ABSTRACT
The model organism Drosophila melanogaster has become a focal system for investigations of rapidly evolving genital morphology as well as the development and functions of insect reproductive structures. To follow up on a previous paper outlining unifying terminology for the structures of the male terminalia in this species, we offer here a detailed description of the female terminalia of D. melanogaster. Informative diagrams and micrographs are presented to provide a comprehensive overview of the external and internal reproductive structures of females. We propose a collection of terms and definitions to standardize the terminology associated with the female terminalia in D. melanogaster and we provide a correspondence table with the terms previously used. Unifying terminology for both males and females in this species will help to facilitate communication between various disciplines, as well as aid in synthesizing research across publications within a discipline that has historically focused principally on male features. Our efforts to refine and standardize the terminology should expand the utility of this important model system for addressing questions related to the development and evolution of animal genitalia, and morphology in general.
Introduction
Animal terminalia (which includes both the genitalia and analia) have a long history of being used for taxonomic and phylogenetic purposes, as well as being studied in the context of functional morphology and morphological evolution. This is because these structures possess a remarkable level of anatomical diversity, making them excellent morphological features for distinguishing species as well as understanding mechanisms of rapid morphological change [Citation1–3]. Past investigations mostly focused on male terminalia, and female terminalia were generally considered to be relatively invariable [Citation1,Citation4]. In the last several decades however, there has been a burgeoning interest in improving our understanding of female genital diversity [Citation5–11]. This interest has been motivated by the realization that some evolutionary hypotheses, for instance with respect to coevolution of genitalia, are best addressed by studying both male and female genital morphology simultaneously [Citation3,Citation11–15]. In addition, the female terminalia can evolve in response to ecological factors, such as the properties of egg-laying substrates [Citation6]. Furthermore, as morphological adaptations in female genitalia are central to the ability of many pest species to damage crops when laying their eggs into plants [Citation16–20], studying female genitalia can potentially lead to practical applications.
In recent years, the genitalia of species in the Drosophila genus have become an important study system to address research questions in ecology, behaviour, evolution, development and taxonomy. A survey of the egg-laying apparatus of Hawaiian drosophilids for example revealed that ovipositor form, and especially length and patterns of sensory structures, differ between species and strongly correlate with adaptations to different oviposition substrates [Citation6]. Similar observations were made for Drosophila suzukii, which has evolved an elongated ovipositor with derived sensory structures, enabling piercing through the skin of still-ripening fruits, which allows this species to access a new ecological niche and simultaneously makes it a pest causing massive agricultural damage [Citation8,Citation16].
Cross-disciplinary communication among researchers investigating different aspects of Drosophila female terminalia has often been impeded by two important challenges. First, many important features are internal, mostly composed of folded soft tissues, which can make it more difficult to identify, delimit and rigorously quantify variation in shape between individuals or species. For example, commonly used terms such as vulva, vagina and uterus have no clear delineations and have been applied to variable portions of the genitalia in different publications (see ). Imaging and dissecting technology developed in recent years has greatly mitigated this technical limitation [Citation5]. For instance, micro-computed tomography (micro CT) scanning can now provide detailed images of internal structures [Citation21]. The second challenge has been that individual structures have often been referred to by several different names. This is most obvious in the long list of synonyms that have been applied to the egg-laying sclerites laterally surrounding the gonopore (e.g. ovipositor, vaginal plates, oviscapt, gonopod, etc. See ).
Table 1. Definition of the terms in the standardized nomenclature
In a previous paper, we delineated the structures of the male terminalia of D. melanogaster and proposed a standard set of terms for these parts [Citation22]. Following a discussion with the members of the consortium, we opted to designate terms that can be homologized across the Diptera, but we recommended that authors also indicate common terms, whenever possible, in their manuscripts in order to guarantee maximum understanding among disciplines. In this paper, we follow the same approach and collectively propose a collection of terms and definitions to unify the terminology associated with the female terminalia in D. melanogaster (). In contrast to our previous paper, which was limited to the external male terminalia, we also include here a comprehensive overview of the internal reproductive structures of females. Many of these structures make contact with intromittent parts of the male genitalia during mating and may therefore be of interest in studies of genital evolution and coevolution [e.g. Citation15, Citation21, Citation23].
Distinguishing between the various parts of the female genitalia can be challenging, especially where clear boundaries (e.g. sutures, joints) do not exist. To achieve maximum clarity in our visual depictions, we have used a combination of bright field images of dissected cuticle (Canton S wild type strain), scanning electron microscopy, and line drawings.
Results and discussion
A visual atlas of adult D. melanogaster female terminalia
We provide below a unified nomenclature of the anatomical parts of the female terminalia of D. melanogaster, together with images to visualize the various structures with as much clarity as possible. We hope that this effort will facilitate the study of female terminalia in D. melanogaster and related species by providing both a common language for cross-reference and delimitations for features of interest.
The female terminalia of D. melanogaster are composed of anatomical structures arising from a fusion of abdominal primordia 8–10 [Citation24,Citation25]. In females, the abdominal segment 8 primordium of the genital disc develops into the majority of the internal and external female genital structures [Citation26,Citation27]. The abdominal segment 9 primordium is reduced in females, giving rise to the internal structure of the female accessory gland and the dorsal surface of the uterus [Citation26,Citation28]. In females, as in males, the abdominal segment 10 primordium of the genital disc develops to become the analia [Citation29]. We divide our descriptions of the terminalia into two regions, internal and external. The external terminalia have prominent roles in oviposition and copulation, while the internal terminalia have roles in ovulation and sperm storage. We use the junction of the oviprovector (external) and vulva (internal) as the division between these two regions.
details our proposed unified nomenclature. Each proposed term is listed along with a description of the structure, previously used alternate names, and references. For ease of conversion, provides the reverse search functionality; previously used terms are listed in the first column, with the corresponding unified nomenclature term we propose here given in the second column. Instances where the same term has been used elsewhere for more than one distinct structure are indicated with an asterisk.
Table 2. Table of correspondence between terms previously used in publications and term of the standardized nomenclature
External structures of the female terminalia
The external structures of the female terminalia () consist of the female analia and external genitalia, both of which harbour sensilla (bristles). In females, the analia (, panel A”) are subdivided into a dorsal plate (the epiproct), and a ventral plate (the hypoproct). The analia are surrounded by the genital tissue of the epigynium (formerly female abdominal tergite 8). The epigynial ventral lobe connects to the paired valves (left and right) of the hypogynium via the perineal membrane. We further subdivide the hypogynium into several parts (, panel A’). The hypogynial posterior lobe and hypogynial anterior lobe are the posterior and anterior parts of each valve of the hypogynium. The ventral side of both valves is connected by the hypogynial anteroventral bridge (, panel B’). The hypogynial mid-dorsal incision is an indentation on the outside of each hypogynial valve. The posterior and anterior hypogynial lobes are delimited by an imaginary line connecting the hypogynial mid-dorsal incision with the hypogynial long sensillum. During copulation, the male surstylus contacts the hypogynium near this incision [Citation30]. The hypogynial posterodorsal pouch is a depression positioned at the apical end of each hypogynial valve (, panel A’), which contacts the male epandrial posterior lobe early in copulation [Citation15,Citation31]. The two hypogynial valves are connected medially by the oviprovector, an eversible membrane whose ventral surface bears the oviprovector scales (, panel C; ), which likely act to prevent bidirectional movement of eggs [Citation32].
Figure 1. The terminalia of female D. melanogaster. [A] Model diagram of posterior female abdomen of D. melanogaster, lateral view. [B] Scanning electron micrograph of D. melanogaster female terminalia, posterior view. T3-T7 = female abdominal tergites 3–7. G* = female genitalia, A = female analia, Eg = epigynium (T8), Hp = hypoproct, Ep = epiproct. The hypoproct and the epiproct together form the female analia. *Note that the female genitalia includes the epigynium, which is indicated separately in this figure.
![Figure 1. The terminalia of female D. melanogaster. [A] Model diagram of posterior female abdomen of D. melanogaster, lateral view. [B] Scanning electron micrograph of D. melanogaster female terminalia, posterior view. T3-T7 = female abdominal tergites 3–7. G* = female genitalia, A = female analia, Eg = epigynium (T8), Hp = hypoproct, Ep = epiproct. The hypoproct and the epiproct together form the female analia. *Note that the female genitalia includes the epigynium, which is indicated separately in this figure.](/cms/asset/321b768e-4397-4979-9a02-8332fb0f01a1/kfly_a_2058309_f0001_oc.jpg)
Figure 2. Visual atlas of the external female terminalia. Light microscopy images showing the whole external terminalia in lateral view (panel A) and the genitalia in posteroventral view (panel B). Individual structures are highlighted below each image, with line drawings to aid identification. Previous FlyBase terms are listed in the left column and revised terms are given in the right column. Panel C is a detail of a lateral view with internal structures extruded (as during egg laying), to highlight interior membranous structures.
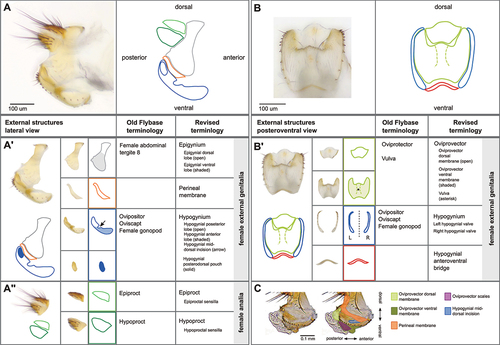
Figure 3. Hypogynial sensilla. [A] Light microscopy image of hypogynial lobes. Inset is a closeup of the posterior tip of one lobe. [B] Line tracing of [A], showing locations of bristle types. Hypogynial short sensilla are barely visible from this angle, but one is shown in the inset (arrow). [C] Scanning electron micrograph of female genitalia, posterior view. Colour-coding of sensilla types is as follows: Red, hypogynial tooth; Green, hypogynial long sensillum; Blue, hypogynial short sensillum. [D] Scanning electron micrograph of female genitalia, posterior view. The region covered with oviprovector scales is indicated with a dashed purple line.
![Figure 3. Hypogynial sensilla. [A] Light microscopy image of hypogynial lobes. Inset is a closeup of the posterior tip of one lobe. [B] Line tracing of [A], showing locations of bristle types. Hypogynial short sensilla are barely visible from this angle, but one is shown in the inset (arrow). [C] Scanning electron micrograph of female genitalia, posterior view. Colour-coding of sensilla types is as follows: Red, hypogynial tooth; Green, hypogynial long sensillum; Blue, hypogynial short sensillum. [D] Scanning electron micrograph of female genitalia, posterior view. The region covered with oviprovector scales is indicated with a dashed purple line.](/cms/asset/01a2bea7-a7c4-4aa9-99da-5a7e00bee37c/kfly_a_2058309_f0003_oc.jpg)
The setation of the external female terminalia has several readily identifiable components (). Sensilla on the epiproct and the hypoproct are referred to as epiproctal sensilla and hypoproctal sensilla, respectively (, panel A). On the genitalia, both the epigynium (epigynial sensilla) and hypogynium (hypogynial sensilla) have characteristic setation. The hypogynial sensilla are subdivided into three types (). Hypogynial short sensilla (previously gonopod sensillum trichodeum; , blue) are small apical bristles at the dorsal tip of the hypogynial posterior lobe. The hypogynial posterior lobe of each valve also possesses a single hypogynial long sensillum (previously gonopod long bristle; , green) at the apical end. Finally, each valve of the hypogynium possesses a row of stout hypogynial teeth (previously gonopod thorn bristles or vaginal teeth; , red).
Internal female genital and reproductive structures
The upper reproductive tract consists of the ovaries and oviducts, which transfer mature eggs to the lower reproductive tract (). The lower reproductive tract is composed of the genital chamber, female accessory glands, seminal receptacle and spermathecae. The seminal receptacle and spermathecae store sperm after mating, while the female accessory glands and the spermathecal secretory glandular cells that surround the spermatheca capsule serve as secretory organs. The genital chamber is subdivided into the uterus (or bursa; anterior) and vagina (posterior) (). It is in the uterus that fertilization of eggs takes place [Citation33]. The posterior opening of the lower reproductive tract consists of the vagina through which sperm is transferred to the female and the vulva, a name which has also previously been used for the oviprovector, and where copulation occurs and where the egg exits the reproductive tract [e.g. Citation34, Citation35].
Figure 4. Visual atlas of internal female genitalia and reproductive structures. Confocal bright-field images and schematic of Drosophila melanogaster female (Canton S strain) reproductive system. Scale bar is 500 µm. The upper box shows the upper reproductive tract (upper RT) and the ovaries, the lower box is the lower reproductive tract (Lower RT). The lower panel displays individual structures with line drawings to aid identification. The internal structures and substructures include the gonad (ovaries), the upper RT (oviduct) and the lower RT (seminal receptacle, spermatheca, female accessory glands, genital chamber). Inset is a detail of the spermatheca to highlight substructures. Previous FlyBase terms are on the left and revised terms are on the right.
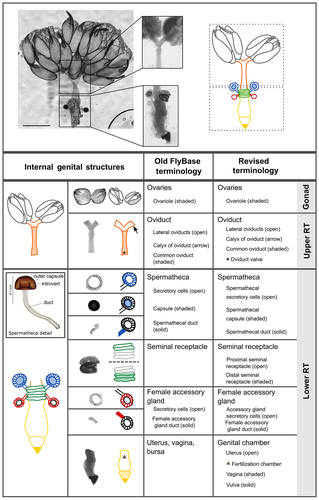
Figure 5. Internal genital structures of the female reproductive tract. [A] The Drosophila female reproductive tract consists of a pair of ovaries (OV) connected to a median common oviduct (CO) by two lateral oviducts (LO), and a uterus (UT) that leads to the vagina, which opens to the exterior through the vulva. The reproductive tract also includes specialized organs: a pair of spermathecae (Spt), seminal receptacle (SR), and a pair of female accessory glands (AG); drawing by Zohar Nir-Amitin. [B, C] The whole system with fat body [C] or without the fat body [B] that covers the spermatheca (Spt-FB) and the female accessory glands (AG-FB) (scale bar is 500 µm). [D-F] Upper RT that includes the lateral and common oviducts (scale bar is 100 µm), [D]. Toluidine blue stained 1 µm thick section of the oviduct that highlight the luminal space (l) and the epithelial cells (Epi) [E]. Upper RT stained with Alexa Fluor 594-phalloidin (red) showing the muscle fibres in different regions of oviduct (scale bar is 50 µm), [F]. [G, H] Lower reproductive tract, including the spermatheca (Spt), seminal receptacle (SR), and female accessory glands (AG). Note the red and blue arrowheads that mark the connection of the Spt and AG stalks to the uterus (scale bar is 50 µm). The panel also presents bright-field, phalloidin and DAPI images: SR showing the proximal (Pro) and distal (Dis) regions and the surrounding layer of visceral muscle (scale bar is 50 µm); Spt showing the spermathecal secretory cells (SSC), the lumen where sperm is stored (L), the stalks (St) (scale bar is 20 µm), the end apparatus (EA), and the fat body (FB, stained with DAPI) that surrounds the Spt; the female accessory glands (AG) showing the secretory cells (SC) (scale bar is 20 µm). [I-L] Zoom-in image of the uterus: [J] layers of circular muscle fibres (UTm) (scale bar is 50 µm), [K] micro-CT of the uterus highlighting the structure of the uterine lumen (L) (scale bar is 50 µm), [L] DsRed expression (magenta) showing the location of the fertilization chamber (FC), a structure to which the stalks of the SR, Spt and AG enter.
![Figure 5. Internal genital structures of the female reproductive tract. [A] The Drosophila female reproductive tract consists of a pair of ovaries (OV) connected to a median common oviduct (CO) by two lateral oviducts (LO), and a uterus (UT) that leads to the vagina, which opens to the exterior through the vulva. The reproductive tract also includes specialized organs: a pair of spermathecae (Spt), seminal receptacle (SR), and a pair of female accessory glands (AG); drawing by Zohar Nir-Amitin. [B, C] The whole system with fat body [C] or without the fat body [B] that covers the spermatheca (Spt-FB) and the female accessory glands (AG-FB) (scale bar is 500 µm). [D-F] Upper RT that includes the lateral and common oviducts (scale bar is 100 µm), [D]. Toluidine blue stained 1 µm thick section of the oviduct that highlight the luminal space (l) and the epithelial cells (Epi) [E]. Upper RT stained with Alexa Fluor 594-phalloidin (red) showing the muscle fibres in different regions of oviduct (scale bar is 50 µm), [F]. [G, H] Lower reproductive tract, including the spermatheca (Spt), seminal receptacle (SR), and female accessory glands (AG). Note the red and blue arrowheads that mark the connection of the Spt and AG stalks to the uterus (scale bar is 50 µm). The panel also presents bright-field, phalloidin and DAPI images: SR showing the proximal (Pro) and distal (Dis) regions and the surrounding layer of visceral muscle (scale bar is 50 µm); Spt showing the spermathecal secretory cells (SSC), the lumen where sperm is stored (L), the stalks (St) (scale bar is 20 µm), the end apparatus (EA), and the fat body (FB, stained with DAPI) that surrounds the Spt; the female accessory glands (AG) showing the secretory cells (SC) (scale bar is 20 µm). [I-L] Zoom-in image of the uterus: [J] layers of circular muscle fibres (UTm) (scale bar is 50 µm), [K] micro-CT of the uterus highlighting the structure of the uterine lumen (L) (scale bar is 50 µm), [L] DsRed expression (magenta) showing the location of the fertilization chamber (FC), a structure to which the stalks of the SR, Spt and AG enter.](/cms/asset/44019de2-0451-47bc-ad6b-e7a1fcd668d0/kfly_a_2058309_f0005_oc.jpg)
Delineation of structures
Some parts of the female genitalia that we outline in this work do not have clear boundaries, such as ridges or clefts. We justify the demarcation of these structures in several ways. In some cases, we note the structure separately because the feature appears to have functional significance. For instance, the hypogynial mid-dorsal incision () does not have clear boundaries with surrounding tissue, but there is evidence to suggest that this depression is a site that makes contact with the male surstylus during copulation [Citation30]. Delimitation of anatomical features can also be aided by considering the distribution of important developmental molecules (e.g. transcription factors), the patterning of which may indicate regions that harbour developmental or evolutionary independence [Citation27,Citation28,Citation36]. Lastly, some identified features are quite subtle in D. melanogaster but are more exaggerated in closely related species, providing reasoning for their designation as notable structures of the female genitalia in this group. For example, the hypogynial posterodorsal pouch is relatively shallow in D. melanogaster but is unambiguous in D. simulans [Citation15], a closely related species which diverged about 2 million years ago [Citation37]. Future work investigating the development and function of these structures will further aid in structural demarcation.
Choice of terms
The term hypogynium was first proposed by Crampton [Citation38] to refer to the abdominal sternite below the genital apparatus of the female, which in the case of Diptera corresponds to sternite 8. In the same paper, Crampton [Citation38] defined the term hypandrium as the abdominal sternite below the genital apparatus of the male, i.e. sternite 9 in Diptera. Whereas the term hypandrium has been used in Drosophila systematics and developmental biology as early as the 1940s [e.g. Citation39, Citation40], ‘hypogynium’ has never been applied to Drosophila. Instead, a variety of non-anatomical terms such as ‘egg-guide’ and ‘ovipositor’ have been applied to the female egg-laying external structures. In entomology, the ovipositor is usually formed from the appendices of the genital segment [Citation41], and indeed Ferris [Citation42] called the external egg-laying structure (in D. melanogaster) the ‘female gonopod’. However, it has been suggested that Diptera females lack an ovipositor, in the proper entomological sense [Citation41]. Indeed, in D. melanogaster the homoeotic gene Abdominal-B represses all leg-development genes in female A8, confirming the sternal nature of the hypogynium [Citation43]. Crampton [Citation44] suggested that specific terms, such as oviscapt, would be more appropriate. Grimaldi [Citation45] has introduced this term in Drosophila systematics, and since then it has been used in multiple systematic and functional morphology studies [Citation15,Citation30,Citation46,Citation47,Citation72]. However, given our conservation of the terms hypandrium and epandrium for the sternite and tergite of abdominal segment 9 in our paper on male terminalia anatomy [Citation22], we prefer here for consistency the usage of the terms hypogynium and epigynium for the sternite and tergite of female abdominal segment 8. As the anatomical term hypogynium is not commonly used in the literature, it would be preferable to cite it alongside the more common functional term ‘ovipositor’ in publications, e.g. hypogynium (ovipositor) or ovipositor (hypogynium).
The analia have formerly been called the proctiger and consequently the sternite and tergite surrounding the anus were called the hypoproct and the epiproct, respectively [Citation30,Citation45,Citation46,Citation72,Citation48,Citation49]. However, in some Dipteran species, two additional lateral plates, called the cerci, also surround the anus. Remarkably, there are no hypo- and epiprocts in males and no cerci in females of D. melanogaster. Nonetheless, it has been observed that in doublesex, transformer-2, hermaphrodite, or intersex mutant females, the hypoproct is reduced and the epiproct shifts laterally, resembling the male cerci, but still usually fused on the dorsal side [Citation50–53]. This suggests that the female epiproct may have the same developmental origin as both male cerci. Females of the subfamily Steganinae have a pair of lateral plates identified as cerci posterior to the epiproct [Citation45]. In the subfamily Drosophilinae, however, these cerci have been lost or possibly fused to the epiproct. In addition, we note that in some insect groups (such as odonates) the terms hypoproct, epiproct, and paraproct describe terminal structures that are not functionally homologous to the structures named here for D. melanogaster and could very well derive from different segment primordia during terminalia development [Citation54,Citation55].
Considering the internal structures, we propose here a term in Drosophila, the furca (). In non-Cyclorrhaphan Diptera, the furca is an internalized sclerite on the dorsal surface of the genital chamber derived from sternite 9 [Citation56], and it was believed to be absent or unrecognizable in most Cyclorrhapha. Interestingly, developmental studies showed that the dorsal wall of the genital chamber in D. melanogaster derives from the A9 primordium [Citation26], suggesting the furca is present in this species though far less sclerotized. The furca has several folds that we choose to define more precisely here, motivated by evidence that some of these may interact with male intromittent organs. For example, the vaginal furcal dorsolateral fold () is the location of one type of copulatory wound described by Kamimura and Mitsumoto [Citation30].
Figure 6. Scanning electron micrographs of the furca and furcal folds. [A] Lateral view with internal structures extruded, [B] lateral view, unextended, [C] posterior view. In each image, the vaginal furca is indicated by the yellow dashed line. a. vaginal furcal dorsal fold, b. vaginal furcal dorsolateral fold, c. vaginal furcal lateral fold, d. uterine furca. Not shown in the figure is the portion of the uterine furca that extends internally until the entry point of the spermathecal and accessory gland ducts into the genital chamber. e. oviprovector scales.
![Figure 6. Scanning electron micrographs of the furca and furcal folds. [A] Lateral view with internal structures extruded, [B] lateral view, unextended, [C] posterior view. In each image, the vaginal furca is indicated by the yellow dashed line. a. vaginal furcal dorsal fold, b. vaginal furcal dorsolateral fold, c. vaginal furcal lateral fold, d. uterine furca. Not shown in the figure is the portion of the uterine furca that extends internally until the entry point of the spermathecal and accessory gland ducts into the genital chamber. e. oviprovector scales.](/cms/asset/4149d01b-39a7-4886-8b98-38db593ed7b7/kfly_a_2058309_f0006_oc.jpg)
Incorporation of our standardized terminology across areas of research and species
A primary goal of this article is to facilitate the flow of information across disciplines and research areas. To this aim, we worked with the FlyBase team to incorporate our unified terminology into their database, updating and adding terms as needed. We understand that there may be good reasons for individual authors to continue using the terminology that they are accustomed to in their own work. In such cases, our suggestion would be to parenthetically reference the unified terminology that we outline here, e.g. parovaria (female accessory glands). In this manner, there will be greater ease in translating across works that employ different terminology for the same feature.
Our work here focused on the terminalia of Drosophila melanogaster females. However, despite the great morphological diversity of the female genitalia in the Drosophilidae, the general ground plan of these structures is fairly well conserved. Therefore, most of the terms we define are easily extensible to other species, facilitating comparison across studies outside D. melanogaster. In cases where structures have been lost in D. melanogaster (and thus are not named here), we hope that this set of unified terms will mitigate potential confusion by giving common references for surrounding structures. We briefly illustrate below two exciting research areas for which our unified terminology may prove useful in facilitating fruitful comparisons across species or in different species groups.
Evolution of genitalia in response to ecological factors
Evolution of the female genitalia has frequently taken place in response to changing oviposition substrates. Adaptations usually involve changes in size and shape of the hypogynium as well as in the number, disposition and shape of the hypogynial sensilla [Citation6,Citation16]. For example, species laying eggs on solid substrates, such as D. suzukii or leaf- and bark-breeding Hawaiian drosophilids [Citation57], often have large and elongate hypogynium with numerous, large teeth-like sensilla. On the other hand, species laying eggs in decaying or soft tissues, such as D. melanogaster, often have a short and roundish hypogynium with fewer and less sharp sensilla. In some cases, female genital evolution in Drosophila has important consequences for agriculture. For example, the evolution of a serrated ovipositor in D. suzukii and closely related species (e.g. D. subpulchrella) has allowed these flies to oviposit in ripening fruit, making them crop-damaging pests, while closely related species such as D. biarmipes, where the ovipositor retains the basal shape and setation, are benign [Citation16]. A common language with respect to anatomical structures will ensure that studies conducted in disparate systems come together to inform our collective understanding of the forces and mechanisms driving such changes in response to ecological factors.
Coevolution of the sexes
The rapid evolution of animal genitalia is a longstanding area of research interest [Citation1–3]. While early work focused specifically on male structures, added emphasis has recently been placed on understanding the evolution of female structures [Citation5,Citation10], and how coevolution of male and female genitalia might contribute to the rapid evolution of these structures in both sexes [Citation11,Citation12]. Adaptations of male genitalia to rapidly evolving female genitalia [Citation58], or vice versa, usually involve changes concerning specific genital features, such as the shape and size of the hypogynial posterodorsal pouch in the melanogaster species complex and the sclerification of some internal walls of the oviprovector (e.g. in D. teissieri), the vulva (e.g. in D. orena) and the vagina (e.g. in D. erecta) [Citation15,Citation59,Citation60]. Some internal sperm-storage organs, such as the seminal receptacle, have co-evolved with the size of the male sperm [Citation61,Citation62]. We hope that the common set of terms we outline here to reference the various parts of the female genitalia, in combination with the previous work outlining terms for the male genital structure [Citation22], will aid in the synthesis of empirical studies of genital evolution and coevolution across Drosophilid species.
Methods
Scanning electron microscopy
The scanning electron micrographs from were collected about 40 years ago, and the exact strain of D. melanogaster, and exact methods used to collect these images are no longer known.
The scanning electron micrograph in was collected as follows: Adult female D. melanogaster were fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate, stained with osmium tetroxide, dried through an ethanol series (35–100%) and the ethanol dried with a Tousimis AutoSamdri 815 critical point dryer. The terminalia were then dissected from the abdomen, mounted on stubs, and coated with gold-palladium using a Tousimis sputter coater. Specimens were visualized with a Hitachi SM-5000 scanning electron microscope.
The scanning electron micrograph in was collected by fixing a female sample from the Oregon-R strain in ice-cold ethanol, followed by a t-butanol wash, and drying by sublimation. The samples were then gold-coated and observed under a scanning electron microscope (Hitachi S-510).
The scanning electron micrographs in , are from L. Tsacas’ collection at the National Museum of Natural History, Paris (Courtesy of the Museum).
Bright field cuticle imaging
For cuticle images in (except , panel C), a Canton S line of Drosophila melanogaster (Bloomington # 64349) was used. Adult females were dissected in 100% EtOH with micro-forceps and mounted in PVA Mounting Medium (BioQuip). Samples were imaged at 10× and 20× magnification on a Leica DM 2000 with a Leica DFC540 C camera. Images were Z stack-compiled with the Leica Application Suite to allow for optimal focus.
For cuticle image in and the image of the spermatheca in (inset), female specimens from a lab-culture strain of Canton S were used. The distal portion of abdomen after the segment 7 including the spermatheca therein was detached from the main body in 70% EtOH, treated with 10% KOH solution at 80–90°C for about 5 min, and mounted in a droplet of glycerine on a cavity slide. After removing the tergite and sternite 7 within glycerine, the dissected and cleaned terminalia and spermatheca were microphotographed at different depths of focus using a DinoLite® Digital Eyepiece Camera attached to an Olympus BX50 microscope. The photos were stacked into an all-in-focus composite using the software CombineZP [Citation63]. The confocal images were edited using Adobe Photoshop CS6 and Adobe Illustrator CS6.
For the cuticle image in , a female from the Canton S strain was used. The sample was mounted in 50:50 Hoyer’s medium and lactic acid. The sample was imaged at 20× magnification using a Zeiss Axioplan with a Manta G609C camera (Allied Vision Technologies). Focus stacking was performed with the software Picolay (www.picolay.de, version 2020–08-13).
Visualization of the upper and lower RT
Reproductive tracts were dissected in Schneider’s Drosophila medium (Sigma) on ice and processed for electron microscopy as described in Citation64. Briefly, tracts were flat-embedded between two sheets of Aclar (Electron Microscopy Sciences), which allowed us to image the entire tract at the light microscopic level prior to sectioning. Sections were cut on a Reichert Ultracut microtome. One-µm thick sections were stained with 1% toluidine blue and viewed with a Zeiss Axioplan microscope.
Immunocytochemistry
Reproductive tracts were dissected in Yamamoto’s Ringer, fixed in 4% paraphormaldehyde in PBS and incubated in blocking solution and stain with Alexa Fluor 594-phalloidin (1:200) and DAPI (Molecular Probes) as described in Citation64.
Reproductive tracts of the different treatments were mounted with Antifade media [Citation65] on a multi-well glass slide.
Reporter constructs
The image in shows the pattern of DsRed expression (magenta) for an enhancer-reporter construct containing 301 bp of sequence between the transcription start site of CG32833 and a distal transcription start site of twist (coordinates 2,2985,299–2,2985,599 in D. melanogaster genome v6.42). Note that this intergenic sequence is also upstream of the transcription start site of miR-4939 (transcribed in the same direction as CG32833) and of the transcription start site of long non-coding RNA gene CR42742 (transcribed in the same direction as twist). It is not known to which gene’s expression pattern the reporter corresponds. The 301-bp fragment was amplified by PCR with primers respectively containing a KpnI site and an XhoI site, for cloning into the KpnI and XhoI sites in the polylinker of pRed H-Stinger [Citation66]. The construct was inserted into strain w1118 by P-element-mediated transformation, and the reproductive tract of a female from the resulting strain was dissected and imaged as done previously [Citation67].
Confocal microscopy
Reproductive tracts were imaged in a Leica TCS SP8 multiphoton (MP) laser scanning confocal microscope operated by the LAS X software. Fluorescence was detected by using argon excitation lasers of 488 nm captured by a conventional photomultiplier (PMT). Image processing was done using Fiji and Imaris 8.4 (Bitplane).
Micro computed tomography (micro-CT)
Reproductive tracts were stained with a mixed contrasting dye [1% crystalline I2 (Merck 376,558) and 1% Tannic acid (Merck 1401–55-4) in 200 proof ethanol] for 24–48 hours at 40°C. Before imaging, the samples were washed two times for ten minutes each in fresh 200 proof ethanol. Micro-CT was done with a Zeiss Xradia micro XCT-400 at X20 magnification and data processing was done using AVIzO and Fiji (Zelinger E, Brumfeld V, Rechav K, Heifetz Y, in prep).
Acknowledgments
We thank Clare Pilgrim and Steven Marygold from FlyBase for working with us to integrate our terminology into the FlyBase terms. We also thank Patricia Rivlin and Anat Kapelnikov for images used in , and Zohar Nir-Amitin for the female reproductive tract drawing in .
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Eberhard WG. Sexual selection and animal genitalia. Cambridge Massachusetts USA: Harvard University Press; 1985.
- Hosken DJ, Stockley P. Sexual selection and genital evolution. Trends Ecol Evol. 2004;19(2):87–93.
- Simmons LW. Sexual selection and genital evolution. Austral Entomol. 2014;53(1):1–17.
- Eberhard WG. Evolution of genitalia: theories, evidence, and new directions. Genetica. 2010;138(1):5–18.
- Ah-King M, Barron AB, Herberstein ME. Genital evolution: why are females still understudied? PLoS Biol. 2014;12(5):e1001851.
- Craddock EM, Kambysellis MP, Franchi L, et al. Ultrastructural variation and adaptive evolution of the ovipositor in the endemic Hawaiian Drosophilidae. J Morphol. 2018;279:1725–1752.
- Crava MC, Zanini D, Amati S, et al. Structural and transcriptional evidence of mechanotransduction in the Drosophila suzukii ovipositor. J Insect Physiol. 2020;125:104088.
- Green JE, Cavey M, Médina Caturegli E, et al. Evolution of ovipositor length in Drosophila suzukii is driven by enhanced cell size expansion and anisotropic tissue reorganization. Curr Biol. 2019;29:2075–2082.e6.
- Méndez V, Córdoba-Aguilar A. Sexual selection and animal genitalia. Trends Ecol Evol. 2004;19(5):224–225.
- Sloan NS, Simmons LW. The evolution of female genitalia. J Evol Biol. 2019;32(9):882–899.
- Yassin A. Unresolved questions in genitalia coevolution: bridging taxonomy, speciation, and developmental genetics. Organ Divers Evol. 2016;16(4):681–688.
- Brennan PL, Prum RO. Mechanisms and evidence of genital coevolution: the roles of natural selection, mate choice, and sexual conflict. Cold Spring Harb Perspect Biol. 2015;7(7):a017749.
- Brennan PL, Prum RO, McCracken KG, et al. Coevolution of male and female genital morphology in waterfowl. PLoS one. 2007;2(5):e418.
- Kuntner M, Coddington JA, Schneider JM. Intersexual arms race? Genital coevolution in nephilid spiders (Araneae, Nephilidae). Evolution. Int J Org Evol. 2009;63(6):1451–1463.
- Yassin A, Orgogozo V. Coevolution between male and female genitalia in the Drosophila melanogaster species subgroup. PloS one. 2013;8(2):e57158.
- Atallah J, Teixeira L, Salazar R, et al. The making of a pest: the evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc R Soc B. 2014;281:20132840.
- Childers CC. Feeding and oviposition injuries to plants. In: Lewis T, editor. Thrips as Crop Pests. New York: CAB International; 1997. p. 505–537.
- Dreves AJ, Walton V,M, Fisher GC. (2009). “A new pest attacking healthy ripening fruit in Oregon: spotted wing Drosophila: Drosophila suzukii (Matsumura)“. Accessed 05 04 2022. https://ir.library.oregonstate.edu/concern/open_educational_resources/st74cq71x
- Omoloye AA, Oladapo OG, Ibitoye O, et al. Effects of field attack by Ceratitis capitata Wiedemann (Diptera: tephritidae) on the morphology and nutritional quality fresh fruit of Citrus sinensis L. Afr J Agric Res. 2016;11(11):967–973.
- Seraj AA. Comparison of plant species as host for cabbage leaf miner in khuzestan province. J Agric Sci Technol. 2000;2(2):127–135.
- Mattei AL, Riccio ML, Avila FW, et al. Integrated 3D view of postmating responses by the Drosophila melanogaster female reproductive tract, obtained by micro-computed tomography scanning. Proc Nat Acad Sci. 2015;112(27):8475–8480.
- Rice G, David JR, Kamimura Y, et al. A standardized nomenclature and atlas of the male terminalia of Drosophila melanogaster. Fly (Austin). 2019Mar-Dec;13(1–4):51–64. Epub 2019 Aug 19. PMID: 31401934; PMCID: PMC6988887.
- Kamimura Y. Correlated evolutionary changes in Drosophila female genitalia reduce the possible infection risk caused by male copulatory wounding. Behav Ecology Sociobiol. 2012;66(8):1107–1114.
- Epper F. Three-dimensional fate map of the female genital disc of Drosophila melanogaster. Wilhelm Roux’s Arch Dev Biol. 1983;192(5):270–274.
- Schüpbach T, Wieschaus E, Nöthiger R. The embryonic organization of the genital disc studied in genetic mosaics of Drosophila melanogaster. Wilhelm Roux’s Arch Dev Biol. 1978;185(3):249–270.
- Keisman EL, Christiansen AE, Baker BS. The sex determination gene doublesex regulates the A/P organizer to direct sex-specific patterns of growth in the Drosophila genital imaginal disc. Dev Cell. 2001;1(2):215–225.
- Sánchez L, Guerrero I. The development of the Drosophila genital disc. Bioessays. 2001;23(8):698–707.
- Estrada B, Casares F, Sánchez‐Herrero E. Development of the genitalia in Drosophila melanogaster. Differentiation. 2003;71(6):299–310.
- Nöthiger R, Dübendorfer A, Epper F. Gynandromorphs reveal two separate primordia for male and female genitalia in Drosophila melanogaster. Wilhelm Roux’s Arch Dev Biol. 1977;181(4):367–373.
- Kamimura Y, Mitsumoto H. Comparative copulation anatomy of the Drosophila melanogaster species complex (Diptera: drosophilidae). Entomol Sci. 2011;14(4):399–410.
- Jagadeeshan S, Singh RS. A time‐sequence functional analysis of mating behaviour and genital coupling in Drosophila: role of cryptic female choice and male sex‐drive in the evolution of male genitalia. J Evol Biol. 2006;19(4):1058–1070.
- Austin AD, Browning TO. A mechanism for movement of eggs along insect ovipositors. Int J Insect Morphol Embryol. 1981;10:93–108.
- Nonidez JF. The Internal Phenomena of Reproduction in Drosophila. Biol Bull. 1920 Oct;39(4):207–230. Published by: The University of Chicago Press in association with the Marine Biological Laboratory Stable URL https://www.jstor.org/stable/1536488
- Bryant PJ. Pattern formation in imaginal discs. In: Ashburner M, Wright TRF, editors. The genetics and biology of Drosophila. New York and London: Academic Press; 1978. p. 229–335.
- Tsacas L, David J. Les Drosophilidae (Diptera) de la Réunion et de l’Ile Maurice. II. Trois nouvelles espèces des genres Drosophila et Zaprionus. Publications de la Société Linnéenne de Lyon. 1975;44(10):373–380.
- Christiansen AE, Keisman EL, Ahmad SM, et al. Sex comes in from the cold: the integration of sex and pattern. Trends Genet. 2002;18(10):510–516.
- Li YJ, Satta Y, Takahata N. Paleo-demography of the Drosophila melanogaster subgroup: application of the maximum likelihood method. Genes Genet Syst. 1999;74(4):117–127.
- Crampton GCV. The terminal abdominal structures of the primitive Australian termite, mastotermes darwinensis Froggalt. Trans Royal Entomol Soc London. 1920;68:137–145.
- Hildreth PE. Doublesex, a recessive gene that transforms both males and females of Drosophila into intersexes. Genetics. 1965;51(4):659.
- Salles H. Sobre a Genitalia dos Drosofilidios (Diptera): I. Drosophila melanogaster E. D. simulans. Summa Brasiliensis Biologiae. 1947;1(15):1–73.
- Snodgrass RE. The abdominal mechanisms of a grasshopper. CITY OF WASHINGTON: Smithsonian Miscellaneous Collections; 1935.
- Ferris GF. External morphology of the adult. In: Demerec M, editor. Biology of Drosophila. New York: Hafner; 1950. p. 368–419.
- Estrada B, Sánchez-Herrero E. The Hox gene Abdominal-B antagonizes appendage development in the genital disc of Drosophila. Development. 2001;128(3):331–339.
- Crampton. The external morphology of the Diptera. In guide to the insects of Connecticut. Part VI. Connecticut Geol Nat History Survey Bull. 1942;64:76.
- Grimaldi DA. A phylogenetic, revised classification of genera in the Drosophilidae (Diptera). Bull Am Mus Nat Hist. 1990;197:1–139.
- Bächli G, Vilela CR, Escher AS, et al. The Drosophilidae (Diptera) of Fennoscandia and Denmark. In Kristensen, NP. (editor). Fauna Entomologica Scandinavica. Vol. 39. Brill Leiden; 2004. p. 362.
- Kamimura Y. Copulation anatomy of Drosophila melanogaster (Diptera: drosophilidae): wound-making organs and their possible roles. Zoomorphology. 2010;129(3):163–174.
- Vilela CR, Bächli G. Taxonomic studies on Neotropical species of seven genera of Drosophilidae (Diptera). Mitteilungen der schweizerischen entomologischen Gesellschaft. 1990;63(Supp.):1–332 .
- Zhang WX, Toda MJ. A new species-subgroup of the Drosophila immigrans species-group (Diptera, Drosophilidae), with description of two new species from China and revision of taxonomic terminology. Jpn J Entomol. 1992;60(4):839–850.
- Belote JM, Baker BS. Sex determination in Drosophila melanogaster: analysis of transformer-2, a sex-transforming locus. Proc Nat Acad Sci. 1982;79(5):1568–1572.
- Garrett-Engele CM, Siegal ML, Manoli DS, et al. intersex, a gene required for female sexual development in Drosophila, is expressed in both sexes and functions together with doublesex to regulate terminal differentiation. Development. 2002;129:4661–4675.
- Li H, Baker BS. Hermaphrodite and doublesex function both dependently and independently to control various aspects of sexual differentiation in Drosophila. Development. 1998;125(14):2641–2651.
- Siegal ML, Baker BS. Functional conservation and divergence of intersex, a gene required for female differentiation in Drosophila melanogaster. Dev Genes Evol. 2005;215(1):1–12.
- Boudinot BE. A general theory of genital homologies for the Hexapoda (Pancrustacea) derived from skeletomuscular correspondences, with emphasis on the Endopterygota. Arthropod Struct Dev. 2018;47(6):563–613.
- Snodgrass RE. A textbook of arthropod anatomy. Ithaca, NY: Comstock Pub.; Cornell University Press; 1952. 363.
- Cumming JM, Wood DM. Adult morphology and terminology. In: Kirk-Spriggs AH, Sinclair BJ, editors. Manual of afrotropical Diptera. v. 1. Introductory chapters and keys to Diptera families. Suricata. Vol. 4. Pretoria: South African National Biodiversity Institute; 2017. p. 89–133.
- O’Grady PM, Lapoint RT, Bonacum J, et al. Phylogenetic and ecological relationships of the Hawaiian Drosophila inferred by mitochondrial DNA analysis. Mol Phylogenet Evol. 2011;58(2):244–256.
- Muto L, Kamimura Y, Tanaka KM, et al. An innovative ovipositor for niche exploitation impacts genital coevolution between sexes in a fruit-damaging Drosophila. Proc R Soc B. 2018;285(1887):20181635.
- Kamimura Y. Significance of constraints in genital coevolution: why does female Drosophila appear to cooperate males by accepting harmful traumatic matings? Evolution. 2016;70(7):1674–1683.
- Kamimura Y, Mitsumoto H. Lock-and-key structural isolation between sibling Drosophila species. Entomol Sci. 2012;15:197–201.
- Joly D, Schiffer M. Coevolution of male and female reproductive structures in Drosophila. Genetica. 2010;138(1):105–118.
- Pitnick S, Marrow T, Spicer GS. Evolution of multiple kinds of female sperm‐storage organs in Drosophila. Evolution. 1999;53(6):1804–1822.
- Hadley A. Combine ZP software .2010. Accessed 5 4 2022. Available from https://combinezp.software.informer.com/
- Kapelnikov A, Rivlin PK, Hoy RR, et al. Tissue remodeling: a mating-induced differentiation program for the Drosophila oviduct. BMC Dev Biol. 2008a;8:114.
- Giloh H, Sedat JW. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982;217:1252–1255.
- Barolo S, Castro B, Posakony JW. New Drosophila transgenic reporters: insulated P-element vectors expressing fast-maturing RFP. Biotechniques. 2004;36(3):436–442.
- Schnakenberg SL, Matias WR, Siegal ML. Sperm-storage defects and live birth in Drosophila females lacking spermathecal secretory cells. PLoS Biol. 2011;9(11):e1001192.
- Casares F, Sánchez L, Guerrero I, et al. The genital disc of Drosophila melanogaster. Dev Genes Evol. 1997;207(4):216–228.
- Epper F, Sánchez L. Effects of engrailed in the genital disc of Drosophila melanogaster. Dev Biol. 1983;100(2):387–398.
- Sánchez L, Casares F, Gorfinkiel N, et al. The genital disc of Drosophila melanogaster. Dev Genes Evol. 1997;207(4):229–241.
- Robertson HM. Mating asymmetries and phylogeny in the Drosophila melanogaster species complex. Pacific Sci. 1988;42:72–80.
- Hu YG, Toda MJ. Polyphyly of Lordiphosa and its relationships in Drosophilinae (Diptera: drosophilidae). Syst Entomol. 2001;26(1):15–31.
- Chen EH, Baker BS. Compartmental organization of the Drosophila genital imaginal discs. Development. 1997;124(1):205–218.
- Gorfinkiel N, Sánchez L, Guerrero I. Drosophila terminalia as an appendage-like structure. Mech Dev. 1999;86(1–2):113–123.
- Sturtevant AH (1921). The North American species of Drosophila (No. 301). Carnegie institution of Washington.
- Miller A. The internal anatomy and histology of the imago of Drosophila melanogaster. In: Demerec M, editor. Biology of Drosophila. New York: Wiley; 1950. p. 420–534.
- Bock IR, Wheeler MR. The Drosophila melanogaster species group. Univ Texas Publ. 1972;7213:1–102.
- Fung STC, Gowen JW. The developmental effect of a sex‐limited gene in Drosophila melanogaster. J Exp Zool. 1957;134(3):515–532.
- Keisman EL, Baker BS. The Drosophila sex determination hierarchy modulates wingless and decapentaplegic signaling to deploy dachshund sex-specifically in the genital imaginal disc. Development. 2001;128(9):1643–1656.
- Okada T. Systematic study of Drosophilidae and allied families of Japan. Tokyo: Gihodo Co; 1956.
- Bodenstein D. The postembryonic development of Drosophila. In: Demerec M, editor. “The Biology of Drosophila”. New York: C. John Wiley & Sons. 1950. p. 275–367.
- Snodgrass RE. The terminal abdominal segments of female Tipulidae. J New York Entomol Soc. 1903;11:177–183.
- Van Emden F, Hennig W. Taxonomist’s glossary of genitalia in insects. In Diptera. Tuxen SL, editor. 2nd. 395. Copenhagen: Munksgaard. p. 130–140. 1970.
- Grimaldi DA. Phylogenetics and taxonomy of Zygothrica (Diptera: drosophilidae). Filogenia y taxonomía de Zygothrica (Diptera: drosophilidae). Bull Am Mus Nat Hist. 1987;186:104–268.
- Al Sayad S, Yassin A. Quantifying the extent of morphological homoplasy: a phylogenetic analysis of 490 characters in Drosophila. Evol Lett. 2019;3:286–298.
- Vilela CR, Prieto D. A new Costa Rican species of Drosophila visiting inflorescences of the hemi-epiphytic climber Monstera lentii (Araceae). Revista Brasileira de Entomologia. 2018;62:225–231.
- Kikkawa H. THE “SUMP” METHOD FOR DROSOPHILA MORPHOLOGY. J Heredity. 1938;29(10):395–397.
- Gorfinkiel N, Sánchez L, Guerrero I. Development of the Drosophila genital disc requires interactions between its segmental primordia. Development. 2003;130(2):295–305.
- Bloch Qazi MC, Heifetz Y, Wolfner MF. The developments between gametogenesis and fertilization: ovulation and female sperm storage in Drosophila melanogaster. Dev Biol. 2003;256:195–211.
- Adams EM, Wolfner MF. Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage. J Insect Physiol. 2007Apr;53(4):319–331. Epub 2006 Dec 29. PMID: 17276455; PMCID: PMC2144743.
- Avila FW, Sánchez-López JA, McGlaughon JL, et al. Nature and functions of glands and ducts in the Drosophila reproductive tract. In: Cohen E, Moussian B, editors. Extracellular composite matrices in arthropods. Cham: Springer; 2016, 411–444. DOI: 10.1007/978-3-319-40740-1_11
- Heifetz Y, Lindner M, Garini Y, et al. Mating regulates neuromodulator ensembles at nerve termini innervating the Drosophila reproductive tract. Curr Biol. 2014;24(7):731–737.
- Heifetz Y, Lung O, Frongillo EA Jr, et al. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr Biol. 2000;10(2):99–102.
- Kapelnikov A, Zelinger E, Gottlieb Y, et al. Mating induces an immune response and developmental switch in the Drosophila oviduct. Proc Natl Acad Sci U S A. 2008b;105:13912–13917.
- Gleichauf R. Anatomie und variabilitat des geschlechtapparates von Drosophila melanogaster (Meigen). Zeitschrift für wissenschaftliche Zoologie. 1936;148:1–66.
- Heifetz Y, Rivlin PK. Beyond the mouse model: using Drosophila as a model for sperm interaction with the female reproductive tract. Theriogenology. 2010;73:723–739.
- Anderson RC. A Study of the Factors Affecting Fertility of Lozenge Females of Drosophila melanogaster. Genetics. 1945;30(3):280–296.
- Dobzhansky T, Pavan C. Studies on Brazilian species of Drosophila. Boletim da Faculdade de Filosofia, Ciências e Letras. Universidade de São Paulo. 1943;36(Biol. Geral, 4):7–72.
- Hardy DE. Diptera: cyclorrhapha II, Series Schizophora. Section Acalypteratae I. Family Drosophilidae. In: Zimmerman EC, editor. Insects of Hawaii. vii +. Vol. 12, Honolulu: The University Press of Hawaii; 1965. p. 814.
- Knight GR. Chromosome number and morphology of Drosophila silvestris Basden — With a descriptive note on the internal genitalia of the adults. Zeitschrift für indukt Abstammllngs- und Vorerbungslehre. 1990;87:430–442.
- Mather WB. The genus Drosophila in eastern Queensland I. Taxonomy. Aust J Zool. 1955;3:545–582.
- Okada T. Cladogenetic differentiation of Drosophilidae in relation to material compensation. Mushi. 1963;37:79–100.
- Patterson JT, Stone WS. Evolution in the genus Drosophila. New York: Macmillan; 1952. p. 610.
- Spieth HT. The behavior and biology of the Hawaiian Drosophila anomalipes species group. Ann Entomol Soc Am. 1975;68(3):506–510.
- Sturtevant AH. The classification of the genus Drosophila, with descriptions of nine new species. Univ Texas Publ. 1942;4213:5–51.
- Sturtevant AH. On the subdivision of the genus Drosophila. Proc Natl Acad Sci U S A. 1939;25:137–141.
- Throckmorton LH. The problem of phylogeny in the genus Drosophila. Vol. 6205. Austin, TX: University of Texas Publishing; 1962. p. 207–343.
- Vaz SC, Vilela CR, Krsticevic FJ, et al. Developmental sites of Neotropical Drosophilidae (Diptera): v. Inflorescences of Calathea cylindrica and Calathea monophylla (Zingiberales: marantaceae). Ann Entomol Soc Am. 2014;107:607–620.
- Wheeler MR. V. Taxonomic studies on American Drosophilidae. Univ Texas Publ. 1954;5422:47–64.
- Yassin A. Molecular and morphometrical revision of the Zaprionus tuberculatus species subgroup (Diptera: drosophilidae), with descriptions of two cryptic species. Ann Entomol Soc Am. 2008;101:978–988.
- Mayhew ML, Merritt DJ. The morphogenesis of spermathecae and spermathecal glands in Drosophila melanogaster. Arthropod Struct Dev. 2013;42(5):385–393.
- Pascini TV, Martins GF. The insect spermatheca: an overview. Zoology. 2017;121:56–71.
- Allen AK, Spradling AC. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development. 2008;135(2):311–321.
- Baker BS, Ridge KA. Sex and the single cell. I. On the action of major loci affecting sex determination in Drosophila. Genetics. 1980;94:383–423.
- Gao J, Watabe H, Toda MJ, et al. The Drosophila obscura species-group (Diptera, Drosophilidae) from Yunnan Province, Southern China. Zoolog Sci. 2003;20(6):773–782.
- Polidori C, Wurdack M. Mg-enriched ovipositors as a possible adaptation to hard-skinned fruit oviposition in Drosophila suzukii and D. subpulchrella. Arthropod Plant Interact. 2019;13:551–560.
