ABSTRACT
In competition for food, territory and mates, male fruit flies (Drosophila melanogaster) engage in agonistic encounters with conspecifics. The fighting strategies used to obtain these resources are influenced by previous and present experience, environmental cues, and the internal state of the animal including hormonal and genetic influences. Animals that experience prior defeats show submissive behavior and are more likely to lose 2nd contests, while animals that win 1st fights are more aggressive and have a higher probability of winning 2nd contests. In a recent report, we examined these loser and winner effects in greater detail and demonstrated that both winners and losers show short-term memory of the results of previous bouts while only losers demonstrate a longer-term memory that requires protein synthesis. The recent findings also suggested that an individual recognition mechanism likely exists that can serve important roles in evaluating the fighting ability of opponents and influencing future fighting strategy. In this article, we follow up on these results by asking how previous defeated and victorious flies change their fighting strategies in the presence of 2nd losing and winning flies, by searching for evidence of territory marking, and discussing the existing literature in light of our findings.
In complex behaviors like courtship and aggression, animals display a limited number of highly stereotypical motor patterns and make decisions about the usage of and transitions between the patterns during social engagement. In Drosophila melanogaster, courtship and aggression both have been quantified by: (i) defining the behavioral patterns involved; (ii) scoring the use of and transition between these patterns; (iii) constructing transition matrices; and (iv), calculating 1st order Markov chains of the probabilities of transitions.Citation1-4 These yield snapshots of average fights and courtship rituals, with no single fights or courtship cycles actually mirroring these snapshots. Instead individual variation yields views of the wide variety of different strategies used by individual animals to obtain desired goals. Variability of this type likely depends on differences in internal state and innate behavioral abilities, previous experience and the strategies used by a second animal, engaged in social dialog with the first one.
Complex behaviors also must be malleable and highly adaptable to allow survival and procreation in what may be hostile environments. These adaptations involve learning new strategies that succeed in gaining desired resources, and storing memories of the new knowledge to drive future behavioral choices. Fruit flies are not included among the “social insects,” however, they engage in many social interactions during which they acquire new information. The learning and memory aspect of this in Drosophila has been studied in a wide variety of social situations, including aggregation,Citation5 courtshipCitation6 and more recently, aggression,Citation7,8
In this article, we focus on aggression and particularly on the learning and memory that accompanies agonistic interactions between pairs of male fruit flies in competition for resources. Although not well known prior to the present millennium, male and female fruit flies compete for resources.Citation1,2,9-14 Once again, it was SturtevantCitation15 who in describing pairs of males courting and competing for a single female noted: “one of them soon gives up and runs away. If the other then runs at him again within the next few minutes, he usually makes off without showing fight.” Was this the first experimental description of short-term “winner” and “loser” effects resulting from competition between pairs of laboratory-reared male fruit flies? Certainly this work was completed well before aggression between laboratory-reared male Drosophila melanogaster and closely-related species were described by Jacobs, Dow and Von Schilcher, and Hoffmann.Citation10-14 Female Drosophila also compete for resources,Citation2,9 but as these do not result in the formation of hierarchical relationships, they are not considered further in this article.
An awakened interest in studies on aggression in Drosophila began in the early 2000s.Citation1,2,16,17 One of these publicationsCitation1 included a simplification of the behavioral protocols used to study aggression with only 2 male flies placed in a fight arena with resources added, including a female or headless female, or a drop of yeast paste on the food surface.Citation1,17 Earlier publications identified the behavioral patterns observed during fights.Citation1,2,9,12,13,16,17 quantified the behavior, and showed that the first fly to “lunge” was 16 times more likely to win a fight.Citation1 In this article, we score the frequency of the commonly used “lunge” behavior as the most important higher intensity element influencing the outcome of a fight.Citation7,8
In many animal species, loser and winner effects result from fights between conspecifics (for review, seeCitation18,19). In loser effects, animals that are defeated in 1st fights are less likely to win 2nd fights; while in winner effects, victorious animals show higher probabilities of winning their next fights. These effects also are seen in fruit flies, with previous fighting experience influencing future fight outcomes.Citation7,8,20 The previous studies demonstrated that flies losing 1st fights also lost 2nd fights against socially naïve, familiar and unfamiliar opponents. These studies, however, did not show any advantages in 2nd fights of winning 1st fights.Citation7 To further investigate the behavioral consequences of losing and winning fights, we developed a behavioral apparatus that allowed transfer of flies to fight arenas without handling the animals.Citation21 This improved the fight conditions allowing for measurement of more accurate kinetics of fights. Thus, flies initiated fighting behavior sooner, delivered higher numbers of lunges, and showed stronger loser and winner effects in 2nd fights.Citation22 Using these newer fight arenas, we demonstrated that a single victory or defeat yielded both short-term winner as well as loser effects. These memories decayed over several hours, with the temporal dynamics depending on the familiarity of the opponents. The latter observation supported the existence of a possible individual recognition mechanism. Experiencing repeated defeats induced long-term loser effects that required protein synthesis, while comparable long-term winner effects were not observed under similar experimental conditions.Citation8
In this article we add further details concerned with the loser and winner effects, by asking what might be the outcome, and how might fighting strategies change when 2 previous winners (WW) or losers (LL) are paired in 2nd fights. These fights were scored at 10 and 60 min after the completion of 1st fights when short-term loser and winner effects were known to be present.Citation8 As shown in , LL pairs fight, lunge and establish dominance relationships in 2nd fights. Fewer encounters () and reduced numbers of lunges () were observed in LL fights compare with WW fights, but comparable percentages of fights ended up with the establishment of new hierarchical relationships (). These results seemingly were in conflict with our earlier results showing that losing flies, when paired with other losers, showed limited lunging behavior and minimal establishment of new hierarchical relationships.Citation7 The discrepancy might be explained by differences in the experimental protocols used in the 2 cases. In the earlier studies the arena used was larger, flies were transferred by aspiration and most importantly, fights lasted 90 min followed by a 30 min rest period for flies in their original vials before starting the 2nd fights. When we exactly duplicated the timing of the previous experiments in the new chambers (90 min 1st fight, 30 min separation followed by 60 min 2nd fight), the results obtained were similar to those reported in the original publication. Indeed, under the original timing protocols, we found that only 38% (5/13) of the pairs of LL flies re-established a dominance relationship during 60 min 2nd fights (compare with 90% with the 20-10-20 protocol, ). These results were significantly different from the results presented in (Fisher's exact test LL20–10–20-LL90–30–60: p = 0.0005). Not surprisingly, in WW pairings, flies also re-established new dominance relationships at 10 and 60 min ().
Figure 1. Aggressive behavioral patterns in loser-loser (LL) and winner-winner (WW) pairs of flies. (A) The numbers of encounters were significantly decreased in LL compare with WW pairings at both 10 and 60 min (LL-WW10 min: p = 0.033, n = 27; LL-WW60 min: p = 0.005, n>17). (B) Significantly fewer lunges were observed in LL compare with WW fights at the 2 time points (LL-WW10 min: p = 0.001, n = 27; LL-WW60 min: p = 0.007, n >17). (C) The percentage of fights performed between LL and WW pairings 10 min and 60 min after the 1st fights in which dominance relationship were established were not statistically different (Fisher's exact test LL-WW10 min: p = 1, n = 27; LL-WW60 min: p = 0.66, n > 19). (D) Significantly more encounters were necessary before the first lunge in LL pairings at 10 min after 1st fights only (LL-WW10 min: p < 0.0001, n = 27; LL-WW60 min: p = 0.237, n > 17). The latencies to lunge (E) and to re-establish dominance (F) were significantly increased between LL compare with WW pairings (Latency to lunge: LL-WW10 min: p < 0.0001, n = 27; LL-WW60 min: p = 0.024, n > 17; Latency to dominance: LL-WW10 min: p = 0.004, n = 26; LL-WW60 min: p = 0.1, n >16).
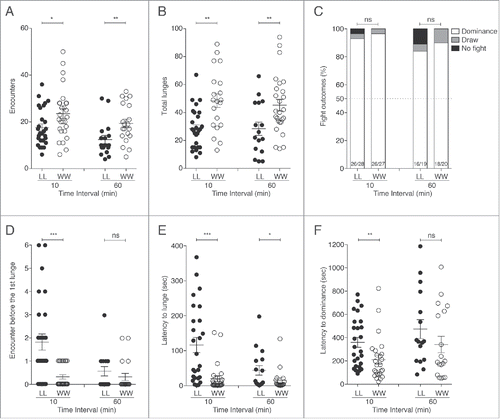
In addition to fewer encounters and reduced numbers of lunges in LL pairings in 2nd fights (), the numbers of meetings taking place between LL flies before the first lunge was delivered (), the latency to lunge () and the latency to establish dominance relationships () also were increased when compare with the values obtained in WW pairings. The latency to lunge was greatly shortened in the WW pairings, with lunging observed during 1st encounters in 66% (18/27) of the WW bouts but only in 25% (7/28) of 1st meetings in the LL fights. Even though the WW pairs were more aggressive and showed more retaliation than the LL pairings, no tussling or boxing (the highest intensity aggressive behaviors) events were seen (data not shown). Thus, previous winning flies did not reach the intensity levels commonly observed in fights between hyper-aggressive strains of flies selected for winning fights over 34–37 generations.Citation20,23
In numerous animal species, territories recently visited or acquired are marked in various ways to signal possession.Citation24-28 In fruit flies, territorial effects have been observed, with the outcomes of aggressive encounters influenced by the resident status and the age of the animals.Citation13,14,23 In ant colonies, cuticular hydrocarbons have been shown to be important in recognition of nest differences in the same species of ants, and to be factors that influence aggression levels between nests.Citation27 Cuticular hydrocarbons are easily removed from the surfaces of fruit flies,Citation29 and in combat situations one might anticipate that these possibly could be used to mark a territory.
Therefore, in the next set of experiments we asked whether territorial marking of some sort might exist in D. melanogaster species, and if so, whether this might influence the outcome of subsequent fights. To test this notion, we asked whether experiencing 1st and 2nd fights in the same or different arenas would offer any competitive advantages or disadvantages for flies in 2nd fights. To do this we examined fight outcomes for winner and loser flies of 1st fights in 2nd fights taking place in home arenas or in new arenas in which neither fly had fought previously.
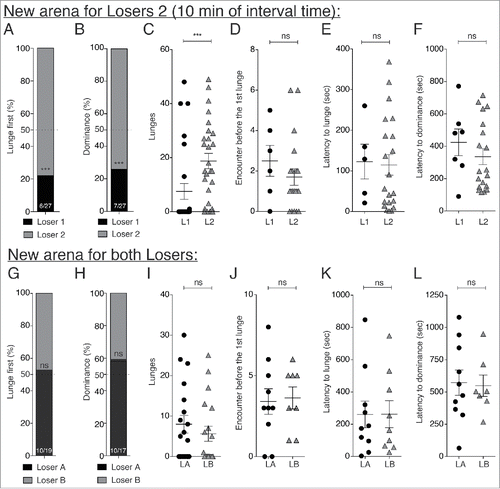
LL pairings: We first examined the outcomes of fights with pairs of losers at 10 min () after the completion of their 1st fights. These flies were designated as L1 (resident losers) who remained in arenas in which they lost their 1st fights and L2 (intruder losers) who were transferred from their original arenas to L1 fight arenas for 2nd fights. At 10 min after the 1st loss, only 22% (6/27) of the L1 flies lunged first () and only 26% (7/27) won their 2nd fights (). L1 flies also delivered significantly fewer lunges in those 2nd fights than L2 flies (). Several other parameters routinely measured in fights like encounter number before the first lunge (), latency to lunge () and latency to dominance () also appeared to be slowed in L1 compare with L2 flies, but these reductions were not statistically significant due to the small numbers of L1 flies initiating 2nd fights. By 60 min after the 1st fights, no residual competitive disadvantage remained for L1 flies in their home arenas (data not shown). In these experiments, only the L2 flies were transferred from their original 1st fight chambers to the 2nd fight arenas. We were concerned that this transfer and the accompanying movement might make L2 flies more aggressive. Therefore, as a control, we transferred L1 flies into and out of their home chambers in the same way as L2 flies were transferred when moved. As in the previous results (), L1 flies were still more submissive than L2 flies delivering significantly fewer lunges (L1-L210 min: p = 0.024) with only 23% (3/13) winning their 2nd fights (Fisher's exact test L1-L2: p = 0.017). As further controls, we performed a 2nd set of experiments in which both L1 and L2 flies were moved to new arenas for 2nd fights. In this case neither loser showed a competitive advantage over the other (). Therefore, previous losing flies present a competitive disadvantage of remaining in arenas where they have just lost a fight. Pheromone deposit in arena by the previous winners during 1st fights might represent a territory mark that losers would recognize, and might play important roles in the formation of loser effects, but further investigations are necessary to demonstrate such a hypothesis.
Figure 2. Loser flies showed a competitive disadvantage when 1st and 2nd fights were performed in the same arena. (A through F) After 10 min of rest, Losers 1 (L1 - “resident” - 1st and 2nd fights performed in the same arena) showed a lower percentage of first lunges (A) (6/27, Fisher's exact test L1-L210 min: p < 0.0001) and won a significantly lower percentage of second fights (B) compare with Losers 2 (L2 - “intruder” - flies moved to a different arena for 2nd fights – 7/27, Fisher's exact test L1-L210 min: p = 0.0009). (C) L1 flies delivered significantly fewer lunges than L2 flies during 2nd fights (L1-L210 min: p = 0.0004, n = 27). However, no differences were seen between L1 and L2 in: the numbers of encounters before the first lunge (D) (L1-L210 min: p = 0.313, nL1 = 6, nL2 = 20); the latencies to lunge (E) (L1-L210 min: p = 0.713, nL1 = 5, nL2 = 21); or the latency to establish dominance (F) (L1-L210 min: p = 0.363, nL1 = 7, nL2 = 20). (G through L) When both losers were moved to new arenas for the 2nd fights, Loser 1 did not show a significant reduction in lunging first (G) (10/19, Fisher's exact test LA-LB10 min: p = 1), and in establishment of dominance relationships (H) (10/17, Fisher's exact test LA-LB10 min: p = 0.493), the numbers of lunges (I) (L1A-LB10 min: p = 0.458, n = 19), the numbers of encounters before the first lunge (J) (LA-LB10 min: p = 0.736, nLA = 10, nLB = 8), the latency to lunge (K) (LA-LB10 min: p > 0.999, nLA = 10, nLB = 9), or the latency to establish dominance (L) (LA-LB10 min: p > 0.999, nLA = 10, nLB = 7).
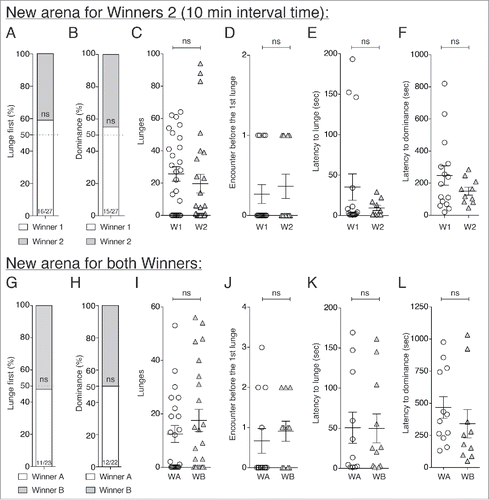
WW pairings: In experiments identical to those performed above, but with winners of 1st fights paired in this case, W1 (resident winners) flies showed no competitive advantage over W2 (intruder winners) flies in 2nd contests at 10 min after the completion of 1st fights (). This despite the existence of territoriality effects demonstrated in different species of flies,Citation13,30 and known winner effects during that time window.Citation8 However, in these experiments in which 2 previous winning male flies were tested, W1 and W2 engaged in fights by displaying the first lunge, and won 2nd fights in equal proportions, demonstrating that winner effects might overcome any territorial effects. Therefore, competitive advantages associated with occupying a territory either declined rapidly or were too small to be observed in the face of the behaviorally induced short-term winner effects.
Figure 3. No territorial effects were observed in fights between pairs of previous winner flies. (A through F) There were no significant differences between Winners 1 (W1 – “resident”) and Winners 2 (W2 –“intruder”) at 10 min after 1st fights in any of the parameters measured. Winner 1 did not significantly lunge first (A) (16/27, Fisher's exact test: W1-W210 min: p = 0.276) and win (B) 2nd fights (15/27, Fisher's exact test: W1-W210 min: p = 0.587). (C) At 10 min, no statistical differences were observed between W1 and W2 flies in the numbers of lunges (C) (W1-W210 min: p = 0.283, n>27), the numbers of encounters before the 1st lunge (D) (W1-W210 min: p = 0.683, nW1 = 15, nW2 = 11), the latencies to lunge (E) (W1-W210 min: p = 0.989, nW1 = 16, nW2 = 10) and to establish dominance (F) (W1-W210 min: p = 0.517, nW1 = 15, nW2 = 10). (G through L) When both winners were moved to new arenas for 2nd fights, no differences were found in any of the parameters measured. These included: who lunged first (G) (11/23, Fisher's exact test: WA-WB10 min: p = 1); who won (H) (12/22, Fisher's exact test: WA-WB10 min: p = 0.763); the numbers of lunges (I) (WA-WB10 min: p = 0.455, n = 23); the numbers of encounters before the 1st lunge (J) (WA-WB10 min: p = 0.33, nWA = 11, nWB = 12); the latencies to lunge (K) (WA-WB10 min: p = 0.883, nWA = 11, nWB = 11); and the latency to establish dominance (L) (WA-WB10 min: p = 0.177, nWA = 12, nWB = 10).
Discussion: Animals learn and store information during social encounters and subsequently use that information in future contests to gain resources. Fruit flies are no exception, and indeed the propagation of the species likely requires acquisition of such information. That fruit flies are capable of learning and memory has been extensively documented,Citation31,32 with the most complete and detailed experiments being performed using classical and operant learning protocols.Citation33,34 Social interactions between animals, by contrast, commonly involve patterned displays initiated by one animal accompanied by responses to those displays by a second animal. In aggression, the displays usually ascend in intensity from viewing and tapping opponents toward actions that are potentially damaging in competition for resources.Citation1,7 Our recent publicationCitation8 demonstrated that short-term loser and winner effects are observed as consequences of competition among male Drosophila melanogaster. These appear to involve individual recognition between flies, as the effects last longer with familiar than non-familiar opponents. In addition, behavioral consequences with formation of long-term loser effects persist one day later in losing male flies. Winner and loser effects resulting from aggression have been observed in many animal species with the causes and consequences of these extensively discussed in the literature.Citation18,19,35,36 Loser effects generally last longer than winner effects and are thought to induce caution in engagement in future male-male competitions and also to affect courtship behaviors.Citation12,37 Winner effects, by contrast, offer immediate rewards to animals, but decay faster, possibly to reduce over-confident behavior in future contests. In these ways as well, the outcomes of fruit fly fights resemble those of other species. One possible way that they differ, however, is that losing flies continue to engage winners in fights, possibly because no dangerous weapons are available to the winners. In the wild, fruit flies are not found alone: instead they exist in a complex world of rotting fruit along with possible pathogens, parasites, predators and competitors.Citation38 In such complex environments, there may be obvious advantages to the development of short-term loser and winner effects as they can alter behavior on a minute-to-minute time scale. It is less clear what the advantage of long-term loser effects would be, unless they are of value in recognizing and avoiding predators.
Despite the tremendous heterogeneity in fight strategies displayed by individual male flies in competition for resources, analyses of multiple fights reveal that limited numbers of behavioral patterns are used during fights and that these bear statistical linkages between them as to their usage.Citation1,2 Of the patterns observed, the lunge, which is commonly observed later in a fight, has been shown to be a highly successful predictor of the outcome of a fight. Fights tend to start at lower levels of intensity with limited physical contact between the animals and move to higher intensity levels as they progress. Hyper-aggressive males we have generated by inbreeding winners for 35-40 generations (“bullies”),Citation20 however, appear to display patterns in different sequences than the original parent stocks. These flies move to higher intensity levels faster than the parent line during fights, lunge and retaliate when lunged at much more frequently, display boxing and tussling, and almost always win fights against the parent line.Citation20 When bullies are paired in fights, however, the losing fly loses all competitive advantage against all other flies. Over the course of the next 24 hrs, the loser bullies revert to the hyper-aggression phenotype (unpublished). In the experiments described here, we found that for short periods of time (min) in winner flies, pattern usage also is different, with winners eliminating preliminary steps like tapping: instead they go directly to higher intensity patterns of behavior like lunging in even the 1st encounters of fights. This makes perfect ethological sense, as the lunge is a predictor of fight outcome: therefore why waste time and energy on earlier less effective patterns of behavior? But how does this happen? How does winning or losing a fight change the fight strategy used by an animal? Do changes take place: at a systems level; a circuit level; a neuronal level? And how do long-term behavioral changes differ from short-term changes?
In addressing such challenging questions, one can ask whether prior explorations of influences on the expression of higher level aggression by male flies in fights might offer some insight. First, we can include studies involving the selection of hyper-aggressive flies from wild type populations. Studies of this type have identified genetic differences between hyper-aggressive and wild type fliesCitation23,39 and follow-up studies on the localizations of these genes and examination of their functional roles can be of great value. Next, a wide variety of neurotransmitters and neuromodulators have been implicated as serving roles in higher-level aggression in male flies. These include: acetylcholineCitation40,41; serotoninCitation42-44; dopamineCitation45; octopamineCitation46-48; and a variety of peptides including tachykininsCitation49 and peptide F.Citation44 In some of these cases, highly restricted populations of neurons have been shown to be involved.Citation43-45,47,50,51 Possibly the best studied of these are 2 pairs of serotonergic neurons (the 5HT-PLP neurons) whose roles seems to be to facilitate going to higher levels of aggression during fights.Citation43,52 Do such neurons change in function during the progression of, or as a consequence of the outcome of fights? If so, are the changes in correct directions to alter the subsequent fighting behavior of flies for varying periods of time? A great advantage of the fruit fly model system is that by combining already existing and rapidly developing molecular, genetic and optogenetic methods with quantitative behavioral studies, one now can address and gain insight into issues of great magnitude like how animals learn, store and later use information to optimize their usage of innate complex behaviors.
Materials and methods
Fly stocks
The CantonS Drosophila melanogaster strain was used in this study. Flies were raised at 25°C with 50% humidity under 12h:12h light/d ark cycles.
Aggression assays
The protocol and experimental setup used in this study were previously described.Citation8,21,22 Briefly, male flies were raised in social isolation since eclosion. Two days prior to the assays, flies were anesthetized using CO2 and a dot of paint was applied on the dorsal thorax for identification purposes. The previously described fighting chambers were used to minimize the handling of flies before and during the behavioral assays.Citation21
Assays were performed with 2 aged and size-matched socially naïve 7 day-old males.
Two experimental protocols were used in this study:
Male flies were allowed to interact for a 1st 20 min fight. After 10 or 60 min of rest, 2 previous winners or losers were paired for a 2nd 20 min fight.
In a second set of experiments, males were paired for 1st fights of 90 min, had 30 min of rest and pairs of loser-loser and winner-winner flies fought 2nd fights of 60 m in.
All the experiments were performed at 25°C with 50% humidity during the 1st hours of day-light.
Behavioral analysis
For all fights, only behavioral patterns observed on food cups were scored: the numbers of encounters (meeting between the 2 flies), numbers of lunges delivered by each fly (high-intensity aggressive behavioral pattern), the latency to lunge (time between the 1st encounter and the 1st lunge), the numbers of encounters before the 1st lunge, and the latency to establish dominance relationships (time between the 1st encounter and dominance). Dominance is established when one fly retreats at least 3 times from the food cup after receiving a lunge. To test the effect of territoriality, we scored which fly initiated higher level fighting (lunged first) and which won the 2nd fights.
Loser flies were defined as flies that retreated 3 times from the food cup after receiving lunges. For , fights in which clear losers and winners were observed were counted as “Dominance.” Fights in which more than 5 lunges were observed but without 3 retreats were considered as “Draw.” Fights in which fewer than 5 lunges were scored were removed from the analysis and scored at “No fight.”
In 2nd fights pairings, Loser 1 (L1) and Winner 1 (W1) were defined as the flies that fought 1st and 2nd fights in the same fighting chamber, while Loser 2 (L2) and Winner 2 (W2) were the flies that fought 1st and 2nd fights in different arenas. As controls, both flies were moved to new arena for 2nd fights. In these control experiments, Losers and Winners were randomly named A and B.
Handling of flies
Using the recently described behavioral chambers,Citation21 the handling of flies was minimized as much as possible before and during the experiments. In these setups, flies enter on each side of an arena by negative geotaxis, but are unable to interact because a divider separates them. To start experiments, dividers that separate the arenas are removed and flies are allowed to interact for 1st fights. At the end of the first 20 min bouts, dividers are reinserted to separate the flies. By negative geotaxis, “Losers 2” were transferred to the arena of Losers 1 for 2nd fights.
To control for any effects of transfer alone on aggressiveness, a set of experiments was done in which Losers 1 were removed by negative geotaxis and then reinserted back into the arena they were just removed from.
Statistical analysis
GraphPad Prism 6 was used for all the statistical analysis. All the data were subjected to a Grubb's test (α = 0.05), and all values considered as outliers were excluded from the analysis. As most of the data set did not pass the Shapiro-Wilk normality test, a nonparametric Mann-Whitney test was used for data analysis. Fisher's exact test was used in to compare fight outcomes between LL and WW and in , to compare the data between L1/L2 and W1/W2 or LA/LB and WA/WB.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the members of Kravitz lab for their helpful comments and their support on this study. We also thank Kenia Lucey who obtained preliminary results on this project.
Funding
This research was support by National Institute of General Medicine Sciences Grants: GM099883; GM074675 and 1R35GM118137–01 (to EAK). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci U S A 2002; 99:5664-8; PMID:11960020; http://dx.doi.org/10.1073/pnas.082102599
- Nilsen SP, Chan YB, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A 2004; 101:12342-7; PMID:15302936; http://dx.doi.org/10.1073/pnas.0404693101
- Yamamoto D, Koganezawa M. Genes and circuits of courtship behaviour in Drosophila males. Nat Rev Neurosci 2013; 14:681-92; PMID:24052176; http://dx.doi.org/10.1038/nrn3567
- Lasbleiz C, Ferveur JF, Everaerts C. Courtship behaviour of Drosophila melanogaster revisited. Anim Behav 2006; 72:1001-12;http://dx.doi.org/10.1016/j.anbehav.2006.01.027
- Bartelt RJ, Schaner AM, Jackson LL. cis-Vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J Chem Ecol 1985; 11:1747-56; PMID:24311338; http://dx.doi.org/10.1007/BF01012124
- Gailey DA, Jackson FR, Siegel RW. Male courtship in Drosophila: the conditioned response to immature males and its genetic control. Genetics 1982; 102:771-82; PMID:17246097
- Yurkovic A, Wang O, Basu AC, Kravitz EA. Learning and memory associated with aggression in Drosophila melanogaster. Proc Natl Acad Sci U S A 2006; 103:17519-24; PMID:17088536; http://dx.doi.org/10.1073/pnas.0608211103
- Trannoy S, Penn J, Lucey K, Popovic D, Kravitz EA. Short and long-lasting behavioral consequences of agonistic encounters between male Drosophila melanogaster. Proc Natl Acad Sci U S A 2016; 113:4818-23; PMID:27071097; http://dx.doi.org/10.1073/pnas.1520953113
- Ueda A, Kidokoro Y. Aggressive behaviours of female Drosophila melanogaster are influenced by their social expriences and food resources. Physiol Entomol 2002; 27:21-8; http://dx.doi.org/10.1046/j.1365-3032.2002.00262.x
- Jacobs ME. Influence of beta-alanine on mating and territorialism in Drosophila melanogaster. Behav Genet 1978; 8:487-502; PMID:103533; http://dx.doi.org/10.1007/BF01067478
- Jacobs ME. Influence of light on mating of Drosophila melanogaster. Ecology 1960; 41:182-8; http://dx.doi.org/10.2307/1931952
- Dow MA, von Schilcher F. Aggression and mating success in Drosophila melanogaster. Nature 1975; 254:511-2; PMID:804664; http://dx.doi.org/10.1038/254511a0
- Hofmann AA. A laboratory study of male territoriality in the sibling species Drosophila melanogaster and Drosophila simulans. Anim Behav 1987; 35:807-18 ; http://dx.doi.org/10.1016/S0003-3472(87)80117-3
- Hofmann AA. The influence of age and experience with conspecifics on territorial behavior in Drosophila melanogaster. J insect Behav 1990; 3:1-12 ; http://dx.doi.org/10.1007/BF01049191
- Sturtevant AH. Experiments on sex recognition and the problem of sexual selection in Drosophila. J Animal Behav 1915; 5:351-66 ; http://dx.doi.org/10.1037/h0074109
- Baier A, Wittek B, Brembs B. Drosophila as a new model organism for the neurobiology of aggression? J Exp Biol 2002; 205:1233-40; PMID:11948200
- Lee G, Hall JC. A newly uncovered phenotype associated with the fruitless gene of Drosophila melanogaster: aggression-like head interactions between mutant males. Behav Genet 2000; 30:263-75; PMID:11206081; http://dx.doi.org/10.1023/A:1026541215546
- Rutte C, Taborsky M, Brinkhof MW. What sets the odds of winning and losing? Trend Ecol Evol 2006; 21:16-21 ; http://dx.doi.org/10.1016/j.tree.2005.10.014
- Hsu Y, Earley RL, Wolf LL. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol Rev Camb Philos Soc 2006; 81:33-74; PMID:16460581; http://dx.doi.org/10.1017/S146479310500686X
- Penn JK, Zito MF, Kravitz EA. A single social defeat reduces aggression in a highly aggressive strain of Drosophila. Proc Natl Acad Sci U S A 2010; 107:12682-6; PMID:20616023; http://dx.doi.org/10.1073/pnas.1007016107
- Trannoy S, Chowdhury B, Kravitz EA. A new approach that eliminates handling for studying aggression and the “Loser” Effect in Drosophila melanogaster. J Vis Exp 2015; 106:e53395.
- Trannoy S, Chowdhury B, Kravitz EA. Handling alters aggression and “loser” effect formation in Drosophila melanogaster. Learn Mem 2015; 22:64-8; PMID:25593291; http://dx.doi.org/10.1101/lm.036418.114
- Dierick HA, Greenspan RJ. Molecular analysis of flies selected for aggressive behavior. Nat Genet 2006; 38:1023-31; PMID:16906161; http://dx.doi.org/10.1038/ng1864
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BJ, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature 2007; 450:899-902; PMID:18064011; http://dx.doi.org/10.1038/nature05997
- Jaeger RG. Pheromonal Markers as Territorial Advertisement by Terrestrial Salamanders. In: Duvall DM-S D, Silverstein R., ed. Chemical Signals in Vertebrates 4. New York: Plenum Press; 1985. p. 191–203.
- Grether GF. The neuroecology of competitor recognition. Integr Comp Biol 2011; 51:807-18; PMID:21700572; http://dx.doi.org/10.1093/icb/icr060
- Kidokoro-Kobayashi M, Iwakura M, Fujiwara-Tsujii N, Fujiwara S, Sakura M, Sakamoto H, Higashi S, Hefetz A, Ozaki M. Chemical discrimination and aggressiveness via cuticular hydrocarbons in a supercolony-forming ant, Formica yessensis. PLoS One 2012; 7:e46840; PMID:23115632; http://dx.doi.org/10.1371/journal.pone.0046840
- Wyatt TD. Introduction to Chemical Signaling in Vertebrates and Invertebrates. In: Mucignat-Caretta C, ed. Neurobiology of Chemical Communication. Boca Raton, FL: CRC Press; 2014.
- Yew JY, Cody RB, Kravitz EA. Cuticular hydrocarbon analysis of an awake behaving fly using direct analysis in real-time time-of-flight mass spectrometry. Proc Natl Acad Sci U S A 2008; 105:7135-40; PMID:18474870; http://dx.doi.org/10.1073/pnas.0802692105
- Benelli G, Romano D, Desneux N, Messing RH, Canale A. Sex differences in fighting-induced hyperaggression in a fly. Anim Behav 2015; 104:165-74; http://dx.doi.org/10.1016/j.anbehav.2015.02.026
- Kahsai L, Zars T. Learning and memory in Drosophila: behavior, genetics, and neural systems. Int Rev Neurobiol 2011; 99:139-67; PMID:21906539; http://dx.doi.org/10.1016/B978-0-12-387003-2.00006-9
- Siwicki KK, Ladewski L. Associative learning and memory in Drosophila: beyond olfactory conditioning. Behav Processes 2003; 64:225-38; PMID:14556954; http://dx.doi.org/10.1016/S0376-6357(03)00137-2
- McGuire SE, Deshazer M, Davis RL. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog Neurobiol 2005; 76:328-47; PMID:16266778; http://dx.doi.org/10.1016/j.pneurobio.2005.09.003
- Guven-Ozkan T, Davis RL. Functional neuroanatomy of Drosophila olfactory memory formation. Learn Mem 2014; 21:519-26; PMID:25225297; http://dx.doi.org/10.1101/lm.034363.114
- Chase I, Bartolomeo C, Dugatkin LA. Aggressive interactions and inter-contest interval: how long do winners keep winning? Anim Behav 1994; 48:393-400 ; http://dx.doi.org/10.1006/anbe.1994.1253
- Benelli G, Desneux N, Romano D, Conte G, Messing RH, Canale A. Contest experience enhances aggressive behaviour in a fly: when losers learn to win. Sci Rep 2015; 5:9347; PMID:25792294; http://dx.doi.org/1 0.1038/s rep09347
- Teseo S, Veerus L, Mery F. Fighting experience affects fruit fly behavior in a mating context. Naturwissenschaften 2016; 103:38; PMID:27108453; http://dx.doi.org/10.1007/s00114-016-1368-x
- Markow TM. The natural history of model organisms: the secret lives of Drosophila flies. Elife 2015; 4:e06793; http://dx.doi.org/10.7554/eLife.06793
- Wang L, Dankert H, Perona P, Anderson DJ. A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc Natl Acad Sci U S A 2008; 105:5657-63; PMID:18408154; http://dx.doi.org/10.1073/pnas.0801327105
- Mundiyanapurath S, Chan YB, Leung AK, Kravitz EA. Feminizing cholinergic neurons in a male Drosophila nervous system enhances aggression. Fly (Austin) 2009; 3:179-84; PMID:19556850; http://dx.doi.org/10.4161/fly.3.3.8989
- Dankert H, Wang L, Hoopfer ED, Anderson DJ, Perona P. Automated monitoring and analysis of social behavior in Drosophila. Nat Methods 2009; 6:297-303; PMID:19270697; http://dx.doi.org/10.1038/nmeth.1310
- Alekseyenko OV, Lee C, Kravitz EA. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS One 2010; 5:e10806; PMID:20520823; http://dx.doi.org/10.1371/journal.pone.0010806
- Alekseyenko OV, Chan YB, Fernandez Mde L, Bulow T, Pankratz MJ, Kravitz EA. Single serotonergic neurons that modulate aggression in Drosophila. Curr Biol 2014; 24:2700-7; PMID:25447998; http://dx.doi.org/10.1016/j.cub.2014.09.051
- Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet 2007; 39:678-82; PMID:17450142; http://dx.doi.org/10.1038/ng2029
- Alekseyenko OV, Chan YB, Li R, Kravitz EA. Single dopaminergic neurons that modulate aggression in Drosophila. Proc Natl Acad Sci U S A 2013; 110:6151-6; PMID:23530210; http://dx.doi.org/10.1073/pnas.1303446110
- Andrews JC, Fernandez MP, Yu Q, Leary GP, Leung AK, Kavanaugh MP, Kravitz EA, Certel SJ. Octopamine neuromodulation regulates Gr32a-linked aggression and courtship pathways in Drosophila males. PLoS Genet 2014; 10:e1004356; PMID:24852170; http://dx.doi.org/10.1371/journal.pgen.1004356
- Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci 2008; 11:1059-67; PMID:19160504; http://dx.doi.org/10.1038/nn.2164
- Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA, Hardie SL, Heisenberg M. Octopamine in male aggression of Drosophila. Curr Biol 2008; 18:159-67; PMID:18249112; http://dx.doi.org/10.1016/j.cub.2007.12.052
- Asahina K, Watanabe K, Duistermars BJ, Hoopfer E, Gonzalez CR, Eyjolfsdottir EA, Perona P, Anderson DJ. Tachykinin-expressing neurons control male-specific aggressive arousal in Drosophila. Cell 2014; 156:221-35; PMID:24439378; http://dx.doi.org/10.1016/j.cell.2013.11.045
- Hoopfer ED, Jung Y, Inagaki HK, Rubin GM, Anderson DJ. P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. Elife 2015; 4:e11346; PMID:26714106; http://dx.doi.org/10.7554/eLife.11346
- Koganezawa M, Kimura KI, Yamamoto D. The neural circuitry that functions as a switch for courtship versus aggression in drosophila males. Curr Biol 2016; 26(11):1395-403; PMID:27185554
- Alekseyenko OV, Kravitz EA. Serotonin and the search for the anatomical substrate of aggression. Fly (Austin) 2014; 8:200-5; PMID:25923771; http://dx.doi.org/10.1080/19336934.2015.1045171
