ABSTRACT
Apoptosis-induced proliferation (AiP) maintains tissue homeostasis following massive stress-induced cell death. During this phenomenon, dying cells induce proliferation of the surviving cells to compensate for the tissue loss, and thus restore organ size. Along with wound healing and tissue regeneration, AiP also contributes to tumor repopulation following radiation or chemotherapy. There are several models of AiP. Using an “undead” AiP model that causes hyperplastic overgrowth of Drosophila epithelial tissue, we recently demonstrated that extracellular reactive oxygen species (eROS) are produced by undead epithelial cells, and are necessary for inducing AiP and overgrowth. Furthermore, hemocytes, the Drosophila blood cells, are seen adjacent to the undead epithelial tissue, and may secrete the TNF ortholog Eiger that signals through the TNF receptor to active Jun-N-terminal kinase (JNK) in the undead tissue and induce proliferation. We propose that undead epithelial tissue triggers an inflammatory response that resembles recruitment of macrophages to human epithelial tumors, and that these tumor-associated macrophages release signals for proliferation and tumor growth of the epithelium. This Extra View article summarizes these recent findings with a focus on the role of eROS for promoting regeneration and inflammation-induced tumorigenesis.
Synopsis of our recently published work
Compensatory proliferation is a mechanism that maintains tissue homeostasis after significant stress-induced cell death.Citation1 Compensatory proliferation initiated by active caspases is termed apoptosis-induced compensatory proliferation (AiP).Citation2,3 The role of AiP in tissue regeneration is demonstrated in multiple models including Hydra, Planaria, Drosophila, Xenopus and mice.Citation4 In mice and potentially in humans, AiP can contribute to tumor repopulation following radiation or chemotherapy, where the dying tumor cells promote the proliferation of surviving tumor cells.Citation5,6 In Drosophila, several studies have identified different factors that contribute to AiP. These studies make use of the “undead” model of AiP, in which the apoptotic cascade is initiated by expressing pro-apoptotic factors such as hid, but the execution of cell death is inhibited by co-expression of the effector caspase inhibitor p35 (ref. Citation7) (). In this experimental setup, the active initiator caspase Dronc (the Drosophila Caspase-9 ortholog), while unable to kill cells due to p35 expression, continues to induce the release of signals for AiP, thus causing hyperplastic overgrowth of the epithelial tissueCitation4 (). Using an eye-specific undead AiP model (ey-Gal4 UAS-hid UAS-p35 (ey>hid-p35)),Citation8 we recently demonstrated that continued signaling by active Dronc in undead cells leads to generation of extracellular reactive oxygen species (eROS) via the activity of NADPH oxidases, in particular Dual Oxidase (Duox) ().Citation9 These eROS drive AiP and cause overgrowth of the undead tissue as loss of Duox or mis-expression of extracellular catalases (hCatS) suppresses the overgrowth phenotype (). Thus, eROS are necessary for AiP; however, if they are sufficient to cause overgrowth of undead tissue needs to be determined. One function of eROS is the activation of hemocytes, Drosophila macrophages, on the undead epithelial tissue. Activated hemocytes attached to the undead cells secrete inflammatory cytokines such as the TNF ortholog Eiger, which triggers activation of JNK back in the undead cells through activation of the TNF receptor Grindelwald ().Citation9 JNK signaling then promotes the release of mitogens such as Wingless (Wg) which triggers AiP. In undead cells, JNK also triggers transcription of the pro-apoptotic gene hid which stimulates an amplification loop for AiP and overgrowthCitation10 (). Whether the eROS have any other function to play in AiP – for example whether they directly activate JNK in undead cells () – remains to be determined.
Figure 1. Schematic view of our current understanding of the cellular mechanisms of apoptosis-induced compensatory proliferation (AiP). Undead cells can be induced by co-expression of the pro-apoptotic gene hid and the effector caspase inhibitor p35. Because P35 specifically inhibits the effector caspase DrICE (and Dcp-1; not shown), the initiator caspase Dronc cannot induce apoptosis (“undead” state), but remains active for apoptosis-independent functions. One of these functions is the activation of the transmembrane NADPH oxidase Duox, which generates extracellular ROS (eROS) such as superoxide and hydrogen peroxide. eROS activate and change the behavior of hemocytes to become proliferation-promoting.Citation3 This is accomplished through release of Eiger which activates the JNK pathway in undead and neighboring surviving cells. JNK stimulates the production of mitogens such as wingless (wg) (and dpp and spi; not shown) for AiP. In undead cells, JNK also induces expression of hid which sets an amplification loop in motion. Factors involved in apoptosis are indicated in blue, in JNK activation in green and in production of ROS in red. Question marks indicate unknown mechanisms.
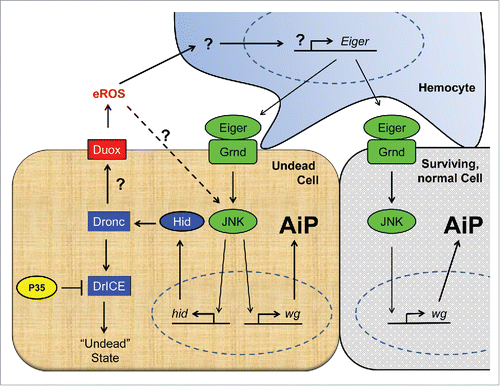
Figure 2. Undead tissue displays eROS-dependent hyperplastic overgrowth. Co-expression of hid and p35 in the developing eye imaginal disc using eyeless-Gal4 (ey-Gal4 UAS-hid UAS-p35 (ey>hid-p35)) promotes hyperplastic overgrowth of head cuticle with pattern duplications compared to wild-type control (A, B). Simultaneous expression of an extracellular catalase (hCatS), which neutralizes H2O2, suppresses overgrowth and normalizes the pattern of the adult head (C). Scale bars, 200μm.

eROS – damage response or redox signaling?
Reactive oxygen species (ROS) are partially reduced metabolites of oxygen, which include superoxide anion (O2−), hydroxyl anion (OH−) and hydrogen peroxide (H2O2).Citation11 ROS are either generated as by-products of aerobic respiration via electron transfer reactions in the mitochondria, or via membrane-associated NADPH oxidases such as Nox and Duox.Citation12 An electron reduction of molecular O2 generates highly reactive and unstable O2−, which is rapidly converted into more stable H2O2, a weak oxidizing agent able to diffuse across cellular membranes. Due to their reactive nature, accumulation of O2− and OH− radicals are more associated with oxidative stress in the cell causing damage to DNA, proteins and lipids which can lead to various pathologies and diseases. However, at optimal concentrations, ROS, in particular H2O2, can function as secondary messengers or signaling molecules to regulate normal cellular functions as part of controlled redox signaling.Citation11,13
Control of hemocyte activity by eROS
Duox-generated eROS, especially H2O2, have a well characterized function during wound repair processes in embryos where they attract and activate hemocytes to the wound site.Citation14 Two recent reports, including our own, demonstrated that repair processes in imaginal discs also require ROS.Citation9,15 In the first report, ROS were generated in response to a short pulse of apoptosis in larval wing imaginal discs. Similar to embryonic wound repair, ROS are exclusively restricted to tissue repair and overgrowth was not observed.Citation15 A role of hemocytes was not addressed in this work. In our report, we demonstrated that eROS trigger over-proliferation of undead epithelial tissue in eye and wing imaginal discs.Citation9 Intracellular ROS – if they are produced – have no or very little contribution to the overgrowth phenotype. In this case, eROS activate hemocytes, which induce proliferation of undead epithelial cells causing overgrowth.
Usually, hemocytes do not control proliferation and instead are involved in non-proliferative processes such as phagocytosis of apoptotic cells, host defense against invading pathogens and tissue repair processes.Citation16 Therefore, in the context of undead tissue, hemocytes appear to adopt novel properties that enable them to promote proliferation.Citation9 Similar observations have also been reported for a Drosophila tumor model.Citation17 How eROS trigger this proliferation-inducing property of hemocytes is unknown. However, because eROS activate hemocytes for non-proliferative wound repair in embryos, it is likely that undead tissue generates additional signals that together with eROS mediate the proliferation-inducing property of hemocytes.
It is interesting to note that hemocytes on undead tissue change their morphology and location. On control eye-antennal imaginal discs, they form large cell aggregates along the morphogenetic furrow at the eye portion and at the antennal portion of the disc. However, in response to exposure to eROS generated by undead tissue, hemocytes separate from the cellular clusters, are less spherical and extend cellular protrusions which make contact with the epithelial tissue of the imaginal discsCitation9 (). Because hemocytes usually do not promote proliferation, these observations suggest that they are “alternatively activated,” similar to tumor-associated macrophages in human cancer.Citation18
Mammalian macrophages are a functionally and phenotypically diverse group of innate immune cells. They are generally classified by their activation states into M1 macrophages, which represent the “classically activated” cells, and M2 macrophages which include the “alternatively activated” macrophages.Citation18 M1 polarized macrophages are primed for pro-inflammatory responses, and display microbicidal and tumoricidal properties. M2 macrophages on the other hand promote anti-inflammatory responses, and are involved with tissue repair and tumor promotion.Citation18,19 Most human solid tumors have high density of macrophage infiltration, which usually correlates with poor patient prognosis. These tumor-associated macrophages (TAMs) show characteristics of alternatively-activated M2 state. TAMs are responsible for promoting tumor inflammation, DNA damage, metastasis and tumor repopulation.Citation18 Like mammalian macrophages, Drosophila hemocytes show functional plasticity; however, whether the hemocytes undergo differential activation is something that is not well understood. Thus, it is tempting to assume that the tumor or the undead epithelium in Drosophila promotes activation of hemocytes to induce tumor progression and overgrowth.
Molecular mechanisms of ROS action
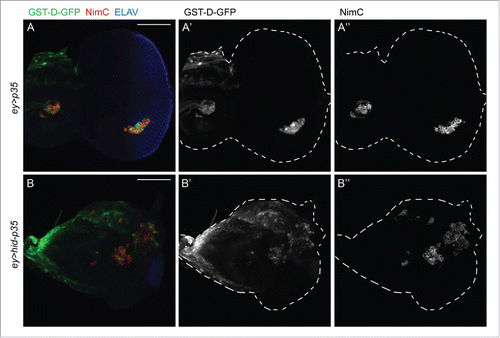
It is unknown how Duox-generated eROS from undead tissue activate hemocytes. Redox sensitive signaling events are often triggered close to the source of ROS production, and because hemocytes are already attached to eye-antennal imaginal discs,Citation9 they may be directly activated by Duox-generated ROS. Indeed, the redox reporter GST-D-GFPCitation20 is active in hemocytes attached to undead eye imaginal discs (). However, the reporter is also induced in hemocytes attached to the control eye discs (). This finding may suggest that hemocytes do not further respond to eROS. Nevertheless, another possibility to explain this result is that hemocytes at control discs are primed for redox signaling and can respond rapidly when they actually are exposed to eROS.
Figure 3. The redox reporter GST-D-GFP is expressed in hemocytes attached to both control and undead eye-antennal imaginal discs. Shown are eye-antennal imaginal discs of (A) ey-Gal4 UAS-p35 (ey > p35; control) and (B) ey-Gal4 UAS-hid UAS-p35 (ey > hid-p35; undead) genotype. Hemocytes are labeled using the NimC antibody (red in A, B; gray in A, “B”). GST-D-GFP is labeled in green in (A, B) and gray in (A’, B’). NimC and GFP labeling overlap. Shown also in (A, B) is labeling with ELAV which marks photoreceptor neurons in the posterior part of the eye disc. ey-Gal4 is only expressed in the anterior portion of the eye disc, but when expressing hid and p35, ey > Gal4 drives overgrowth of anterior tissue into the posterior part at the expense of photoreceptors. Scale bars, 100μm.
ROS can modify proteins by oxidizing key residues in proteins causing alterations in protein structure and/or function. Oxidative modification of cysteine residues is the most common protein modification mediated by H2O2.Citation11 This modification can cause formation of intra- and/or inter-disulfide bridges and can lead to changes in the protein activity levels, or may facilitate formation of protein complexes.Citation11 H2O2 can also regulate several cell adhesion molecules like P-selectins, E-selectins, ICAM-1 and VCAM-1, either by direct oxidation or transcriptionally via redox-sensitive transcription factors, thereby regulating adhesion and migration of inflammatory blood cells.Citation21 Along with cell adhesion molecules, H2O2 also affects junction proteins, thereby causing changes in cell-cell adhesion.Citation21 H2O2 also catalyzes the dityrosine-dependent crosslinking of extra-cellular matrix (ECM).Citation11 For example, a homolog of Duox in C. elegans has been shown to induce dityrosine crosslinks of collagen to stabilize the ECM.Citation22 In Drosophila, H2O2 generated by Duox is involved in maturation of the wing during the last day of pupal development, likely by physically crosslinking the dorsal and ventral surface cuticles.Citation23,24 It will be an interesting focus for further study to examine whether the eROS from undead cells cause any oxidative modifications on cell adhesion molecules or the ECM of undead cells, or if they directly modify the activity of hemocytes.
Stress activated signaling cascades that activate JNK or p38 kinases are also responsive to ROS. For example, ROS can activate Apoptosis signal-regulating kinase 1 (ASK1), a MAPKKK, by causing its dissociation from its inhibitor Thioredoxin, and recruitment of TRAF2 and TRAF6 to form a multimeric complex with ASK1, leading to activation of downstream signaling pathways, including JNK and p38.Citation25,26 ASK1 thus acts as an important molecular switch, responding to oxidative changes and activating stress response signaling pathways. It is involved in mediating apoptosis via TNFα-induced JNK activation, or ATP-induced activation of p38. (ref. Citation27,28) ASK1 also causes production of inflammatory cytokines in response to ROS in macrophages and other innate immune cells, and is required for recruitment and activation of macrophages to skin wounds in mice, which then mediate hair regeneration.Citation29 While we have not found a requirement of ASK1 in the undead epithelial cells for activation of JNK,Citation8 we have observed active JNK in hemocytes (unpublished data), and an interesting follow-up question would be to examine if eROS from the undead tissue enters the cytosol of hemocytes stimulating activation of ASK1 and downstream JNK signaling activity. This could be one of the mechanisms by which eROS activate hemocytes on the undead tissue, ultimately leading to production of TNF Eiger from hemocytes.
Differential requirement of ROS for wound repair, regeneration and over-proliferation/cancer
Many recent studies explored the requirement of ROS to induce regenerative responses, not only in flies but also other organismsCitation14,15,30-32 indicating an universal function of redox signaling for regeneration and tissue repair. These studies indicate that ROS act in the initial stage of wound detection, causing attraction of inflammatory cells to wounds and initiating an inflammatory response, culminating in regeneration and repair of the wound. Along with regeneration, ROS have also been shown to be important for driving tumorigenesis. Cancer cells show high levels of ROS, which can cause an increase in activation of mitogenic signaling pathways in these cells promoting tumor formation.Citation13 As the production of ROS is observed in both the regenerative response as well as cancer cells, it will be important to understand what distinguishes both responses in terms of ROS action. Whether it is the duration of ROS production – short pulse in regeneration vs. sustained production in cancer cells – or the threshold levels of ROS will be an interesting area for further studies. Very relevant in this respect is an old theory that classifies tumors as “wounds that do not heal.”Citation33 Potentially, the level, type and duration of ROS shift the response from wound healing to cancer progression.
A recent study demonstrated that damaged cells can induce organ-level quorum sensing wherein stress beyond a particular threshold leads to regeneration via recruitment of macrophages to the wounded skin in mice, which then secrete TNFα and other inflammatory cytokines.Citation34 It is tempting to speculate that ROS may act as a quorum sensing molecule determining the degree of stress, and signaling to macrophages to induce either a regenerative response or promoting overgrowth as is seen in the undead model.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank our colleagues in the Bergmann lab especially Caitlin E. Fogarty, Jillian L. Lindblad, Meghana Tare and Alla Amcheslavsky for fruitful discussions during the course of this work.
Funding
This work was supported by MIRA grant R35 GM118330 from the National Institute of General Medical Sciences (NIGMS) of the NIH.
References
- Haynie JL, Bryant PJ. The effects of X-rays on the proliferation dynamics of cells in the imaginal wing disc ofDrosophila melanogaster. Wilhelm Roux's Arch Dev Biol 1977; 183:85-100; http://dx.doi.org/10.1007/BF00848779
- Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell! Trends Cell Biol 2008; 18:467-73
- Mollereau B, Perez-Garijo A, Bergmann A, Miura M, Gerlitz O, Ryoo HD, Steller H, Morata G. Compensatory proliferation and apoptosis-induced proliferation: a need for clarification. Cell Death Differ 2012; 20:181; PMID:22722336; http://dx.doi.org/10.1038/cdd.2012.82
- Ryoo HD, Bergmann A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb Perspect Biol 2012; 4:1-17; http://dx.doi.org/10.1101/cshperspect.a008797
- Li F, Huang Q, Chen J, Peng Y, Roop DR, Bedford JS, Li CY. Apoptotic cells activate the ‘phoenix rising' pathway to promote wound healing and tissue regeneration. Sci Signal 2010; 3:ra13; PMID:20179271
- Huang Q, Li F, Liu X, Li W, Shi W, Liu FF, O'Sullivan B, He Z, Peng Y, Tan AC, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med 2011; 17:860-6; PMID:21725296; http://dx.doi.org/10.1038/nm.2385
- Martín FA, Peréz-Garijo A, Morata G. Apoptosis in Drosophila: compensatory proliferation and undead cells. Int J Dev Biol 2009; 53:1341-7; http://dx.doi.org/10.1387/ijdb.072447fm
- Fan Y, Wang S, Hernandez J, Yenigun VB, Hertlein G, Fogarty CE, Lindblad JL, Bergmann A. Genetic models of apoptosis-induced proliferation decipher activation of JNK and identify a requirement of EGFR signaling for tissue regenerative responses in Drosophila. PLoS Genet 2014; 10:e1004131; http://dx.doi.org/10.1371/journal.pgen.1004131
- Fogarty CE, Diwanji N, Lindblad JL, Tare M, Amcheslavsky A, Makhijani K, Brückner K, Fan Y, Bergmann A. Extracellular reactive oxygen species drive apoptosis-induced proliferation via drosophila macrophages. Curr Biol 2016; 26(5):575-84; PMID:26898463; http://dx.doi.org/10.1016/j.cub.2015.12.064
- Shlevkov E, Morata G. A dp53/JNK-dependant feedback amplification loop is essential for the apoptotic response to stress in Drosophila. Cell Death Differ 2012; 19:451-60; PMID:21886179; http://dx.doi.org/10.1038/cdd.2011.113
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 2000; 279:L1005-28; PMID:11076791
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004; 4:181-9; PMID:15039755; http://dx.doi.org/10.1038/nri1312
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 2014; 24:R453-62; PMID:24845678; http://dx.doi.org/10.1016/j.cub.2014.03.034
- Razzell W, Evans IR, Martin P, Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr Biol 2013; 23:424-9; PMID:23394834; http://dx.doi.org/10.1016/j.cub.2013.01.058
- Santabárbara-Ruiz P, López-Santillán M, Martínez-Rodríguez I, Binagui-Casas A, Pérez L, Milán M, Corominas M, Serras F. ROS-Induced JNK and p38 signaling is required for unpaired cytokine activation during Drosophila regeneration. PLoS Genet 2015; 11:1-26; http://dx.doi.org/10.1371/journal.pgen.1005595
- Evans IR, Wood W. Drosophila blood cell chemotaxis. Curr Opin Cell Biol 2014; 30C:1-8; http://dx.doi.org/10.1016/j.ceb.2014.04.002
- Cordero JB, Macagno JP, Stefanatos RK, Strathdee KE, Cagan RL, Vidal M. Oncogenic Ras diverts a host TNF tumor suppressor activity into tumor promoter. Dev Cell 2010; 18:999-1011
- Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: Functional diversity, clinical significance, and open questions. Semin Immunopathol 2013; 35:585-600; PMID:23657835; http://dx.doi.org/10.1007/s00281-013-0367-7
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958-69; PMID:19029990; http://dx.doi.org/10.1038/nri2448
- Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in drosophila. Dev Cell 2008; 14:76-85
- Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 2014; 20:1126-67; PMID:23991888; http://dx.doi.org/10.1089/ars.2012.5149
- Edens WA, Sharling L, Cheng G, Shapira R, Kinkade JM, Lee T, Edens HA, Tang X, Sullards C, Flaherty DB, et al. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J Cell Biol 2001; 154:879-91; PMID:11514595; http://dx.doi.org/10.1083/jcb.200103132
- Anh NT, Nishitani M, Harada S, Yamaguchi M, Kamei K. Essential role of duox in stabilization of Drosophila wing. J Biol Chem 2011; 286:33244-51; PMID:21808060; http://dx.doi.org/10.1074/jbc.M111.263178
- Hurd TR, Liang FX, Lehmann R. Curly encodes dual oxidase, which acts with heme peroxidase curly su to shape the adult Drosophila Wing. PLoS Genet 2015; 11:1-15; http://dx.doi.org/10.1371/journal.pgen.1005625
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 1998; 17:2596-606; PMID:9564042; http://dx.doi.org/10.1093/emboj/17.9.2596
- Noguchi T, Takeda K, Matsuzawa A, Saegusa K, Nakano H, Gohda J, Inoue J, Ichijo H. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J Biol Chem 2005; 280:37033-40; PMID:16129676; http://dx.doi.org/10.1074/jbc.M506771200
- Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of Apoptosis by ASK1, a Mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997; 275:90-4; PMID:8974401; http://dx.doi.org/10.1126/science.275.5296.90
- Noguchi T, Ishii K, Fukutomi H, Naguro I, Matsuzawa A, Takeda K, Ichijo H. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J Biol Chem 2008; 283:7657-65; PMID:18211888; http://dx.doi.org/10.1074/jbc.M708402200
- Osaka N, Takahashi T, Murakami S, Matsuzawa A, Noguchi T, Fujiwara T, Aburatani H, Moriyama K, Takeda K, Ichijo H. ASK1-dependent recruitment and activation of macrophages induce hair growth in skin wounds. J Cell Biol 2007; 176:903-9; PMID:17389227; http://dx.doi.org/10.1083/jcb.200611015
- Gauron C, Rampon C, Bouzaffour M, Ipendey E, Teillon J, Volovitch M, Vriz S. Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci Rep 2013; 3:2084; PMID:23803955; http://dx.doi.org/10.1038/srep02084
- Love NR, Chen Y, Ishibashi S, Kritsiligkou P, Lea R, Koh Y, Gallop JL, Dorey K, Amaya E. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat Cell Biol 2013; 15:222-8; PMID:23314862; http://dx.doi.org/10.1038/ncb2659
- Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009; 459:996-9; PMID:19494811; http://dx.doi.org/10.1038/nature08119
- Dvorak HF. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315:1650-9; PMID:3537791; http://dx.doi.org/10.1056/NEJM198612253152606
- Chen CC, Wang L, Plikus MV, Jiang TX, Murray PJ, Ramos R, Guerrero-Juarez CF, Hughes MW, Lee OK, Shi S, et al. Organ-Level quorum sensing directs regeneration in hair stem cell populations. Cell 2015; 161:277-90; PMID:25860610; http://dx.doi.org/10.1016/j.cell.2015.02.016
