Abstract
In regulated exocytosis vesicular and plasma membranes merge to form a fusion pore in response to stimulation. The nonselective cation HCN channels are involved in the regulation of unitary exocytotic events by at least 2 mechanisms. They can affect SNARE-dependent exocytotic activity indirectly, via the modulation of free intracellular calcium; and/or directly, by altering local cation concentration, which affects fusion pore geometry likely via electrostatic interactions. By monitoring membrane capacitance, we investigated how extracellular cation concentration affects fusion pore diameter in pituitary cells and astrocytes. At low extracellular divalent cation levels predominantly transient fusion events with widely open fusion pores were detected. However, fusion events with predominately narrow fusion pores were present at elevated levels of extracellular trivalent cations. These results show that electrostatic interactions likely help determine the stability of discrete fusion pore states by affecting fusion pore membrane composition.
Introduction
The merger between the vesicle and the plasma membranes is present in the majority of eukaryotic cells in the form of constitutive exocytosis.Citation1 Specialized cells, like neurons and neuroendocrine cells, additionally exhibit regulated exocytosis, triggered by a physiologic stimulus,Citation2 usually in the form of increased free intracellular calcium ([Ca2+]i).Citation3 Membrane merger leads to the formation of a fusion pore, a water-filled channel, which may eventually reversibly close (transient exocytosis) or fully widen, allowing the integration of the vesicle membrane with the plasma membrane (full-fusion exocytosis).Citation4,5 A large body of evidence suggests that the transition from transient fusion, where the fusion pore diameter fluctuates between a wide open and a virtually closed state, to a fully-fused state, is the rate limiting process.Citation6-8 A fusion pore is formed by deforming membranes into highly curved regions and the fact that they can persist in this apparently energetically unfavourable stateCitation9 indicates that certain stabilization factors are likely present.
The pivotal contribution from an energetics standpoint, especially in the early stages of the fusion pore formation, is likely provided by the SNARE (Soluble N-ethylmaleimide sensitive factor attachment protein receptor) complex formation.Citation10 Nevertheless, several other SNARE-binding proteins, be they individual or in complexes, influence the formation and the expansion of fusion pores; including synaptotagmins,Citation11 complexins,Citation12 and Sec1/Munc18 (SM) proteins.Citation13 Lipids also affect the fusibility of lipid bilayersCitation14 and can regulate the fusion process by manipulating the SNARE complex.Citation15 In addition to specific interactions, membrane constituents (lipids, proteins or their complexes) can affect the energy landscape via their shape. If their shape is non-axisymmetric (anisotropic), membrane constituents can stabilize the highly curved fusion pore,Citation16 similarly as voussoirs (wedge-shaped stones) in a Roman arch. Moreover, membrane constituents usually contain one or more polar groups, which may be ionized.Citation17 The accumulation of ions at polarized membrane regions may affect local curvature due to lipid demixing. If such a region is associated with the fusion pore, then the accumulation of ions (at the cytoplasmic or extracellular side) could affect the stability and local curvature of the pore, as proposed by Kabaso et al.Citation18
Recently, we reported that Hyperpolarization-activated Cyclic Nucleotide-gated (HCN) channels modulate fusion pore properties.Citation19 HCN channels are permeable to cations (Na+, K+ and Ca2+)Citation20-22 and likely affect exocytosis indirectly by increasing the local [Ca2+]i, but may also contribute directly via electrostatic interactions with charged membrane constituents near the fusion pore. Here, we assessed the contribution of electrostatic interactions, mediated by extracellular di- and trivalent cations, to changes in fusion pore conductance, a parameter reporting pore geometry and in particular fusion pore diameter.Citation23 For this we have first modified the extracellular concentration of cations by removing Ca2+ from or by adding Al3+ to the extracellular solution and then studied the discrete states of fusion pore.
Results and Discussion
HCN2 channels modulate exocytosis
It was shown previously that an increase in intracellular second messenger cAMP affects exocytotic events in cultured pituitary lactotrophsCitation19 and that some of the modulations are mediated by HCN channels, which are expressed in the plasma membrane and in the membrane of secretory vesicles.Citation24 If the plasma membrane-resident HCN channels are activated, then this may increase the local [Ca2+]i, a stimulus known to increase the exocytotic activity.Citation25 However, in lactotrophs overexpressing HCN2 channels, the overall [Ca2+]i was lower compared to non-transfected lactotrophs and an increase in intracellular cAMP did not significantly affect [Ca2+]i,Citation24 consistent with previous results.Citation26 A possible explanation is that the activation of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA)Citation27 decreased global [Ca2+]i, as depicted in the model (). How, then, did exocytotic activity increase in the study by Calejo et al.Citation24? It is possible that Ca2+ is still increased locally, in the proximity of the fusion pore. Alternatively, HCN channels could modulate exocytosis through a more general local cloud of positively charged ions that may potentially lead to lipid demixing at the region of the fusion pore.Citation18 To date, the exact proteo-lipidic composition of the fusion pore remains unclear.Citation28 However, it is safe to assume that anisotropic membrane constituents (proteins, lipids or other nanodomains) can attribute to its stability.Citation29,30 The constituents of biological membranes (lipids, glycoproteins, glycolipids, etc.) frequently carry one or more ionized or polar groupsCitation17 and are influenced by local cation clouds.Citation31,32 Prime candidates are widespread anisotropic anionic lipids, known to bind di- and trivalent cations, such as phosphoinositides (PI), phosphatidic acid (PA) and, particularly, phosphatidylserine (PS).Citation33 The interaction of anionic lipids with cations (especially Ca2+) can dehydrate anionic lipid head groups and consequently alter local membrane curvature and lipid molecule packing into the local membrane regions (e.g. in the fusion pore region),Citation32 leading to lateral phase separation of membrane components.Citation34 These changes can then affect anisotropic neutral (e.g., cholesterol) and zwitterionic (e.g. phosphatidylethanolamine) lipids,Citation33 known to participate in regulated exocytosis.Citation35 Therefore, changes in cation concentration in the pore area likely influence the membrane fusion process during exocytosis.
Figure 1. A model demonstrating how HCN channels affect fusion of secretory vesicles. HCN channel activation in the plasma membrane and/or in the membrane of fused vesicles increases the intracellular concentration of cations (due to their established role in the process of exocytosis, only calcium ions are drawn) in the close proximity of the fusion pore. cAMP directly facilitates the opening of HCN channels, however, cAMP can also activate sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) through protein kinase A – dependent mechanism, by reducing the association between SERCA and its inhibitor phospholamban in some cell types.Citation47 As a consequence, HCN channels may promote a local decrease in extracellular cation concentration. Increased intracellular cation levels affect fusion pore properties either through SNARE complex or by electrostatic interactions as proposed by Kabaso et al.Citation18 Low extracellular divalent cation concentration promotes the formation of wide fusion pores (A) and high extracellular divalent cation concentration supports the formation of narrow fusion pores (B). Gray areas denote the intracellular domain adjacent to a fusion pore.
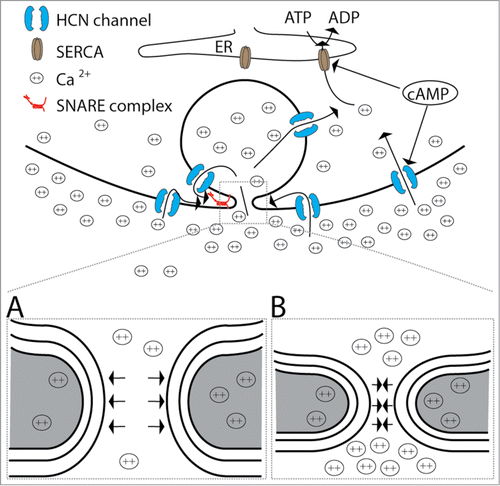
Removal of extracellular divalent cations results in fusion pores with relatively wide diameters
HCN2 channels have been shown to reside in or near vesicles in lactotrophs.Citation24 Manipulation of HCN channels, where their presence was either increased by HCN2 overexpression or their rhythmic re-opening was accelerated by cAMP, amplifies HCN-specific Ih current,Citation24 which likely increases local cytoplasmic cation concentration near fusion pores (). Simultaneously, the proportion of narrow fusion pores was decreased.Citation24 To assess if the observed effect may in part be attributed to the electrostatic interactions, we designed a conceptually similar experiment, where instead of increasing divalent cation concentration intracellularly, we removed Ca2+ ions from the extracellular space. Then, cell-attached patch-clamp technique was employed to measure reversible discrete steps in the membrane capacitance (Cm), corresponding to unitary, transient fusion events of vesicles with the plasma membrane in real time.Citation36
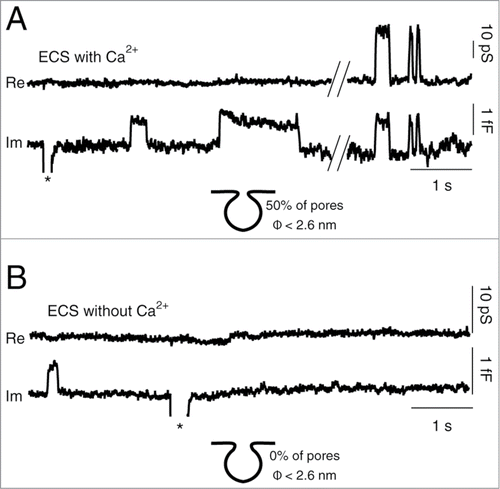
To test the robustness of our predictions on the general importance of electrostatic interactions in regulated exocytosis, we performed these experiments on a different cell type – astrocytes. Astrocytes are electrically silent and abundant glial cells in the brain, which actively contribute to information processing in the central nervous system by releasing gliotransmitters.Citation37 In astrocytes, reversible discrete steps in Cm were observed in controls with 2 mM Ca2+ () and in conditions without Ca2+ (). Here, we focused only on reversible exocytotic events, which likely represent transient fusion pore openings.Citation38 A fraction of reversible events exhibit a measurable (narrow) fusion pore conductance, which is discerned by the projection between the imaginary (Im) and the real (Re) parts of admittance signals.Citation29 In controls half of the reversible events exhibited projections to the Re trace (). For these events the average fusion pore conductance of 35 ± 4 pS was calculated, which corresponds to the average fusion pore diameter of 0.73 ± 0.05 nm (n = 12 cells) (see Materials and Methods for details). In contrast, in astrocytes that were bathed in Ca2+-free ECS, reversible exocytotic events exhibited no projections to the Re trace (), indicating fusion pores with relatively wide diameters. Experimentally determined detection limit for projected exocytotic events with our recording system was determined at ∼2.6 nm. Non-projected exocytotic events therefore exhibit fusion pores wider than ∼2.6 nm in diameter. Moreover, the frequency of all reversible exocytotic events was significantly lower in Ca2+-free ECS (0.14 ± 0.06 events/min, n = 12 cells) compared to ECS with 2 mM Ca2+ (2.2 ± 0.2 events/min, n = 12 cells, P < 0.001, U-test).
Figure 2. Calcium removal from ECS results in wide fusion pores in astrocytes. (A) Representative discrete steps in membrane capacitance (Cm) denote transient fusion exocytotic events. The top trace shows the real (Re) part and the bottom one the imaginary (Im) part of the admittance signal in controls bathed in extracellular solution (ECS) containing 2 mM Ca2+ (ECS with Ca2+). The Im trace exhibits 2 types of reversible exocytotic events: those without projections (left) and those with projections to the Re trace (right). In controls, overall, 50% of reversible events in the Im trace exhibited projections to the Re trace. (B) Representative discrete steps in Cm from astrocytes bathed in Ca2+-free ECS (ECS without Ca2+). No reversible events, detected in the Im trace, exhibited a projection to the Re trace, indicating wide diameter fusion pores. Asterisks denote truncated calibration pulses.
This experiment shows that the removal of extracellular Ca2+ reduces exocytotic activity. However, fusion pores that are formed have relatively wide diameters, which is in line with the proposed model A (). Moreover, compared to HCN2 overexpressing cells, where [Ca2+]i was also shown to be reduced,Citation24 the observed effect was even stronger, since none of the events exhibited projected (narrow) fusion pore (compared to the 17% of narrow fusion pores observed in cells overexpressing HCN2).
Extracellular trivalent cations stabilize fusion pores with relatively narrow diameters
In lactotrophs exposed to HCN2 blocker (ZD7288), the HCN-specific Ih current was decreased, indicating a reduction in local cytoplasmic cation concentration ().Citation24 In this case the proportion of narrow fusion pores recorded was increased.Citation24 This effect was even more profound after the addition of cAMP, which likely triggered the activation of SERCA pumps, subsequently decreasing cytoplasmic cation (Ca2+) concentration.Citation24 To further validate our model, we conducted conceptually the opposite experiments, as depicted in model B (). To increase the local cation concentration in the extracellular space, we monitored discrete changes in Cm of lactotrophs bathed in ECS containing 30 μM Al3+.
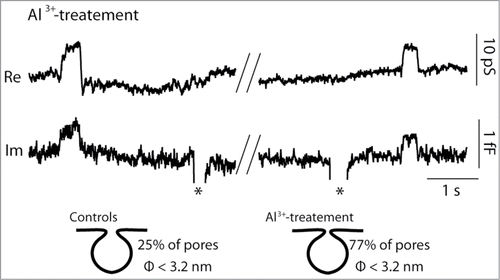
Here, the majority of reversible exocytotic events were projected to the Re trace of the admittance signal (). Compared to the previous reports, where ∼25% of reversible events exhibited projections to the Re trace in conditions where normal ECS was used,Citation24 Al3+-treatment significantly increased the percentage of reversible events to 77% (), suggesting strong stabilization of narrow exocytotic fusion pores (). Although Al3+ has a wide range of modus operandi,Citation39 electrostatic interactions could, as proposed in the model (), be responsible for this outcome. The average frequency of all reversible exocytotic events was significantly lower in Al3+ treated lactotrophs (0.45 ± 0.09 events/min, n = 8 cells) compared to controls (2.5 ± 0.9 events/min, n = 8 cells; P < 0.001, U-test), as previously reported.Citation40
Figure 3. Incubation in Al3+-enriched ECS results in narrow fusion pores in lactotrophs. Representative discrete steps in membrane capacitance (Cm) in lactotrophs bathed in 30 μM Al3+. The top trace shows the real (Re) part and the bottom trace shows the imaginary (Im; proportional to Cm) part of the admittance signals. Two representative transient fusion events with projections to the Re trace are shown. Previous reports indicate that ∼25% of reversible events are projected to the Re trace in Al3+-free ECS.Citation24 When lactotrophs were bathed in Al3+, we detected 77% projected events, indicating that most transient exocytotic events exhibited only narrow fusion pores.
Local cation concentration modulates discrete fusion pore state
We propose that changes in divalent cation concentration near fusion pores determines the extent of cation binding with charged membrane constituents, which affects membrane curvature and affects lipid demixing. These changes then, in turn, provide a framework responsible for the stabilization of fusion pore configurations, as summarized in . This is consistent with results acquired on chromaffin cells where an increase in extracellular calcium concentration shifts the mode of exocytosis to kiss-and runCitation41 and reduces the quantum content of a single exocytotic event.Citation42 The results presented in this work show a role of electrostatic interactions in affecting the transitions between discrete states of fusion pores. However, they do not argue against the necessity of protein-protein interactions (such as the formation of the SNARE complex) in this process.
Figure 4. Summary of electrostatic modulation due to the presence/absence of extracellular cations (di- and trivalent) on fusion pore conductance (i.e., pore diameter). From the previously published data by Calejo et al.Citation24 and from the new data in this paper, we conclude that fusion pore conductance (a parameter related to pore morphology) is modulated by cations adjacent to the fusion pore. A reduction of extracellular cation concentration (or an increase of intracellular cation concentration) stabilizes fusion pore in more conductive, wider configuration. Conversely, the increase of extracellular cation concentration (or a decrease of intracellular cation concentration) stabilizes fusion pores in less conductive, narrower configuration. (Control; HCN2, HCN2 transfected cells; HCN/dbcAMP, HCN2 transfected dbcAMP treated cells; noCa2+, ECS without Ca2+; ZD, ZD7288 treated cells; ZD/dbcAMP, ZD7288 and dbcAMP treated cells). Numbers indicate the fraction of exocytotic events (in%) with narrow fusion pores compared to all observed events.
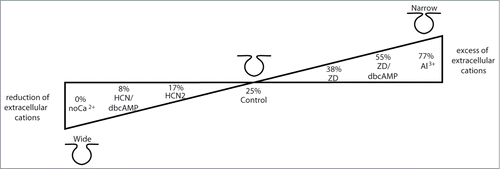
In summary, our results show that changes in the extracellular concentration of cations directly modulate fusion pore conductance, a parameter related to the fusion pore diameter. We propose that the fusion pore stability in either a wide or a narrow configuration is affected by electrostatic interactions mediated by cations adjacent to the fusion pore. These observations bear physiological significance, since extracellular calcium concentration is reduced during activity in the nervous system, which may regulate synaptic activity via sensing extracellular Ca2+ via GPCR receptorsCitation43 and as shown in this study by directly affecting the fusion pore properties as well.
Materials and Methods
Materials and solutions
Extracellular solution (ECS) for astrocytes contained (in mM): 130 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 D-glucose, 10 HEPES and pH 7.2 (with NaOH). In experiments without extracellular Ca2+, CaCl2 was replaced with NaCl. Extracellular solution used for lactotrophs contained (in mM): 130 NaCl, 5 KCl, 8 CaCl2, 1 MgCl2, 10 D-glucose, 10 HEPES (N-2-Hydroxyethylpiperazine-N'-2-ethanesulfonic acid) and pH 7.2 (with NaOH). AlCl3 was prepared as a stock solution and was added to the growth medium and ECS at 30 μM (final concentration). Osmolarity of solutions was ∼300 mOsm. All chemicals were purchased from Sigma, unless stated otherwise.
Cell cultures
Astrocytes were isolated from cortices of 2–3-day-old Wistar ratsCitation36 and maintained in growth medium (high-glucose Dulbecco's modified Eagle's medium) containing 10% fetal bovine serum (FBS), 1 mM pyruvate, 2 mM glutamine and 25 μg/ml penicillin/streptomycin at 37°C, 95% air/5% CO2). Confluent cultures were shaken overnight (225 rpm); the medium was changed the next morning and the process was repeated 3 times. After the third shaking, the cells were trypsinized and cultured in flat culture tubes until confluence.
Lactotroph primary cultures were prepared as described.Citation36 After isolation, lactotrophs were plated onto poly-l-lysine coated glass coverslips and kept in high-glucose Dulbecco's modified Eagle's medium (Invitrogen) with 10% newborn calf serum and l-glutamine at 37°C with 95% humidity and 5% CO2.
All experiments were performed within a period of one to 3 d after cell isolation.
Animals were euthanized according to the International Guiding Principles for Biomedical Research Involving Animals developed by the Council for International Organizations of Medical Sciences and Animal Protection Act (Official Gazette of the RS, No. 38/13). The protocol for the euthanization of the animals used in our study was approved by the Veterinary Administration of the Ministry for Agriculture and the Environment of the RS (permit No: 34401–29/2009/2).
Electrophysiologic measurements of Cm
Cell-attached membrane capacitance measurements (Cm) were performed with a dual-phase lock-in patch-clamp amplifier (SWAM IIC, Celica, Ljubljana, Slovenia) as describedCitation36 at room temperature. We used fire-polished pipettes, heavily coated with Sylgard (to reduce stray capacitance), and with the resistance of 2–5 MΩ. The bath and pipettes contained ECS. A sine wave (111 mV rms and 1591 Hz for lactotrophs or 6364 Hz for astrocytes) was applied to the pipette and the holding steady state pipette potential was held at 0 mV. During the experiments, the phase angle was adjusted to nullify the changes in the real (Re) trace in response to the manually generated 10 fF calibration pulses.
Data analysis
We used custom-made MATLAB (Math Works, Natick, MA, USA) subroutine (CellAn, Celica, Slovenia) to analyze exocytotic events. Fusion event was considered detectable, if the signal-to-noise ratio exceeded 3:1. We analyzed only reversible exocytotic events, where an off-step in Im followed an on-step within 3 s. Vesicle diameter was assessed for all exocytotic events by determining vesicle capacitance (Cv): Cv = (Re2 + Im2)/Im/ω, where Im denotes the amplitude change in the imaginary part of the admittance trace, Re is the amplitude change in the real part of the admittance trace and ω is the angular frequency.Citation44 Vesicle diameter was calculated assuming spherical geometry and using a specific membrane capacitance of 8 fF/μm2 (lactotrophs)3 and of 10 fF/μm2 (astrocytes).Citation45 For transient exocytotic events in the Im that exhibited measurable projections to the Re, we calculated fusion pore conductance (Gp): Gp = (Re2 + Im2)/Re. Gp was used to estimate the fusion-pore radius by using the equation Gp = (πr2)/(ρλ), where r denotes the fusion-pore radius, ρ is the estimated resistivity of the saline (100 Ωcm) and λ is the estimated length of a gap junction channel (15 nm).Citation46
SigmaPlot was used for the statistical analyses. Values are presented as mean ± SEM. Differences between samples were tested with the Mann-Whitney U-test, considering P < 0.05 (*), P < 0.01 (**) and P < 0.001 (***).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Slovenian Research Agency grants: P3 310, J3 6790, J3 4051, J3 4146, L3 3654; J3 3236, CIPKEBIP, COST Nanonet).
References
- Kelly RB. Pathways of protein secretion in eukaryotes. Science 1985; 230:25-32; PMID:2994224; http://dx.doi.org/10.1126/science.2994224
- Burgoyne R, Morgan A. Secretory granule exocytosis. Physiol Rev 2003; 83:581-632; PMID:12663867
- Zorec R, Sikdar S, Mason W. Increased cytosolic calcium stimulates exocytosis in bovine lactotrophs. Direct evidence from changes in membrane capacitance. J Gen Physiol 1991; 97:473-97; PMID:2037838; http://dx.doi.org/10.1085/jgp.97.3.473
- Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nat Struct Mol Biol 2008; 15:675-83; PMID:18596814; http://dx.doi.org/10.1038/nsmb.1455
- Coorssen JR, Zorec R. Regulated exocytosis per partes. Cell Calcium 2012; 52:191-5; PMID:22784668; http://dx.doi.org/10.1016/j.ceca.2012.06.003
- Vardjan N, Stenovec M, Jorgacevski J, Kreft M, Zorec R. Subnanometer fusion pores in spontaneous exocytosis of peptidergic vesicles. J Neurosci 2007; 27:4737-46; PMID:17460086; http://dx.doi.org/10.1523/JNEUROSCI.0351-07.2007
- Elhamdani A, Azizi F, Artalejo C. Double patch clamp reveals that transient fusion (kiss-and-run) is a major mechanism of secretion in calf adrenal chromaffin cells: high calcium shifts the mechanism from kiss-and-run to complete fusion. J Neurosci 2006; 26:3030-6; PMID:16540581; http://dx.doi.org/10.1523/JNEUROSCI.5275-05.2006
- Lollike K, Borregaard N, Lindau M. Capacitance flickers and pseudoflickers of small granules, measured in the cell-attached configuration. Biophys J 1998; 75:53-9; PMID:9649367; http://dx.doi.org/10.1016/S0006-3495(98)77494-6
- Sandre O, Moreaux L, Brochard-Wyart F. Dynamics of transient pores in stretched vesicles. Proc Natl Acad Sci U S A 1999; 96:10591-6; PMID:10485870; http://dx.doi.org/10.1073/pnas.96.19.10591
- Jahn R, Scheller R. SNAREs–engines for membrane fusion. Nat Rev Mol Cell Biol 2006; 7:631-43; PMID:16912714; http://dx.doi.org/10.1038/nrm2002
- Lai Y, Diao J, Liu Y, Ishitsuka Y, Su Z, Schulten K, Ha T, Shin YK. Fusion pore formation and expansion induced by Ca2+ and synaptotagmin 1. Proc Natl Acad Sci U S A 2013; 110:1333-8; PMID:23300284; http://dx.doi.org/10.1073/pnas.1218818110
- Dhara M, Yarzagaray A, Schwarz Y, Dutta S, Grabner C, Moghadam PK, Bost A, Schirra C, Rettig J, Reim K, et al. Complexin synchronizes primed vesicle exocytosis and regulates fusion pore dynamics. J Cell Biol 2014; 204:1123-40; PMID:24687280; http://dx.doi.org/10.1083/jcb.201311085
- Jorgačevski J, Potokar M, Grilc S, Kreft M, Liu W, Barclay JW, Bückers J, Medda R, Hell SW, Parpura V, et al. Munc18-1 tuning of vesicle merger and fusion pore properties. J Neurosci 2011; 31:9055-66; PMID:21677188; http://dx.doi.org/10.1523/JNEUROSCI.0185-11.2011
- Rituper B, Flašker A, Guček A, Chowdhury HH, Zorec R. Cholesterol and regulated exocytosis: a requirement for unitary exocytotic events. Cell Calcium 2012; 52:250-8; PMID:22726879; http://dx.doi.org/10.1016/j.ceca.2012.05.009
- Darios F, Wasser C, Shakirzyanova A, Giniatullin A, Goodman K, Munoz-Bravo JL, Raingo J, Jorgačevski J, Kreft M, Zorec R, et al. Sphingosine facilitates SNARE complex assembly and activates synaptic vesicle exocytosis. Neuron 2009; 62:683-94; PMID:19524527; http://dx.doi.org/10.1016/j.neuron.2009.04.024
- Churchward MA, Rogasevskaia T, Brandman DM, Khosravani H, Nava P, Atkinson JK, Coorssen JR. Specific lipids supply critical negative spontaneous curvature – An essential component of native Ca2+-triggered membrane fusion. Biophys J 2008; 94:3976-86; PMID:18227127; http://dx.doi.org/10.1529/biophysj.107.123984
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem 1989; 18:113-36; PMID:2660821; http://dx.doi.org/10.1146/annurev.bb.18.060189.000553
- Kabaso D, Calejo AI, Jorgačevski J, Kreft M, Zorec R, Iglič A. Fusion pore diameter regulation by cations modulating local membrane anisotropy. ScientificWorldJournal 2012; 2012:983138; PMID:22489211
- Calejo AI, Jorgačevski J, Kucka M, Kreft M, Gonçalves PP, Stojilkovic SS, Zorec R. cAMP-mediated stabilization of fusion pores in cultured rat pituitary lactotrophs. J Neurosci 2013; 33:8068-78; PMID:23637196; http://dx.doi.org/10.1523/JNEUROSCI.5351-12.2013
- Gauss R, Seifert R, Kaupp UB. Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature 1998; 393:583-7; PMID:9634235; http://dx.doi.org/10.1038/31248
- Yu X, Duan KL, Shang CF, Yu HG, Zhou Z. Calcium influx through hyperpolarization-activated cation channels (I(h) channels) contributes to activity-evoked neuronal secretion. Proc Natl Acad Sci U S A 2004; 101:1051-6; PMID:14724293; http://dx.doi.org/10.1073/pnas.0305167101
- Michels G, Brandt MC, Zagidullin N, Khan IF, Larbig R, van Aaken S, Wippermann J, Hoppe UC. Direct evidence for calcium conductance of hyperpolarization-activated cyclic nucleotide-gated channels and human native If at physiological calcium concentrations. Cardiovasc Res 2008; 78:466-75; PMID:18252758; http://dx.doi.org/10.1093/cvr/cvn032
- Breckenridge LJ, Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. Nature 1987; 328:814-7; PMID:2442614; http://dx.doi.org/10.1038/328814a0
- Calejo AI, Jorgačevski J, Rituper B, Guček A, Pereira PM, Santos MA, Potokar M, Vardjan N, Kreft M, Gonçalves PP, et al. Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels and cAMP-Dependent Modulation of Exocytosis in Cultured Rat Lactotrophs. J Neurosci 2014; 34:15638-47; PMID:25411492; http://dx.doi.org/10.1523/JNEUROSCI.5290-13.2014
- Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature 1994; 371:513-5; PMID:7935764; http://dx.doi.org/10.1038/371513a0
- Sikdar SK, Kreft M, Zorec R. Modulation of the unitary exocytic event amplitude by cAMP in rat melanotrophs. J Physiol 1998; 511(Pt 3):851-9; PMID:9714865; http://dx.doi.org/10.1111/j.1469-7793.1998.851bg.x
- Schmidt U, Hajjar RJ, Kim CS, Lebeche D, Doye AA, Gwathmey JK. Human heart failure: cAMP stimulation of SR Ca(2+)-ATPase activity and phosphorylation level of phospholamban. Am J Physiol 1999; 277:H474-80; PMID:10444471
- Lindau M, Alvarez de Toledo G. The fusion pore. Biochim Biophys Acta 2003; 1641:167-73; PMID:12914957; http://dx.doi.org/10.1016/S0167-4889(03)00085-5
- Jorgačevski J, Fošnarič M, Vardjan N, Stenovec M, Potokar M, Kreft M, Kralj-Iglič V, Iglic A, Zorec R. Fusion pore stability of peptidergic vesicles. Mol Membr Biol 2010; 27:65-80; PMID:20334578; http://dx.doi.org/10.3109/09687681003597104
- Hammond GRV, Dove SK, Nicol A, Pinxteren JA, Zicha D, Schiavo G. Elimination of plasma membrane phosphatidylinositol (4,5)-bisphosphate is required for exocytosis from mast cells. J Cell Sci 2006; 119:2084-94; PMID:16687737; http://dx.doi.org/10.1242/jcs.02912
- Boettcher JM, Davis-Harrison RL, Clay MC, Nieuwkoop AJ, Ohkubo YZ, Tajkhorshid E, Morrissey JH, Rienstra CM. Atomic View of Calcium-Induced Clustering of Phosphatidylserine in Mixed Lipid Bilayers. Biochemistry 2011; 50:2264-73; PMID:21294564; http://dx.doi.org/10.1021/bi1013694
- Yang HY, Xu YC, Gao ZB, Mao YY, Du Y, Jiang HL. Effects of Na+, K+, and Ca2+ on the Structures of Anionic Lipid Bilayers and Biological Implication. J Phys Chem B 2010; 114:16978-88; http://dx.doi.org/10.1021/jp1091569
- van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 2008; 9:112-24; PMID:18216768; http://dx.doi.org/10.1038/nrm2330
- Coorssen JR, Rand RP. Structural Effects of Neutral Lipids on Divalent Cation-Induced Interactions of Phosphatidylserine-Containing Bilayers. Biophys J 1995; 68:1009-18; PMID:7756521; http://dx.doi.org/10.1016/S0006-3495(95)80276-6
- Salaun C, James DJ, Chamberlain LH. Lipid rafts and the regulation of exocytosis. Traffic 2004; 5:255-64; PMID:15030567; http://dx.doi.org/10.1111/j.1600-0854.2004.0162.x
- Rituper B, Guček A, Jorgačevski J, Flašker A, Kreft M, Zorec R. High-resolution membrane capacitance measurements for the study of exocytosis and endocytosis. Nat Protoc 2013; 8:1169-83; PMID:23702833; http://dx.doi.org/10.1038/nprot.2013.069
- Guček A, Vardjan N, Zorec R. Exocytosis in astrocytes: transmitter release and membrane signal regulation. Neurochem Res 2012; 37:2351-63; PMID:22528833; http://dx.doi.org/10.1007/s11064-012-0773-6
- Alvarez de Toledo G, Fernández-Chacón R, Fernández JM. Release of secretory products during transient vesicle fusion. Nature 1993; 363:554-8; PMID:8505984; http://dx.doi.org/10.1038/363554a0
- Kawahara M. Effects of aluminum on the nervous system and its possible link with neurodegenerative diseases. J Alzheimers Dis 2005; 8:171-82; discussion 209-15; PMID:16308486
- Calejo AI, Jorgačevski J, Silva VS, Stenovec M, Kreft M, Gonçalves PP, Zorec R. Aluminium-induced changes of fusion pore properties attenuate prolactin secretion in rat pituitary lactotrophs. Neuroscience 2012; 201:57-66; PMID:22123165; http://dx.doi.org/10.1016/j.neuroscience.2011.11.015
- Alés E, Tabares L, Poyato JM, Valero V, Lindau M, Alvarez de Toledo G. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat Cell Biol 1999; 1:40-4; http://dx.doi.org/10.1038/9012
- Shang S, Wang C, Liu B, Wu Q, Zhang Q, Liu W, Zheng L, Xu H, Kang X, Zhang X, et al. Extracellular Ca2⁺ per se inhibits quantal size of catecholamine release in adrenal slice chromaffin cells. Cell Calcium 2014; 56:202-7; PMID:25103334; http://dx.doi.org/10.1016/j.ceca.2014.07.006
- Chen W, Bergsman JB, Wang X, Gilkey G, Pierpoint CR, Daniel EA, Awumey EM, Dauban P, Dodd RH, Ruat M, et al. Presynaptic external calcium signaling involves the calcium-sensing receptor in neocortical nerve terminals. PLoS One 2010; 5:e8563; PMID:20052292; http://dx.doi.org/10.1371/journal.pone.0008563
- Lollike K, Borregaard N, Lindau M. The exocytotic fusion pore of small granules has a conductance similar to an ion channel. J Cell Biol 1995; 129:99-104; PMID: 7535305; http://dx.doi.org/10.1083/jcb.129.1.99
- Gentet LJ, Stuart GJ, Clements JD. Direct measurement of specific membrane capacitance in neurons. Biophys J 2000; 79:314-20; PMID:10866957; http://dx.doi.org/10.1016/S0006-3495(00)76293-X
- Spruce AE, Breckenridge LJ, Lee AK, Almers W. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron 1990; 4:643-54; PMID:2344404; http://dx.doi.org/10.1016/0896-6273(90)90192-I
- MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 2003; 4:566-77; PMID:12838339; http://dx.doi.org/10.1038/nrm1151
