ABSTRACT
Neurons form precise patterns of connections. The cellular recognition mechanisms regulating the selection of synaptic partners are poorly understood. As final mediators of cell-cell interactions, cell surface and secreted molecules (CSMs) are expected to play important roles in this process. To gain insight into how neurons discriminate synaptic partners, we profiled the transcriptomes of 7 closely related neurons forming distinct synaptic connections in discrete layers in the medulla neuropil of the fly visual system. Our sequencing data revealed that each one of these neurons expresses a unique combination of hundreds of CSMs at the onset of synapse formation. We show that 21 paralogs of the defective proboscis extension response (Dpr) family are expressed in a unique cell-type-specific fashion, consistent with the distinct connectivity pattern of each neuron profiled. Expression analysis of their cognate binding partners, the 9 members of the Dpr interacting protein (DIP) family, revealed complementary layer-specific expression in the medulla, suggestive of interactions between neurons expressing Dpr and those expressing DIP in the same layer. Through coexpression analysis and correlation to connectome data, we identify neurons expressing DIP as a subset of the synaptic partners of the neurons expressing Dpr. We propose that Dpr-DIP interactions regulate patterns of connectivity between the neurons expressing them.
The proper assembly of neural circuits ultimately depends on the establishment of specific connections between synaptic partners. Recognition between synaptic partners is no simple feat considering that axons and dendrites of numerous different cell types coalesce to form densely packed neuropils. In this environment, the processes of a given neuron are in contact with those of many other neurons. How neurites discriminate synaptic partners remains a central question in neurobiology.
In its simplest formulation, Sperry's chemoaffinity hypothesisCitation1 suggests that neurons interact through specific surface labels. In this scenario, each neuronal cell type would express a particular surface label, and interactions would occur between the neurons that express labels that are binding partners. Surface labels are expressed during development and therefore the initial wiring of circuits would be determined by the specific gene-expression profile of each neuronal cell type. While this hypothesis was developed primarily to explain axon guidance, one can envision that this type of “lock and key” mechanism could also mediate recognition between synaptic partners.
Over the last few decades, biochemical and genetic approaches have identified, as Sperry hypothesized, cell recognition molecules that regulate axon guidance and the establishment of topographic maps. These aspects of wiring are regulated by a conserved set of cell surface and secreted molecules (CSMs) both in vertebrates and invertebrates. However, rather than being unique to just one set of neurons, this limited set of molecules is used in many different regions of the brain, and sometimes in a combinatorial fashion. These molecules include netrins, slits, semaphorins and their respective cognate cell surface receptors, as well as cadherins and immunoglobulin (Ig) superfamily proteins.Citation2 Notably, Ephs and Ephrins as well as Wnts regulate the formation of topographic maps though gradients.Citation3-5 Our knowledge of synaptic partner selection is more limited: so far, few examples of CSMs that regulate synaptic specificity have been identified. These include Syg1 and Syg2 in the worm,Citation6,7 Toll and Teneurin proteins in the fly olfactory systemCitation8,9 and Sidekick (Sdk) proteins in the mouse retina.Citation10 Families of CSMs are of potential interest since groups of proteins with similar structure could have similar functions. Importantly, divergence of their binding specificities could provide sufficient molecular diversity for complex recognition tasks between cells. Indeed, studies in the chick retina have raised the possibility that related Ig-superfamily proteins, with unique binding specificities and expressed in a cell-type specific fashion, regulate layer-specific patterns of connections between different neurons.Citation11-13
In order to expand our knowledge of the molecular logic underlying synaptic specificity we use the fly visual system as a model; in particular, the medulla neuropil. The medulla is structured in columns, which represent the processing units of discrete points in the visual space. Each one of these columns contains the processes of more than a 100 different types of neurons (Citation14 and A. Nern, personal communication). Each neuronal cell type has a unique morphology, and elaborates processes in particular layers of the medulla. Landmark studies using serial section electron microscopic reconstruction have recently determined the connectivity between neurons in several medulla columns.Citation15-17 That work revealed that these patterns of connectivity are complex, specific and reproducible. Within a layer, neurons form synapses only with a restricted set of neuronal types with processes in that layer.
In our recent studyCitation18 we addressed the issue of whether differences in CSMs between developmentally and functionally related neurons would account for their distinct patterns of connectivity. We focused on the R7 and R8 photoreceptors, and the 5 lamina monopolar neurons L1-L5, each of which elaborates processes and makes connections in a particular set of layers, with specific synaptic partners. To obtain their transcriptomes through RNA-seq, we developed markers and protocols to isolate these neuronal populations in a highly purified form at a developmental time just prior to synaptogenesis. These cell-type-specific transcriptomes allowed us to answer a long-standing question in the field: How many CSMs does a neuron express? The fly genome contains some 976 genes encoding CSMs, representing more than 80 different types of protein domains that could possibly mediate cell recognition events.Citation19 Using a stringent threshold (RPKM > 5 and an adjusted p-value <0.05) we observed that each cell type expressed a quarter to a third (i.e., between 247 for the R7 and 322 for the L3) of the genes encoding CSMs in the genome. While these neurons expressed roughly the same number of CSM genes, each neuron exhibited a unique pattern of expression. In addition, pairwise comparisons gave us insight into the CSM differences, and revealed marked differences between neurons, ranging from 49 (between R7 and R8) to 168 (between R7 and L4) differentially expressed CSM genes. Further analysis revealed that only a small fraction of genes is selectively enriched in only one of the 7 cell types profiled. Thus, each neuron has a complex and unique complement of CSMs, with marked differences between cell types.
The next challenge was to address how this astonishing complexity could be translated into specific patterns of connectivity. Since it had been suggested that members of gene families could play a role in regulating synaptic specificity,Citation11-13,20 we observed the distribution of the members of gene superfamilies and subfamilies in our cell-type-specific data set. Of the families analyzed, the 2-Ig domain defective proboscis extension response (Dpr) family, with 21 members aroused our attention.Citation21 Dprs have recently been shown to interact in trans in an ELISA-based in vitro assay with the 9 members of the 3-Ig domain family of Dpr interacting proteins (DIPs).Citation22 Their complex pattern of interactions includes examples of one Dpr paralog interacting with more than one DIP and vice versa (). While their functional significance remained unclear, they were expressed in the embryonic nervous system.Citation22,23 Our sequencing data indicated that each of the cell types analyzed expressed a particular combination of Dpr molecules; 10 of which we verified using genetically engineered protein trap reporters. While Dprs were found in the R and L cells analyzed, DIPs were not but for the exception of 2 (DIP-β in L4 and DIP-γ in L1 and L2). This observation suggested that DIPs could be expressed in other medulla neurons that interact with R7, R8 and L1-L5. Expression analysis of 6 of the 9 DIPs revealed strikingly specific layer patterns. Moreover, DIPs interacting with the Dprs expressed in R7 and L1-L5 neurons were expressed in the same layers where R7 and L1-L5 neurons made synaptic connections. This remarkable in vivo spatial correlation to in vitro Dpr-DIP interacting pairs led us to seek the medulla neurons expressing specific DIPs. Through colocalization experiments, using a panel of markers for medulla neurons and DIP reporters, we determined the respective DIP expression in a subset of medulla neurons. These included several synaptic partners for L1-L5, as revealed by the connectome data. We identified a total of 10 instances in which at least one synaptic partner for each lamina neuron can be correlated to Dpr-DIP interacting pairs (). We also observed that R7 neurons and their synaptic partner Dm8 express the Dpr11-DIP-γ pair. Indeed, in an accompanying study focusing on the Dpr11-DIP-γ expression, Carrillo and colleagues,Citation24 also detected their respective expression in the R7 and Dm8 synaptic pair in the medulla. Their study also shows that these Dpr-DIP interacting molecules are expressed in T4 and T5 medulla neurons (Dpr11) and lobula plate tangential cells (DIP-γ), which are synaptically connected in specific lobula plate layers.
Figure 1. Summary of cognate Dpr-DIP expression in L1-L5 neurons and a subset of their synaptic partners. (A.) Color coded Dpr-DIP interactions between lamina monopolar neurons and a subset of their synaptic partners. Single headed arrow indicates that the cell of origin is presynaptic to the receiving cell, which is the postsynaptic one. Thus, synaptic input goes in just one direction. Double headed arrows denote that both cells make connections onto each other. A subset of the Dprs expressed in each lamina neuron is annotated. Synaptic partners to the left of lamina neurons express at least a cognate DIP to the Dprs annotated in the corresponding lamina neuron. Synaptic partners to the right of lamina neurons do not show expression of analyzed cognate DIPsCitation18 (Tan, Xiao and Zipursky unpublished). Question marks indicate that the expression analysis of these DIPs, which could interact with annotated Dprs, is in progress. See L3 as an example. L3 expresses Dpr6 and Dpr10. Dpr10 can only interact with DIP-α, however Dpr6 can interact with DIPs –α, -β,−ζ and –ϵ. Tm9 and Tm20 do not express DIP-α or DIP-β. DIP-ζ and DIP-ϵ expression is being analyzed. Note that among the 10 Dpr-DIP predicted interactions between synaptic partners, DIP is expressed in the postsynaptic cell with one exception: Dm1, which expresses DIP-α, is presynaptic to L2. (B.) Summary of the Dpr-DIP interactomeCitation22,24. This diagram depicts in vitro interactions between Dprs and DIPs. Note that one Dpr can interact with more that one DIP.
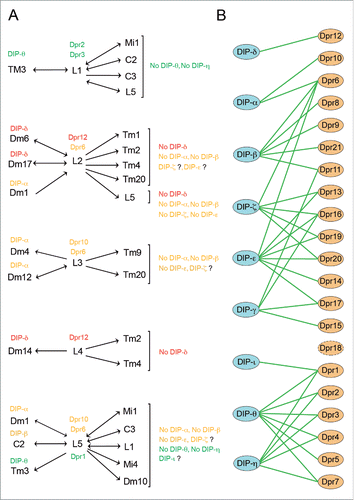
Based on these 12 examples, it is tempting to speculate that different combinations of Dpr-DIP proteins specify synaptic connections with in layers in the fly optic lobe. These observations are reminiscent of the molecular strategy suggested to bias connectivity in the vertebrate inner plexiform layer. In that layered neuropil, it has been proposed that Ig superfamily members from the Dscam, Sdk and Contactin (Cntn) subfamilies, expressed in mostly non-overlapping populations, regulate synaptic pairing between distinct sets of retinal neurons.Citation11-13 Support for this strategy comes from recent studies demonstrating the requirement for Sdk2 homophilic interactions for synapse establishment between a specific pair of amacrine and retinal ganglion neurons.Citation10 The similarities between the medulla and the inner plexiform layer suggest a conserved mechanism of synaptic pairing based on matched codes in presynaptic and postsynaptic neurons. The analysis of Dpr and DIP expression has focused on the R7, R8, lamina neurons and a set of medulla neurons for which drivers are available. However, both Dpr and DIP reporters show expression in other neurons in the optic lobe, suggesting that Dpr-DIP interactions between synaptic partners could take place between other synaptic pairs, and thus represent a widespread strategy in the optic lobe. A way to thoroughly evaluate the expression of Dpr and DIPs in the medulla, and generate a complete list of neurons expressing each Dpr and DIP, is the multi-color flip out (MCFO) methodCitation25 combined with Gal4 derivatives of Dpr and DIP reporter lines. Gene-specific Gal4 drivers, in combination with conditional FLP-mediated excision of stop cassettes, would result in stochastic expression of different combinations of MCFO reporters in scattered driver-expressing cells. Individual neurons of different colors can then be traced and identified by their morphologies. While these Gal4 lines are derived from genetic modifications on the genomic loci of Dprs and DIPs, and are expected to recapitulate the endogenous gene expression patterns, in situ hybridization or antibody staining would be needed to confirm this assumption. Combining expression data with the connectivity patterns in the medulla will reveal the extent of Dpr-DIP interactions between synaptic partners in the optic lobe.
So far, our sequencing results suggest that Dpr and DIPs are rarely co-expressed in the 7 cell types analyzed, posing the question of whether this is a consistent observation throughout the visual system, and more generally in the nervous system. Our data indicates that L1, L2 and L4 express DIP-γ and DIP-β respectively in addition to their specific sets of Dprs. The catalog generated through the MCFO approach will shed light on the level of Dpr-DIP co-expression in other medulla neurons. Outside the visual system, co-expression of Dprs and DIPs has been observed in interneurons and motorneurons in the ventral nerve cord.Citation24 In addition, given that most neurons are both presynaptic and postsynaptic to other neurons it is reasonable to speculate that some neurons could co-express Dpr and DIP paralogs, which could be used in different synaptic contacts. In such scenario Drps and DIPs could be expressed all over the membrane of the neurons and determine synaptic pairing between them, but not the location of the synaptic connection. Alternatively, Dprs and DIPs localization could rely on mechanisms regulating their targeting to specific subcellular membrane regions where connections are made (i.e. axon versus dendrites, or presynaptic active zones vs. postsynaptic densities). It is also unclear whether and how Dprs and DIPs determine the directionality of synaptic contacts. Among the 10 Dpr-DIP interactions between synaptic pairs presented in , 6 are observed between neurons that are both presynaptic and postsynaptic to each other, and thus do not provide information on whether Dpr expression determines presynaptic identify and DIP postsynaptic identity of the contact, or vice versa. In one case (Dm1→L2) DIP is expressed in the presynaptic cell, while we observed 3 instances (L3→Dm4, L4→Dm14 and L5→Tm3) where Dprs are expressed in the presynaptic cell. Tagging these proteins through CRISPR-based knock-in to their genomic loci combined with immunohistochemistry and electron microscopy would be necessary to explore the subcellular localization of cognate Dprs and DIPs in synaptic partners.
Nevertheless, it should be mentioned that in the case of the NMJ (see later in the text), both Dpr11 and its interacting partner DIP-γ have been detected presynaptically in motorneurons and postsynaptically in the muscle.Citation24 Such expression pattern suggests that there might be certain Dpr-DIP interacting pairs, for which Dpr and DIP molecules can localize both to pre- and post- synaptic domains when they are co-expressed in the same cell. This type of expression pattern still supports Dpr-DIP trans interactions between the motorneuron and the muscle, but cannot discard the existence of cis interactions in the motorneuron or in the muscle. In addition, either cis or trans interactions with this Dpr-DIP expression pattern could have different functions from interactions in which Dpr and DIP expression give presynaptic or postsynaptic identity respectively to a contact, or vice versa.
The exact role of Dpr-DIP interactions between synaptic partners is still unclear. So far, the only interacting pair studied is Dpr11-DIP-γ. Studies from the Zinn laboratory report abnormalities in Dpr11 and DIP-γ loss-of-function mutants.Citation24 These mutations affect the yellow-subtype R7 photoreceptor terminal morphology both in Dpr11 and DIP-γ mutants; consistent with a potential role in regulating synaptic specificity. While their possible role in synaptic pairing is attractive, they might regulate other aspects of circuit assembly. Interestingly, a substantial reduction in Dm8 numbers was observed in the analysis of DIP-γ mutants.Citation24 This is similar to our reported observation of a reduction in DIP-α expressing neurons in DIP-α mutantsCitation18 (L. Tan, S.L. Zipursky, unpublished data). Based on the analysis of several reporters for the same cell type, the Zinn group suggested that the reduction in Dm8 neurons is probably due to cell death.Citation24 In 9 of the 10 Dpr-DIP interactions observed between lamina neurons and their synaptic partners, DIPs are expressed in postsynaptic partners with one exception (). One possibility is that DIPs function as receptors mediating trophic support. Indeed, a similar mechanism regulates L3 survival through Jeb/Alk signaling.Citation26 Jeb is secreted by photoreceptor cells and binds to Alk expressed in L3 neurons. The absence of either Jeb or Alk causes L3 neurons to die. In addition to trophic support, Dpr-DIP interactions could have a second function regulating the development of synaptic-terminals, as it has been observed in the case of the neuromuscular junction.Citation24 Both Dpr11 and DIP-γ mutants present many small clustered boutons and defects in synaptic transmission. The satellite bouton phenotype is similar to that observed in mutations resulting in an increase in retrograde bone morphogenetic protein (BMP) signaling in motoneurons.Citation27 Indeed, Dpr11 and DIP-γ genetically interact with genes in this mediator of synaptic growth pathway. Interestingly, these phenotypes can be rescued by presynaptic Dpr11 and postsynaptic DIP-γ expression, and vice versa; suggesting that both complexes have equivalent functions in presynaptic-terminal maturation. Given the variety of defects observed in this single Dpr-DIP pair and the number of possible Dpr-DIP interactions, detailed phenotypic analysis of mutations in genes coding for Dpr-DIP is essential. In many cases, a given DIP can interact with several Dprs and vice versa. Thus, interactions with different partners could have either redundant or independent functions. To distinguish between these possibilities the use of individual null mutants and combinations of them, when necessary, will be essential. The CRISPR-Cas9 mediated gene knock-out approach allows for the generation of these mutations in a fast and reliable manner.
Dprs and DIPs are likely to be just one set of players involved in synaptic specificity in the medulla. In fact, our work identified other families of genes encoding CSMs known to mediate cell-cell interactions that were enriched in a cell-type-specific fashion. Those include: Ig –superfamily members,Citation28,29 among which we also observed differential expression of paralogs in subfamilies such as the BeatsCitation30 and SidesCitation31; leucine-rich repeat (LRR)Citation32 and epidermal growth factor (EGF)Citation33,34 domain containing proteins; as well as members of the Tetraspanin family.Citation35,36
The laboratory of Dr. Garcia probed interactions between the extracellular domains of 202 proteins from the Ig-superfamily, and the LRR and fibronectin III families. Of the 20,503 combinations tested in their ELISA-based assay, they identified 106 interactions; 83 of which had never been reported before, including for example Dpr-DIP interactions.Citation22 In addition, the connectome project of Janelia Research Campus has generated extensive data concerning the area of contact between neurons in a column and the existence of synapses between neurons in contact, as well as the number, position and directionality of synapses in the adult column.Citation15-17 Superimposing interactome and connectome data on our cell-type-specific gene expression profile has revealed putative CSM interactions between R7, R8 and L1-L5, which could shape the adult morphology of these neurons, membrane contacts between apposing neurons and their synaptic patterns. An intriguing case is the relationship between R7, R8 and L3 neurons. These neurons are developmentally dependent upon each other and display intricate physical interactions with each other. While in the adult column the R7 and L3 membranes barely contact, the R8 has roughly the same contact area with R7 and L3Citation16 (S. Takemura, personal communication). However, interestingly, the R8 makes synapses with R7 but not with L3.Citation16 Based on our RNA-seq, data we have identified 14 putative CSM interactions that could take place between these neurons, and that may positively or negatively regulate contact and/or synaptic specificity (L. Tan, M. Morey and S.L. Zipursky, unpublished observations). Addressing some of these questions is technically challenging at the moment, but it is expected that the convergence of improved histological and genetic tools, together with advances in light microscopy imaging, will help unravel the molecular logic behind synaptic specificity.
Abbreviations
| CSM | = | cell surface and secreted molecule |
| Dpr | = | defective proboscis extension |
| DIP | = | Dpr interacting protein |
| Ig | = | Immunoglobulin |
| Sdk | = | Sidekick |
| RNA-seq | = | RNA sequencing |
| Dpr | = | defective proboscis extension response |
| Cntn | = | Contactin |
| MCFO | = | multi-color flip out |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
I thank L. Tan, A. Nern, S.L. Zipursky, and an anonymous reviewer for critical reading and thoughtful comments on this manuscript.
References
- Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci U S A [Internet] 1963 [cited 2015 Sep 27]; 50:703-10. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=221249&tool=pmcentrez&rendertype=abstract; PMID:14077501; http://dx.doi.org/10.1073/pnas.50.4.703
- O'Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci 2009; 32:383-412; PMID:19400716; http://dx.doi.org/10.1146/annurev.neuro.051508.135614
- Cang J, Feldheim DA. Developmental mechanisms of topographic map formation and alignment. Annu Rev Neurosci [Internet] 2013 [cited 2015 Sep 27]; 36:51-77. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23642132; PMID:23642132; http://dx.doi.org/10.1146/annurev-neuro-062012-170341
- Schmitt AM, Shi J, Wolf AM, Lu C-C, King LA, Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature [Internet] 2006 [cited 2015 Sep 27]; 439:31-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16280981; PMID:16280981; http://dx.doi.org/10.1038/nature04334
- Triplett JW, Feldheim DA. Eph and ephrin signaling in the formation of topographic maps. Semin Cell Dev Biol [Internet] 2012 [cited 2015 Sep 27]; 23:7-15. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3288406&tool=pmcentrez&rendertype=abstract; PMID:22044886; http://dx.doi.org/10.1016/j.semcdb.2011.10.026
- Shen K, Bargmann CI. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell [Internet] 2003 [cited 2015 Sep 27]; 112:619-30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12628183; PMID:12628183; http://dx.doi.org/10.1016/S0092-8674(03)00113-2
- Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell [Internet] 2004 [cited 2015 Sep 27]; 116:869-81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15035988; PMID:15035988; http://dx.doi.org/10.1016/S0092-8674(04)00251-X
- Hong W, Mosca TJ, Luo L. Teneurins instruct synaptic partner matching in an olfactory map. Nature [Internet] 2012 [cited 2015 Sep 27]; 484:201-7. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3345284&tool=pmcentrez&rendertype=abstract; PMID:22425994; http://dx.doi.org/10.1038/nature10926
- Ward A, Hong W, Favaloro V, Luo L. Toll receptors instruct axon and dendrite targeting and participate in synaptic partner matching in a Drosophila olfactory circuit. Neuron [Internet] 2015 [cited 2015 Aug 28]; 85:1013-28. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25741726; PMID:25741726; http://dx.doi.org/10.1016/j.neuron.2015.02.003
- Krishnaswamy A, Yamagata M, Duan X, Hong YK, Sanes JR. Sidekick 2 directs formation of a retinal circuit that detects differential motion. Nature [Internet] 2015 [cited 2015 Aug 19]; 524:466-70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26287463; PMID:26287463; http://dx.doi.org/10.1038/nature14682
- Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell [Internet] 2002 [cited 2016 May 18]; 110:649-60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12230981; PMID:12230981; http://dx.doi.org/10.1016/S0092-8674(02)00910-8
- Yamagata M, Sanes JR. Expanding the Ig superfamily code for laminar specificity in retina: expression and role of contactins. J Neurosci [Internet] 2012 [cited 2015 Sep 27]; 32:14402-14. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3488879&tool=pmcentrez&rendertype=abstract; PMID:23055510; http://dx.doi.org/10.1523/JNEUROSCI.3193-12.2012
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature 2008; 451:465-9; PMID:18216854; http://dx.doi.org/10.1038/nature06469
- Fischbach KF, Dittrich AP. The optic lobe of Drosophila melanogaster. I: A. Golgi analysis of wild-type structure. Cell Tissue Res 1989; 258:441-75; PMID:6187612; http://dx.doi.org/10.1007/BF00218858
- Takemura S, Xu CS, Lu Z, Rivlin PK, Parag T, Olbris DJ, Plaza S, Zhao T, Katz WT, Umayam L, et al. Synaptic circuits and their variations within different columns in the visual system of Drosophila. Proc Natl Acad Sci U S A [Internet] 2015 [cited 2016 Apr 18]; 112:13711-6. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4640747&tool=pmcentrez&rendertype=abstract; PMID:26483464; http://dx.doi.org/10.1073/pnas.1509820112
- Takemura SY, Lu Z, Meinertzhagen IA. Synaptic circuits of the Drosophila optic lobe: The input terminals to the medulla. J Comp Neurol 2008; 509:493-513.; PMID:18537121; http://dx.doi.org/10.1002/cne.21757
- Takemura S, Bharioke A, Lu Z, Nern A, Vitaladevuni S, Rivlin PK, Katz WT, Olbris DJ, Plaza SM, Winston P, et al. A visual motion detection circuit suggested by Drosophila connectomics. Nature [Internet] 2013; 500:175-81. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3799980&tool=pmcentrez&rendertype=abstract; PMID:23925240; http://dx.doi.org/10.1038/nature12450
- Tan L, Zhang KX, Pecot MY, Nagarkar-Jaiswal S, Lee PT, Takemura SY, McEwen JM, Nern A, Xu S, Tadros W, et al. Ig superfamily ligand and receptor pairs expressed in synaptic partners in drosophila. Cell [Internet] 2015 [cited 2016 Apr 17]; 163:1756-69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26687360; PMID:26687360; http://dx.doi.org/10.1016/j.cell.2015.11.021
- Kurusu M, Cording A, Taniguchi M, Menon K, Suzuki E, Zinn K. A screen of cell-surface molecules identifies leucine-rich repeat proteins as key mediators of synaptic target selection. Neuron [Internet] 2008 [cited 2016 May 18]; 59:972-85. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2630283&tool=pmcentrez&rendertype=abstract; PMID:18817735; http://dx.doi.org/10.1016/j.neuron.2008.07.037
- Goodman CS, Bastiani MJ, Doe CQ, du Lac S, Helfand SL, Kuwada JY, Thomas JB. Cell recognition during neuronal development. Science [Internet] 1984 [cited 2016 May 18]; 225:1271-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6474176; PMID:6474176; http://dx.doi.org/10.1126/science.6474176
- Nakamura M, Baldwin D, Hannaford S, Palka J, Montell C. Defective proboscis extension response (DPR), a member of the Ig superfamily required for the gustatory response to salt. J Neurosci 2002; 22:3463-72; PMID:11978823; http://dx.doi.org/20026336
- Özkan E, Carrillo R a, Eastman CL, Weiszmann R, Waghray D, Johnson KG, Zinn K, Celniker SE, Garcia KC. An extracellular interactome of immunoglobulin and LRR proteins reveals receptor-ligand networks. Cell 2013; 154; PMID:23827685; http://dx.doi.org/10.1016/j.cell.2013.06.006
- Tomancak P, Beaton A, Weiszmann R, Kwan E, Shu S, Lewis SE, Richards S, Ashburner M, Hartenstein V, Celniker SE, et al. Systematic determination of patterns of gene expression during Drosophila embryogenesis. Genome Biol [Internet] 2002; [cited 2016 Jul 28]; 3:RESEARCH0088. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12537577
- Carrillo RA, Özkan E, Menon KP, Nagarkar-Jaiswal S, Lee P-T, Jeon M, Birnbaum ME, Bellen HJ, Garcia KC, Zinn K. Control of Synaptic Connectivity by a Network of Drosophila IgSF Cell Surface Proteins. Cell [Internet] 2015 [cited 2016 Apr 27]; 163:1770-82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26687361; PMID:26687361; http://dx.doi.org/10.1016/j.cell.2015.11.022
- Nern A, Pfeiffer BD, Rubin GM. Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. Proc Natl Acad Sci U S A [Internet] 2015 [cited 2015 May 13]; 112:E2967-76. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4460454&tool=pmcentrez&rendertype=abstract; PMID:25964354; http://dx.doi.org/10.1073/pnas.1506763112
- Pecot M, Chen Y, Akin O, Chen Z, Tsui CYK, Zipursky SL. Sequential axon-derived signals couple target survival and layer specificity in the drosophila visual system. Neuron [Internet] 2014; 82:320-33. Available from: http://dx.doi.org/10.1016/j.neuron.2014.02.045; PMID:24742459; http://dx.doi.org/10.1016/j.neuron.2014.02.045
- O'Connor-Giles KM, Ganetzky B. Satellite signaling at synapses. Fly (Austin) [Internet] 2008 [cited 2016 May 24]; 2:259-61. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3744159&tool=pmcentrez&rendertype=abstract; PMID:20798607; http://dx.doi.org/10.4161/fly.7133
- Fischbach KF, Linneweber GA, Andlauer TFM, Hertenstein A, Bonengel B, Chaudhary K. The irre cell recognition module (IRM) proteins. J Neurogenet [Internet] 2009 [cited 2015 Mar 26]; 23:48-67. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19132596; PMID:19132596; http://dx.doi.org/10.1080/01677060802471668
- Zipursky SL, Wojtowicz WM, Hattori D. Got diversity? Wiring the fly brain with Dscam. Trends Biochem Sci [Internet] 2006 [cited 2015 Mar 26]; 31:581-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16919957; PMID:16919957; http://dx.doi.org/10.1016/j.tibs.2006.08.003
- Pipes GC, Lin Q, Riley SE, Goodman CS. The Beat generation: a multigene family encoding IgSF proteins related to the Beat axon guidance molecule in Drosophila. Development 2001; 128:4545-52; PMID:11714679
- Sink H, Rehm EJ, Lee R, Bulls YM, Goodman CS. Sidestep encodes a target-derived attractant essential for motor axon guidance in Drosophila. Cell 2001; 105:57-67; PMID:11301002; http://dx.doi.org/10.1016/S0092-8674(01)00296-3
- de Wit J, Hong W, Luo L, Ghosh A. Role of leucine-rich repeat proteins in the development and function of neural circuits. Annu Rev Cell Dev Biol [Internet] 2011 [cited 2016 May 24]; 27:697-729. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21740233; PMID:21740233; http://dx.doi.org/10.1146/annurev-cellbio-092910-154111
- Kenzelmann D, Chiquet-Ehrismann R, Tucker RP. Teneurins, a transmembrane protein family involved in cell communication during neuronal development. Cell Mol Life Sci [Internet] 2007 [cited 2015 Mar 11]; 64:1452-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17502993; PMID:17502993; http://dx.doi.org/10.1007/s00018-007-7108-9
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell [Internet] 1994 [cited 2015 Mar 28]; 78:409-24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8062384; PMID:8062384; http://dx.doi.org/10.1016/0092-8674(94)90420-0
- Fradkin LG, Kamphorst JT, DiAntonio A, Goodman CS, Noordermeer JN. Genomewide analysis of the Drosophila tetraspanins reveals a subset with similar function in the formation of the embryonic synapse. Proc Natl Acad Sci U S A 2002; 99:13663-8; PMID:12370414; http://dx.doi.org/10.1073/pnas.212511099
- Kopczynski CC, Davis GW, Goodman CS. A neural tetraspanin, encoded by late bloomer, that facilitates synapse formation. Science [Internet] 1996 [cited 2015 Feb 25]; 271:1867-70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8596956; PMID:8596956; http://dx.doi.org/10.1126/science.271.5257.1867
