WRC is a pentameric complex that contains SCAR, Abi, Kette/Nap1, Hspc300, and Sra1/Cyfip1. In vitro experiments using purified proteins suggest that when not associated with the complex, SCAR exists in an active conformation and can stimulate Arp2/3 activity through its VCA domain.Citation25 However, in the WRC, the VCA domain of SCAR is bound by Sra-1, and SCAR's activity is inhibited.Citation12 Rac-GTP activates SCAR by competitively binding to Sra1, triggering exposure of the VCA domain of SCAR.Citation26 In vitro experiments confirm that, when activated by Rac-GTP, SCAR does not dissociate from WRC complex, only that its C-terminal VCA domain is no longer bound by Sra1.Citation12,26 In addition to regulating SCAR activity, the formation of the WRC is crucial for SCAR localization and stability.Citation8,27,28 Removal of WRC components, such as Abi or Kette/Nap1, results in SCAR degradation.Citation29
Abi is a key component of the WRC that is important for the stability, localization, and activity of SCAR.Citation27,28,30 Recent experiments have shown that Abi also interacts physically with Dia to regulate actin dynamics and adhesion in A431 and 293T cells.Citation31 Moreover, these experiments indicate that Dia and SCAR physically bind to partially overlapping regions of Abi.Citation31 Since the interaction between Abi and SCAR is critical in localizing and stabilizing SCAR,Citation27,30 the authors suggest that Dia regulates SCAR activity through competition for Abi binding. In this Extra View article, we investigate whether Dia regulates SCAR during Drosophila myoblast fusion through the mechanisms described in mammalian cell culture. We also report an unexpected fusion phenotype that is associated with excessive SCAR activity in embryos.
Results
Excessive Scar activity blocks myoblast fusion
Mammalian tissue culture experiments using different cell types suggested that the binding of Abi to SCAR is required for several aspects of SCAR activity, including stability,Citation27 localization, and activation.Citation30 We hypothesized that this relationship between Abi and SCAR also exists during Drosophila myoblast fusion. To test our hypothesis and investigate how Abi regulates SCAR, we employed a bimolecular fluorescence complementation (BiFC) technique to visualize Abi-SCAR complex formation in vivo.Citation32 In the BiFC system, YFP is split into 2 non-fluorescent fragments: N-terminal YFP (NYFP) and C-terminal YFP (CYFP), and each fragment is fused to either SCAR or Abi. YFP is reconstituted when the 2 fragments are brought in close proximity.Citation32 To visualize SCAR and Abi interaction during fusion, we expressed these split YFP-tagged SCAR and Abi constructs in the developing muscles using the muscle specific driver, Dmef2-Gal4. To control for background fluorescence, we used confocal microscopy to detect fluorescent levels when untagged NYFP and CYFP were similarly expressed. We did not observe fluorescent signal in the muscles expressing untagged split-YFP fragments simultaneously (); this indicated that the untagged split-YFP did not interact spontaneously to reconstitute YFP. We then visualized the interaction between SCAR and Abi by expressing both Abi-NYFP and SCAR-CYFP () or the converse, SCAR-NYFP and Abi-CYFP () simultaneously in muscles. With either combination, we observed strong fluorescent YFP signal at the fusion site, which is marked by the F-actin focus (). Together, these data confirmed our hypothesis that SCAR and Abi physically interact at the fusion site during muscle formation.
Figure 1. Visualization of Abi-SCAR complex formation using split YFP during myoblast fusion. Stage 15 embryo stained for F-actin (phalloidin, white) to label fusion site, and YFP (GFP antibody, green) to detect YFP reconstitution, FCM (magenta, false colored), and FC/myotube (turquoise, false colored). (Ai-Aiii) To visualize the background fluorescent level, UAS-myc-NYFP and UAS-HA-CYFP were expressed in the muscles under the control of muscles specific driver DMef2-Gal4. (Bi-Biii) UAS-Abi-myc-NYFP and UAS-SCAR-HA-CYFP; or (Ci-Ciii) UAS-SCAR-myc-NYFP and UAS-Abi-HA-CYFP were expressed in the muscles under the control of DMef2-Gal4. The reconstituted YFP signals indicate sites of Abi-SCAR interaction. Scale bar: 5 μm.
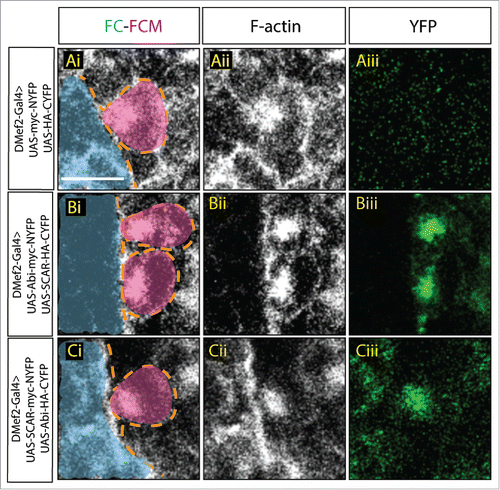
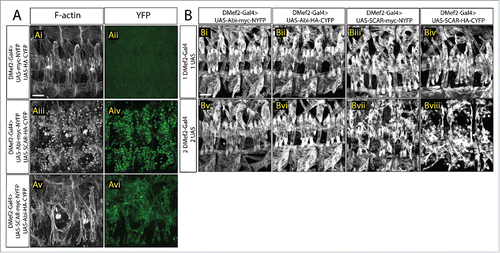
In tissue culture, Abi binding is known to enhance SCAR stability and activity.Citation27,29,30 As the actin polymerization activity of SCAR is required during fusion,Citation8,9 it is possible that the interaction between Abi and SCAR is critical for controlling SCAR levels and activity, and thus actin polymerization, at the fusion site. To test the function of the Abi-SCAR interaction at the fusion site, we next examined the impact of tagged Abi-SCAR expression on muscle development. Analysis of developing muscle cells which overexpress both the SCAR- and Abi- tagged split YFP constructs revealed that myoblast fusion at stage 16 was impaired (). To quantify the fusion block, we examined the fusion index in late stage 16 embryos. In contrast to control embryos in which 5-7 fusion events occur to form each Lateral Transverse (LT) muscle,Citation33 we found that expression of Abi-NYFP and SCAR-CYFP resulted in severe fusion block with only 0-3 rounds of fusion per LT muscle (p < 0.001). A fusion block was also observed in muscles expressing the reverse combination (SCAR-NYFP and Abi-CYFP), but it was less severe (0-4 rounds of fusion per LT, p < 0.001), possibly due to lower expression levels of these specific transgenic constructs. This block in myoblast fusion was not seen when expressing untagged split-YFP constructs simultaneously ().
Figure 2. Increased SCAR activity results in fusion block. A. Three hemisegments from a stage 16 embryo. Embryos are stained for F-actin (phalloidin, white) to show the muscle pattern, and YFP (GFP antibody, green) to detect YFP reconstitution. (Ai–Aii) In control embryos, UAS-myc-NYFP and UAS-HA-CYFP were expressed in muscles under the control of DMef2-Gal4. Phalloidin staining shows wild-type muscle pattern. Background fluorescent level was visualized with antibody against GFP. UAS-Abi-myc-NYFP and UAS-SCAR-HA-CYFP (Aiii–Aiv) or UAS-SCAR-myc-NYFP and UAS-Abi-HA-CYFP (Av–Avi) were expressed in muscles under the control of DMef2-Gal4. Phalloidin staining shows impaired fusion and the actin focus at the fusion site. YFP shows the localization of Abi-SCAR interaction. (B) Muscle pattern from stage 16 embryos (antibody against Myosin Heavy Chain. white). Three hemisegments are shown from each embryo. One (Bi–Biv) or 2 copies (Bv–Bviii) of split-YFP labeled Abi or SCAR were expressed in the muscles under the control of DMef2-Gal4. Abi overexpression does not change muscle pattern. Increased expression of SCAR results in myoblast fusion block in a dosage dependent manner. Scale bar: 20 μm.
The block in fusion detected upon expression of both SCAR and Abi could be explained in 2 ways: First, co-expression of SCAR and Abi leads to excessive SCAR activity. This results in unregulated Arp2/3 activity at the fusion site, and a fusion block. Alternatively, expression of both SCAR and Abi produce a dominant negative effect by preventing interactions between endogenous SCAR, Rac-GTP, and Arp2/3. Due to this reduction in Arp2/3 activation at the fusion site, a fusion block occurs.
To test these 2 models, we measured actin focus size in stage 15 embryos. If overexpressed Abi and SCAR produce a dominant negative effect, we would expect to see enlarged actin foci similar to SCAR loss of function mutant embryos. In muscles with Abi-NYFP and SCAR-CYFP overexpression, actin foci had an average diameter of 2.14 ± 0.05 μm, which is similar to and within the range of control foci (1.99 ± 0.09 μm, N = 11). The wild-type size of the actin focus, therefore, suggested that over-expression of Abi and SCAR did not cause a dominant negative effect that suppresses SCAR activity. In addition, since it is Sra1, not SCAR or Abi, which binds to Rac-GTP, overexpression of SCAR and Abi should not sequester Rac-based WRC activation. When measuring the actin focus size, we observed that nearly every FCM in Abi-SCAR overexpressing embryos had an actin focus, not just those that were adhered to an FC/Myotube and were undergoing myoblast fusion. This suggested that actin polymerization is misregulated by Abi and SCAR co-expression. Altogether, these observations indicated that fusion is blocked in these Abi and SCAR expressing embryos due to more SCAR activity, which in turn leads to more Arp2/3 activity.
To further test our model, we overexpressed SCAR or Abi alone in the muscle-forming mesoderm and examined the muscle pattern in stage 16 embryos. As described earlier, in vitro experiments suggested that SCAR itself can activate Arp2/3.Citation25 Therefore, if our model is correct, namely that fusion is blocked due to upregulated Arp2/3 activity, just increasing the levels of SCAR itself should impair fusion. Indeed, when we overexpressed SCAR using 2 copies of the transgene, fusion was impaired (). The size of the actin focus was similar to controls (2.02 ± 0.09 μm, N = 11) and to simultaneous expression of both tagged SCAR and Abi. Expression of only one copy of SCAR was not sufficient to cause a fusion block (), possibly due to the instability of SCAR when not associated with the WRC. Nevertheless, the fusion phenotype in the low-level SCAR overexpression background can be enhanced by co-expression of Abi (). This observation is consistent with the report that Abi can bind to SCAR and enhance its stability and activity.Citation27,29,30 We also observed that expressing one or 2 copies of Abi did not result in a fusion block (), suggesting that increasing the availability of Abi alone is not sufficient to misregulate Arp2/3 activity and block fusion. Altogether we conclude that excessive SCAR activity impairs myoblast fusion.
Constitutively active Dia mislocalizes the Abi-SCAR complex during muscle formation
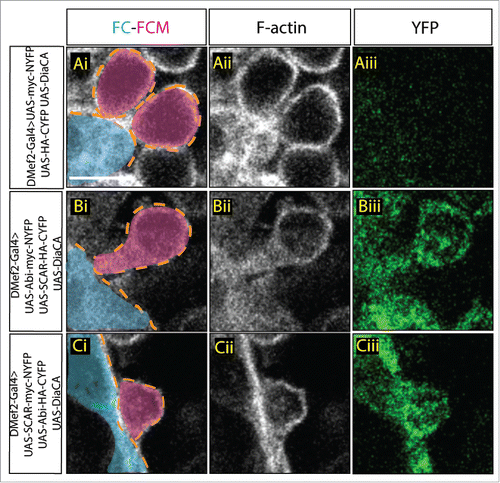
The split-YFP system also allowed us to interrogate Abi-SCAR interactions during myoblast fusion in other genetic backgrounds. Our previous work indicated that Dia regulated SCAR localization during fusion, as in both Dia loss and gain of function, SCAR was no longer restricted to the fusion site.Citation24 Data from mammalian tissue culture indicated that the Dia-binding SNARE domain in Abi partially overlaps with the SCAR binding WAB domain, suggesting that Dia and SCAR compete for Abi binding.Citation31 Since binding to Abi is required for SCAR localization,Citation27 this competition might account for SCAR mislocalization in the Dia gain of function background during myoblast fusion. To test if competition between Dia and SCAR for Abi exists during myoblast fusion, we investigated whether Dia activity altered the Abi-SCAR interaction detected by the split-YFP constructs at the fusion site. To do this, we expressed constitutively active Dia (Dia.CA) specifically in the muscle-forming mesoderm together with SCAR-NYFP and Abi-CYFP and examined the distribution of the YFP signal. We used 2 Dia.CA constructs, one with and the second, without, the putative Abi-binding domain. In controls where there was no Dia.CA being expressed in the myoblasts, YFP reconstitution occurred through Abi-SCAR interaction, and the YFP signal accumulated at the fusion site (). When we expressed Dia.CA constructs together with split-YFP tagged SCAR and Abi, however, we found a diffuse cytoplasmic localization of the YFP signal (, compare ). No particular enrichment was found at between FC/Myotube and FCMS. We observed similar results with both constructs. The presence of the YFP signal suggested that the Abi-SCAR complex still formed in a constitutively active Dia-expressing background; however, the diffuse YFP signal indicated that the Abi-SCAR complex was no longer recruited to the fusion site. The mislocalization of the Abi-SCAR complex was consistent with our earlier report that constitutively active Dia changes the localization of SCAR.Citation24 Since constitutively active Dia without the putative Abi binding domain also led to a diffuse YFP signal, we suggest that Dia changes the localization of Abi-SCAR complex through its actin polymerization activity. As we are unable to accurately quantify the diffuse YFP signal, it remains unclear, however, if Dia.CA only changes the localization of the Abi-SCAR complex or disrupts both Abi-SCAR interaction and localization.
Figure 3. Constitutively active Dia changes Abi-SCAR localization and actin structure at the fusion site. Stage 16 embryo stained for F-actin (phalloidin, white) and YFP (GFP antibody, green), FCM (magenta, false colored), FC/myotube (turquoise/false colored). (Ai–Aiii) As control, constitutively active Dia (UAS-Dia.CA) was expressed together with UAS-myc-NYFP and UAS-HA-CYFP in the muscles under the control of DMef2-Gal4. Fusion is blocked in this context. Background YFP fluorescent level was visualized with antibody against GFP. (B-C) UAS-Dia.CA was expressed together with UAS-Abi-myc-NYFP and UAS-SCAR-HA-CYFP (Bi-Biii) or UAS-SCAR-myc-NYFP and UAS-Abi-HA-CYFP (Ci-Ciii) in the muscles. Actin morphology at the fusion site was visualized by Phalloidin staining of F-actin. Abi-SCAR complex was visualized by YFP reconstitution and was labeled by antibody against GFP. Scale bar: 5 μm.
Dia functions upstream of Arp2/3 to regulate actin at the fusion site
In our previous work, we have demonstrated, using both loss and gain of Dia activity, that Dia functions upstream of Arp2/3 at the fusion site to polymerize linear actin filaments and to regulate the localization of SCAR and WASp.Citation24 To further confirm these data, we performed epistasis experiments in embryos that overexpress Dia.CA, Abi, and SCAR. Both the morphology and size of the actin focus provides insight to which step in fusion is disrupted in a particular fusion mutant.Citation8,24,34 Fusion mutants that impair SCAR-regulated Arp2/3 activation result in enlarged, rounded foci. Fusion mutants that impair WASp regulated Arp2/3 activation result in normal-sized, rounded foci. Fusion mutants that impair both SCAR and WASp-regulated Arp2/3 activation result in no actin focus.Citation23 Likewise, Dia loss of function results in no actin focus; expression of constitutively active Dia (Dia.CA), however, changes actin dynamics during fusion and generates a diffuse distribution of F-actin at the fusion site.Citation24 When Dia.CA is expressed together with SCAR-NYFP and Abi-CYFP in myoblasts, we found that myoblast fusion is still blocked in this context with F-actin organized into diffuse structures (). The morphology of these F-actin structures are similar to those seen in myoblasts expressing Dia.CA alone (), rather than the round foci seen in a SCAR-NYFP/Abi-CYFP background (). These findings reinforce our conclusions from our PLoS Genetics paper,Citation24 namely that Dia functions upstream of Arp2/3 to regulate actin organization at the fusion site.
Discussion
In this Extra View article, we show that Abi and SCAR interact at the fusion site to promote Arp2/3 activity. Excessive SCAR activity results in upregulated Arp2/3 activity and blocked fusion. Dia functions upstream of Arp2/3 to regulate actin polymerization. Excessive Dia leads to mislocalization of the Abi-SCAR complex, possibly through its actin polymerization activity.
The importance of Arp2/3 activity for myoblast fusion has been demonstrated in many studies.Citation8,9,14-16 Both SCAR and WASp can activate Arp2/3 and are known to regulate myoblast fusion in a cell type dependent manner: WASp is specifically required in the FCM,Citation34 while SCAR is required for both FC/myotube and FCM.Citation17 It has also been speculated that SCAR and WASp are playing distinct roles in FCMs, with SCAR mediating the migration of myoblasts and initiating fusionCitation17 and WASp promoting the formation of podosome-like structures and subsequent fusion pore expansion.Citation34 The balance and switch between SCAR and WASp would allow for finely controlled regulation of Arp2/3 activation, which, in turn, controls the formation of the F-actin structures in the FC/myotube and FCM. In this study, we provided evidence that SCAR activity is highly regulated during myoblast fusion. Excessive SCAR activity results in mis- and upregulated Arp2/3 function and blocks myoblast fusion. It is still unclear how excessive SCAR mechanistically blocks fusion. However, the normal-sized actin focus found when SCAR is overexpressed suggests a possible model of SCAR-WASp interaction. In a WASp loss of function background, the size of the actin focus is similar to wild-type foci. A similar sized actin focus is also seen in SCAR overexpressing background. A possible explanation is that SCAR and WASp compete with each other for Arp2/3 binding. Therefore, SCAR overexpression leads to suppressed WASp activity, resulting in a normal-sized actin focus that phenocopies WASp loss of function. Similarly, in a SCAR loss of function background, where Arp2/3 activity is regulated by WASp only, the actin focus is enlarged, as WASp is the major factor that promotes the formation of podosome-like structures at the FCM side.Citation34 Together, these data suggest a possible mutual inhibition model /competition model for SCAR and WASP.
We also provide evidence for 2 distinct ways to control SCAR activity. The first is through direct regulation of SCAR stability, and the second is through the regulation of SCAR localization in a Dia-dependent manner.Citation24 When we overexpress just one copy of SCAR, fusion is unaffected. We suggest that this may be a result of SCAR degradation when it is not associated with the WRC and reflects the cell's capacity to buffer SCAR activity. However, when one copy of Abi is added to this background, it stabilizes exogenous SCAR, leading to an increase in both the level and activity of SCAR. This, in turn, results in a fusion block. Similarly, as the majority of endogenous SCAR is either part of the WRC or degraded, overexpression of Abi alone does not lead to a significant increase in endogenous SCAR activity, and therefore fusion is unaffected. Thus, overall SCAR activity during myoblast fusion depends on the expression level of SCAR and whether it is associated with WRC components.
In our recent PLoS Genetics paper, “The formin Diaphanous regulates myoblast fusion through actin polymerization and Arp2/3 regulation,“ we reported that Dia functions upstream of SCAR, and that both Dia loss of and gain of function results in mislocalized SCAR. However, we did not show whether SCAR mislocalization is due to SCAR dissociation from the WRC or due to mislocalization of the entire complex. In this study, we report that in the DiaCA background, Scar remains bound to Abi and that this complex is mislocalized. Experiments in mammalian tissue culture suggested that Dia and SCAR may compete for Abi binding.Citation31 Since Abi is one of the components that regulate SCAR localization, these tissue culture findings suggest that Dia.CA overexpression could result in dissociation of SCAR from Abi. Our data, however, show that in a Dia.CA overexpression background, at least a portion of the mislocalized SCAR is still associated with Abi. We could not, however, exclude completely the competition model, as we are unable to measure whether the overall level of reconstituted YFP is altered.
It has also been reported that SCAR and Arp2/3 can function together to inhibit Dia activity in HeLa cells.Citation35 As Dia activity is required during myoblast fusion, these data suggested to us that increased SCAR activity may impair fusion through inhibiting Dia. Our results, however, do not support this hypothesis. We found that Dia loss of function resulted in actin focus absence,Citation24 while SCAR overexpression led to normal sized actin foci. Together, our data in this paper and the PLoS Genetics paper suggest that Dia and Arp2/3 collaborate to build an actin focus at the fusion site.Citation24
In summary, we have demonstrated that Abi physically interacts with SCAR at the fusion site and enhances SCAR activity. We also showed that constitutively active Dia polymerizes actin upstream of SCAR function and changes the distribution of the Abi-SCAR complex. Lastly, we found that SCAR activity is highly regulated during fusion. Our data suggest that a balance between Dia, SCAR, and WASp activities exist to spatially and temporally control Arp2/3 activity during myoblast fusion.
Materials and methods
Fly strains: UAS-SCAR-HA-CYFP, UAS-Abi-HA-CYFP, UAS-SCAR-Myc-NYFP, UAS-Abi-Myc-NYFP, UAS-HA-CYFP, UAS-Myc-NYFP,Citation32 UAS-DiaCA-HA, UAS-DiaΔDAD-HA.Citation36,37 Embryos are staged, fixed, stained and imaged as described in Deng et al.Citation24
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank S. Bogdan, M. Peifer and Bloomington Stock Center for fly stocks.
Funding
This study was supported by the National Institute of Health (NIH) [GM078318, AR108981] to MKB and NCI [P30 CA 008748] core grant to MSKCC.
References
- Kim JH, Jin P, Duan R, Chen EH. Mechanisms of myoblast fusion during muscle development. Curr Opin Genet Dev 2015; 32:162-70; PMID:25989064; http://dx.doi.org/10.1016/j.gde.2015.03.006
- Abmayr SM, Pavlath GK. Myoblast fusion: lessons from flies and mice. Development 2012; 139:641-56; PMID:22274696; http://dx.doi.org/10.1242/dev.068353
- Simionescu A, Pavlath GK. Molecular mechanisms of myoblast fusion across species. Adv Exp Med Biol 2011; 713:113-35; PMID:21432017; http://dx.doi.org/10.1007/978-94-007-0763-4_8
- Rai M, Nongthomba U, Grounds MD. Skeletal muscle degeneration and regeneration in mice and flies. Curr Top Dev Biol 2014; 108:247-81; PMID:24512712; http://dx.doi.org/10.1016/B978-0-12-391498-9.00007-3
- Onel S-F, Rust MB, Jacob R, Renkawitz-Pohl R. Tethering membrane fusion: common and different players in myoblasts and at the synapse. J Neurogenet 2014; 28:302-15; PMID:24957080; http://dx.doi.org/10.3109/01677063.2014.936014
- Dobi KC, Schulman VK, Baylies MK. Specification of the somatic musculature in Drosophila. Wiley Interdiscip Rev Dev Biol 2015; 4:357-75; PMID:25728002; http://dx.doi.org/10.1002/wdev.182
- Schulman VK, Dobi KC, Baylies MK. Morphogenesis of the somatic musculature in Drosophila melanogaster. Wiley Interdiscip Rev Dev Biol 2015; 4:313-34; PMID:25758712; http://dx.doi.org/10.1002/wdev.180
- Richardson BE, Beckett K, Nowak SJ, Baylies MK. SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodeling at the site of myoblast fusion. Development 2007; 134:4357-67; PMID:18003739; http://dx.doi.org/10.1242/dev.010678
- Sens KL, Zhang S, Jin P, Duan R, Zhang G, Luo F, Parachini L, Chen EH. An invasive podosome-like structure promotes fusion pore formation during myoblast fusion. J Cell Biol 2010; 191:1013-27; PMID:21098115; http://dx.doi.org/10.1083/jcb.201006006
- Rodal AA, Del Signore SJ, Martin AC. Drosophila comes of age as a model system for understanding the function of cytoskeletal proteins in cells, tissues, and organisms. Cytoskeleton (Hoboken) 2015; 72:207-24; PMID:26074334; http://dx.doi.org/10.1002/cm.21228
- Berger S, Schäfer G, Kesper DA, Holz A, Eriksson T, Palmer RH, Beck L, Klämbt C, Renkawitz-Pohl R, Onel S-F. WASP and SCAR have distinct roles in activating the Arp2/3 complex during myoblast fusion. J Cell Sci 2008; 121:1303-13; PMID:18388318; http://dx.doi.org/10.1242/jcs.022269
- Ismail AM, Padrick SB, Chen B, Umetani J, Rosen MK. The WAVE regulatory complex is inhibited. Nat Struct Mol Biol 2009; 16:561-3; PMID:19363480; http://dx.doi.org/10.1038/nsmb.1587
- Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature 2002; 418:790-3; PMID:12181570; http://dx.doi.org/10.1038/nature00859
- Schäfer G, Weber S, Holz A, Bogdan S, Schumacher S, Müller A, Renkawitz-Pohl R, Onel S-F. The Wiskott-Aldrich syndrome protein (WASP) is essential for myoblast fusion in Drosophila. Dev Biol 2007; 304:664-74; PMID:Can't; http://dx.doi.org/10.1016/j.ydbio.2007.01.015
- Massarwa R, Carmon S, Shilo B-Z, Schejter ED. WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev Cell 2007; 12:557-69; PMID:17419994; http://dx.doi.org/10.1016/j.devcel.2007.01.016
- Kim S, Shilagardi K, Zhang S, Hong SN, Sens KL, Bo J, Gonzalez GA, Chen EH. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev Cell 2007; 12:571-86; PMID:17419995; http://dx.doi.org/10.1016/j.devcel.2007.02.019
- Gildor B, Massarwa R, Shilo B-Z, Schejter ED. The SCAR and WASp nucleation-promoting factors act sequentially to mediate Drosophila myoblast fusion. EMBO Rep 2009; 10:1043-50; PMID:19644501; http://dx.doi.org/10.1038/embor.2009.129
- Kaipa BR, Shao H, Schäfer G, Trinkewitz T, Groth V, Liu J, Beck L, Bogdan S, Abmayr SM, Onel S-F. Dock mediates Scar- and WASp-dependent actin polymerization through interaction with cell adhesion molecules in founder cells and fusion-competent myoblasts. J Cell Sci 2013; 126:360-72; PMID:22992459; http://dx.doi.org/10.1242/jcs.113860
- Haralalka S, Shelton C, Cartwright HN, Katzfey E, Janzen E, Abmayr SM. Asymmetric Mbc, active Rac1 and F-actin foci in the fusion-competent myoblasts during myoblast fusion in Drosophila. Development 2011; 138:1551-62; PMID:21389053; http://dx.doi.org/10.1242/dev.057653
- Kesper DA, Stute C, Buttgereit D, Kreisköther N, Vishnu S, Fischbach K-F, Renkawitz-Pohl R. Myoblast fusion in Drosophila melanogaster is mediated through a fusion-restricted myogenic-adhesive structure (FuRMAS). Dev Dyn 2007; 236:404-15; PMID:17146786; http://dx.doi.org/10.1002/dvdy.21035
- Gruenbaum-Cohen Y, Harel I, Umansky K-B, Tzahor E, Snapper SB, Shilo B-Z, Schejter ED. The actin regulator N-WASp is required for muscle-cell fusion in mice. Proc Natl Acad Sci USA 2012; 109:11211-6; PMID:22736793; http://dx.doi.org/10.1073/pnas.1116065109
- Nowak SJ, Nahirney PC, Hadjantonakis A-K, Baylies MK. Nap1-mediated actin remodeling is essential for mammalian myoblast fusion. J Cell Sci 2009; 122:3282-93; PMID:19706686; http://dx.doi.org/10.1242/jcs.047597
- Bothe I, Deng S, Baylies M. PI(4,5)P2 regulates myoblast fusion through Arp2/3 regulator localization at the fusion site. Development 2014; 141:2289-301; PMID:24821989; http://dx.doi.org/10.1242/dev.100743
- Deng S, Bothe I, Baylies MK. The Formin Diaphanous Regulates Myoblast Fusion through Actin Polymerization and Arp2/3 Regulation. PLoS Genet 2015; 11:e1005381; PMID:26295716; http://dx.doi.org/10.1371/journal.pgen.1005381
- Machesky LM, Mullins RD, Higgs HN, Kaiser DA, Blanchoin L, May RC, Hall ME, Pollard TD. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc Natl Acad Sci USA 1999; 96:3739-44; PMID:10097107; http://dx.doi.org/10.1073/pnas.96.7.3739
- Chen Z, Borek D, Padrick SB, Gomez TS, Metlagel Z, Ismail AM, Umetani J, Billadeau DD, Otwinowski Z, Rosen MK. Structure and control of the actin regulatory WAVE complex. Nature 2010; 468:533-8; PMID:21107423; http://dx.doi.org/10.1038/nature09623
- Echarri A, Lai MJ, Robinson MR, Pendergast AM. Abl interactor 1 (Abi-1) wave-binding and SNARE domains regulate its nucleocytoplasmic shuttling, lamellipodium localization, and wave-1 levels. Mol Cell Biol 2004; 24:4979-93; PMID:15143189; http://dx.doi.org/10.1128/MCB.24.11.4979-4993.2004
- Davidson AJ, Ura S, Thomason PA, Kalna G, Insall RH. Abi is required for modulation and stability but not localization or activation of the SCAR/WAVE complex. Eukaryotic Cell 2013; 12:1509-16; PMID:24036345; http://dx.doi.org/10.1128/EC.00116-13
- Kunda P, Craig G, Dominguez V, Baum B. Abi, Sra1, and Kette control the stability and localization of SCAR/WAVE to regulate the formation of actin-based protrusions. Curr Biol 2003; 13:1867-75; PMID:14588242; http://dx.doi.org/10.1016/j.cub.2003.10.005
- Leng Y, Zhang J, Badour K, Arpaia E, Freeman S, Cheung P, Siu M, Siminovitch K. Abelson-interactor-1 promotes WAVE2 membrane translocation and Abelson-mediated tyrosine phosphorylation required for WAVE2 activation. Proc Natl Acad Sci USA 2005; 102:1098-103; PMID:15657136; http://dx.doi.org/10.1073/pnas.0409120102
- Ryu JR, Echarri A, Li R, Pendergast AM. Regulation of cell-cell adhesion by Abi/Diaphanous complexes. Mol Cell Biol 2009; 29:1735-48; PMID:19158278; http://dx.doi.org/10.1128/MCB.01483-08
- Gohl C, Banovic D, Grevelhörster A, Bogdan S. WAVE forms hetero- and homo-oligomeric complexes at integrin junctions in Drosophila visualized by bimolecular fluorescence complementation. J Biol Chem 2010; 285:40171-9; PMID:20937809; http://dx.doi.org/10.1074/jbc.M110.139337
- Metzger T, Gache V, Xu M, Cadot B, Folker ES, Richardson BE, Gomes ER, Baylies MK. MAP and kinesin-dependent nuclear positioning is required for skeletal muscle function. Nature 2012; 484:120-4; PMID:22425998; http://dx.doi.org/10.1038/nature10914
- Jin P, Duan R, Luo F, Zhang G, Hong SN, Chen EH. Competition between Blown fuse and WASP for WIP binding regulates the dynamics of WASP-dependent actin polymerization in vivo. Dev Cell 2011; 20:623-38; PMID:21571220; http://dx.doi.org/10.1016/j.devcel.2011.04.007
- Beli P, Mascheroni D, Xu D, Innocenti M. WAVE and Arp2/3 jointly inhibit filopodium formation by entering into a complex with mDia2. Nat Cell Biol 2008; 10:849-57; PMID:18516090; http://dx.doi.org/10.1038/ncb1745
- Bilancia CG, Winkelman JD, Tsygankov D, Nowotarski SH, Sees JA, Comber K, Evans I, Lakhani V, Wood W, Elston TC, et al. Enabled negatively regulates diaphanous-driven actin dynamics in vitro and in vivo. Dev Cell 2014; 28:394-408; PMID:24576424; http://dx.doi.org/10.1016/j.devcel.2014.01.015
- Nowotarski SH, McKeon N, Moser RJ, Peifer M. The actin regulators Enabled and Diaphanous direct distinct protrusive behaviors in different tissues during Drosophila development. Mol Biol Cell 2014; 25:3147-65; PMID:25143400; http://dx.doi.org/10.1091/mbc.E14-05-0951
