Abstract
Initiated by the activation of various nociceptors, pain is a reaction to specific stimulus modalities. The μ-opioid receptor (MOR) agonists, including morphine, remain the most potent analgesics to treat patients with moderate to severe pain. However, the utility of MOR agonists is limited by the adverse effects associated with the use of these drugs, including analgesic tolerance and physical dependence. A strong connection has been suggested between the expression of the transient receptor potential vanilloid type 1 (TRPV1) ion channel and the development of inflammatory hyperalgesia. TRPV1 is important for thermal nociception induction, and is mainly expressed on sensory neurons. Recent reports suggest that opioid or TRPV1 receptor agonist exposure has contrasting consequences for anti-nociception, tolerance and dependence. Chronic morphine exposure modulates TRPV1 activation and induces the anti-nociception effects of morphine. The regulation of many downstream targets of TRPV1 plays a critical role in this process, including calcitonin gene-related peptide (CGRP) and substance P (SP). Additional factors also include capsaicin treatment blocking the anti-nociception effects of morphine in rats, as well as opioid modulation of TRPV1 responses through the cAMP-dependent PKA pathway and MAPK signaling pathways. Here, we review new insights concerning the mechanism underlying MOR-TRPV1 crosstalk and signaling pathways and discuss the potential mechanisms of morphine-induced anti-nociception, tolerance and dependence associated with the TRPV1 signaling pathway and highlight how understanding these mechanisms might help find therapeutic targets for the treatment of morphine induced antinociception, tolerance and dependence.
Introduction
The μ-opioid receptor (MOR) agonists are the class of analgesics most widely used to treat moderate and severe chronic pain. This class of analgesics includes morphine, the prototypical MOR agonist, which produces its analgesic effect at clinically relevant doses primarily through the G-protein coupled receptor MOR. It has been reported that most clinical opioids act on MOR. In particular, MOR expressed in the superficial dorsal horn of the spinal cordCitation1,2 is essential for the analgesic effects of MOR agonists.Citation3,4
Chronic morphine administration inevitably results in the development of high tolerance. This propensity of morphine treated patients to develop tolerance, and the related loss of analgesic effectiveness, limits the use of morphine for chronic pain conditions. In addition, prolonged morphine use can lead to physical dependence, defined as a need for continuing drug use to prevent the symptoms of withdrawal. Although the mechanisms underlying morphine tolerance are not fully understood, many studies have reported that repeated morphine exposure opposes the analgesic effects of morphine by increasing the expression and release of chemokines, pro-inflammatory cytokines,Citation5 and pronociceptive neurotransmitters in the spinal cordCitation6 and DRG.Citation7 It has been reported that sustained morphine administration results in numerous pronociceptive changes, including increased capsaicin evoked release and elevated concentrations of pronociceptive neurotransmitters within the spinal dorsal horn.Citation8,9 A prominent feature of opioid-induced hyperalgesia is enhanced responsiveness to noxious thermal stimulation, suggesting that transient receptor potential vanilloid type 1 (TRPV1) channels may be an important element of this response.Citation10
TRPV1 is a nonselective cation (Ca2+) channel that is involved in a variety of nociceptive processesCitation11 and can be activated by multiple stimuli, including acidic pH (≤ 5.9), noxious heat (>42°C), endocannabinoids, endogenous lipids, and capsaicin.Citation12-15 TRPV1 is widely distributed in the sensory terminals of central and peripheral neurons.Citation16-18 The effects of TRPV1 on thermal hyperalgesia and mechanical allodynia have been demonstrated in various diseases.Citation14,19 Additionally, TRPV1 receptors are present in regions of the brain that regulate the transmission and modulation of pain.Citation20 Chronic morphine administration increases TRPV1 expression in the spinal cord, DRG, and sciatic nerve.Citation10,21 In morphine resistant bone cancer pain, TRPV1 receptors are up-regulated in DRG neurons.Citation22 In these patients, morphine may induce the expression of the TRPV1 receptor through the activation of the mitogen-activated protein kinase signaling pathway, including up-stream TRPV1 regulators.Citation21 Blocking TRPV1 receptors via intrathecal administration of SB366971 significantly attenuated morphine tolerance in rats.Citation21 Similarly, the destruction of TRPV1 receptor-expressing sensory neurons by resiniferatoxin, an ultrapotent capsaicin analog, blocked morphine tolerance.Citation23 In accordance with the results of previous studies, a recent report indicated blocking TRPV1 receptors with capsazepine (2.5 mg kg−1) inhibited morphine tolerance induced by 5 days of morphine treatment.Citation24 The results of these studies suggest that sustained opioid exposure also enhances TRPV1 receptor function in the periphery and plays an additional and essential role in sustained morphine induced thermal and tactile hypersensitivity.
Activation of MOR leads to dissociation of the inhibitory Gi/o-protein complex into Gα- and Gβγ-subunits, which then have an important impact on downstream signaling pathways.Citation25-27 Opioids reduce adenylyl cyclase (AC) activity through Gai-subunits. AC catalyzes adenosine triphosphate (ATP) conversion to cyclic adenosine monophosphate (cAMP) ,Citation28 and this modulates the activation of protein kinase A (PKA) or cyclic nucleotide-gated ion channels. Morphine induces expression of the TRPV1 receptor via TRPV1 up-stream regulator activation in the mitogen-activated protein kinase (MAPK) signaling pathway.Citation21 Current evidence emphasizes the importance of TRPV1 in morphine tolerance, dependence and morphine-induced antinociception.Citation10,29,30
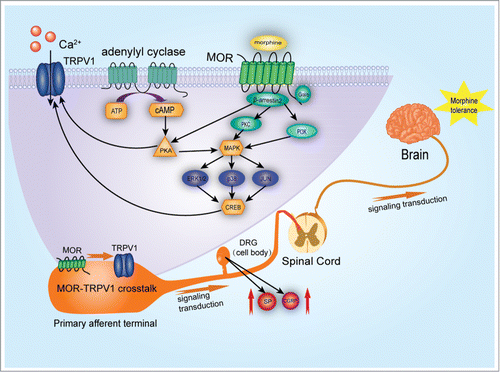
Here, we review the current knowledge concerning these phenomena, focusing on morphine-induced TRPV1 activation. Furthermore, we highlight evidence characterizing downstream TRPV1 signaling molecules and their role in morphine tolerance, dependence and morphine-induced antinociception ().
Figure 1. Signal transduction of TRPV1 activation in morphine induced antinociception, tolerance and dependence. By acting on μ- opioid receptors (MOR), primarily through Gai-subunits, morphine reduces adenylyl cyclase (AC) activity. AC catalyzes the conversion of adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP), which regulates protein kinase A (PKA) or cyclic-nucleotide-gated ion channels. TRPV1 activation results in sensitization of TRPV1 responses through a β- arrestin2 and PKA-dependent manner. Decreased association of β-arrestin2 and constitutive phosphorylation of TRPV1 may underlie enhanced pain perception and hyperalgesia. Chronic administration of morphine activates the MAPK pathway, including ERK, p38 and JNK. This possibly occurs via protein kinase A (PKA), protein kinase C (PKC) and phosphatidylinositol 3-kinase (PI3K). The nuclear translocation of phosphorylated MAPK results in the phosphorylation of transcription factors, such as CREB and c-Jun. This leads to TRPV1 activation through modulation of neurotransmitters such as glutamate, CGRP and SP released from DRG neurons and further contributes to the antinociception, tolerance and dependence associated thermal hyperalgesia.
TRPV1 localization and function
TRPV1 is predominantly expressed in unmyelinated neurons and in both the central and peripheral sensory terminals of primary sensory neurons.Citation17,18 In the central nervous system (CNS), TRPV1 is located in regions that modulate nociceptionCitation20,31 and control autonomic functions.Citation32 TRPV1 receptor is an important integrator of various types of pain stimuli in vivo. Therefore, the properties of polymodal nociceptors might be explained by TRPV1responsiveness to various noxious stimuli. Chimeric and site-directed mutation studies of the TRPV1 channel have demonstrated that the phosphorylation of unique amino acid sites regulates the response of the channel to various stimuli, including capsaicin.Citation33-35 Correspondingly, TRPV1 dephosphorylation can cause pharmacological desensitization of the channel.Citation36,37
TRPV1 appears to be critical for the transmission of noxious stimuli by nociceptive peripheral neurons and the development of inflammatory hyperalgesia.Citation12,14,19,38 Accordingly, axonal transport of TRPV1 mRNA and TRPV1 protein expression are significantly increased in inflamed tissues.Citation39,40 TRPV1 knockdown mice develop less thermal hyperalgesiaCitation14 and TRPV1 antagonists reverse inflammatory thermal hyperalgesia.Citation41 Various factors modulate the response of TRPV1 to inflammatory stimuli, including growth factors, neurotransmitters, peptides or small proteins, lipids, chemokines and cytokines.Citation38 TRPV1 receptor activation by chemokines and cytokines may lead to nociceptive effects that reverse the antinociceptive effects of morphine.Citation38 Furthermore, while capsaicin treatment inhibits morphine induced antinociception in rats,Citation42 capsaicin-induced thermal allodynia is alleviated by MOR activation in the central and peripheral nervous systems of rhesus monkeys.Citation43,44
Recently, a significant increase in TRPV1 immunoreactivity was demonstrated in the spinal cords, dorsal root ganglion (DRG) neurons and sciatic nerves of morphine-tolerant rats given daily intraperitoneal injections of 10 mg/kg morphine. Tolerance to morphine and tolerance-induced thermal hyperalgesia in the rats was suppressed by a 30 μg dose of the selective TRPV1 antagonist SB366791.Citation21 Additionally, Niiyama et al.Citation10 reported data indicating that TRPV1 antagonists acutely enhance morphine analgesia. In agreement with previous data from studies investigating rats,Citation21,23 Nguyen's research on mice also indicated that TRPV1 antagonists effectively prevent the development of morphine tolerance. Furthermore, our data first demonstrated that TRPV1 antagonists significantly reduced withdrawal symptoms in morphine-dependent mice.Citation24
MOR and TRPV1 interaction
TRPV1 and MOR co-localize in DRG neurons and the spinal cord.Citation4,21 TRPV1 can be both sensitized and upregulated during inflammationCitation13 and plays an essential role in the development of inflammation associated thermal hyperalgesia.Citation45 A substantial increase in TRPV1 and MOR positive DRG neurons is caused by induced paw inflammation.Citation46-48 Jeannette Endres-Becker et al. demonstrated that TRPV1 activity could be regulated by MOR ligands.Citation49 TRPV1's contribution to inflammatory hyperalgesia has been established through observations indicating that TRPV1 antagonists dose-dependently reverse both thermal and mechanical inflammatory hyperalgesia.Citation41,50 In addition, thermal inflammatory hyperalgesia is significantly reduced in TRPV1 knock-out mice.Citation14
Jeannette Endres-Becker et al. also found a significant morphine-induced decrease in capsaicin-mediated TRPV1 activity in DRG neurons from complete Freund's adjuvant treated animals.Citation49 Therefore, it has been well established that TRPV1 expression plays an important role in the development of inflammation-induced hyperalgesia.Citation14,45,46 Inflammation and morphine-induced hypesensitivity share many common characteristics, including hyperalgesia, allodynia and similar pronociceptive neuroadaptive changes.
MOR are presynaptically expressed on the terminals of primary afferent neuronsCitation51-53 and on the postsynaptic neurons in the dorsal horn of the spinal cord.Citation54,55 The primary afferent neurons and the spinal cord participate in pain transmission and modulation and are the primary sites for the analgesic activity of MOR agonists.Citation3,4,52,56,57 Treatment of adult rats with the capsaicin analog resiniferatoxin (RTX), a potent TRPV1 agonist, destroys TRPV1-expressing DRG and impairs thermal nociception in adult rats.Citation4 Although RTX induces a significant reduction in presynaptic MOR, it potentiates and prolongs the analgesic effect produced by systemic or intrathecal injection of MOR agonists, including morphine and [D-Ala,Citation2 N-Me-Phe,Citation4 Gly-olCitation5]-enkephalin (DAMGO).Citation4 Additionally, TRPV1 antagonists have been demonstrated to decrease mechanical nociception in acute and chronic pain models.Citation50,58 The reduction of TRPV1-expressing sensory neurons induced by RTX attenuates the development of morphine analgesic toleranceCitation59 and alters the presynaptic effects of the MOR agonist in the spinal cord.Citation60 Furthermore, the thermal hyperalgesia and mechanical allodynia that are normally induced by chronic morphine administration were absent in mice lacking TRPV1 expression.Citation10 These effects were also opposed by treatment with the TRPV1 antagonist AMG0347 (3 mg/kg).Citation10 These results suggest that TRPV1 channels are involved in the development of the thermal hypersensitivity associated with tissue inflammation. MOR and TRPV1 expression in primary afferent neurons and the activity of TRPV1 in DRG neurons can be inhibited by MOR. This inhibition is increased after thermal and mechanical inflammation. Furthermore, the effect of presynaptic MOR on TRPV1-expressing sensory neurons is particularly sensitive to down-regulation by μ opioid agonists during opioid tolerance development.
MOR Sensitizes TRPV1 via β-Arrestin2
The scaffolding protein PKA-anchoring protein 150 (AKAP150) mediates TRPV1 phosphorylation by protein kinases A and C.Citation61-64 A role for anchoring and scaffolding proteins in the mediation of efficient downstream signaling cascades by organizing specific proteins and enzymes near their respective substrates has been established.Citation65,66 β-arrestins, regarded as critical scaffold proteins,Citation67 can form scaffolding complexes with a wide variety of proteins to regulate the strength and duration of diverse signaling pathways, such as Src kinase and phosphodiesterase 4D (PDE4D).Citation68-71 Of particular interest, β-arrestins selectively associate with PDE4D to locally modulate subcellular cyclic AMP availability and subsequently activate PKA.Citation70,72,73 Other research has presented evidence that β-arrestins contribute to ligand-activated β2 -adrenergic receptors by scaffolding PDE4D isoforms that hydrolyze cAMP to regulate PKA activity and consequent receptor sensitivity.Citation72
β-arrestin molecules were originally identified as important mediators of metabotropic receptor desensitization that govern internalization of G-protein coupled receptors (GPCRs) following agonist exposure.Citation74-76 Extensive research by numerous groups has revealed the contributions of β-arrestins to multiple physiological functions and processes.Citation67,77,78 Recent research has demonstrated that β-arrestins are novel regulators that can regulate the function of several TRP channels and desensitize ionotropic receptors.Citation11,77,79 Por ED et al. provided the first evidence that β-arrestin2 regulates TRPV1 receptor through its role as a scaffolding protein.Citation11 Regulation of TRPV1 by β-arrestin2 induces PKA phosphorylation and effectively desensitizes the ionotropic receptor, which is implicated in a variety of inflammatory and pain conditions.Citation11 Por ED et al. also reported that β-arrestin2 inhibited TRPV1 induced PKA phosphorylation, consequentially reducing receptor response to agonist-mediated stimulation.Citation11
β-arrestin2 desensitizes TRPV1 in sensory neurons.Citation11 MOR agonists, such as morphine and DAMGO, sequester β-arrestin2, reduce TRPV1/β-arrestin2 interactions and increase TRPV1 activity in peripheral sensory neurons.Citation30 Previous studies showed that endogenous β-arrestin2 is required for the development of morphine tolerance,Citation80,81 and mice lacking β-arrestin2 demonstrate increased sensitivity to the antinociceptive effects of morphine.Citation82 Furthermore, hyperalgesia may rebound due to overactive PKA and result in phosphorylation and sensitization of TRPV1 during the process of patients ceasing opioid therapy.Citation29 Hence, Rowan et al. speculate that the development of opioid induced hyperalgesia (OIH) is due to chronic ligand treatment recruiting β-arrestin2 away from TRPV1 in sensory neurons.Citation30
cAMP/PKA
The MOR agonist morphine has well documented anti-inflammatory effects when injected directly into inflamed tissues in both animal models and human studies.Citation83-86 Activation of TRPV1 by capsaicin induces hyperalgesia that can be inhibited by peripherally applied MOR agonists, such as morphine.Citation87-89 Opioid withdrawal symptoms are associated with cAMP activity and increased concentrations of AC, PKA and the transcription factor cAMP response element binding protein.Citation90 Phosphorylation mediated by cAMP/PKA can both sensitize TRPV1 and protect it from desensitization.Citation33,91,92 Vetter I et al. reported that the anti-inflammatory action of peripheral opioids is mediated by the interaction of MOR and PKA-sensitized TRPV1.Citation92-95 Phosphorylation of TRPV1 by numerous kinases, such as cAMP-dependent PKA, can regulate the function of the receptor.Citation91,96,97 cAMP levels are elevated in inflamed tissuesCitation98,99 and the cAMP/PKA pathway appears to be important for inflammatory nociception. Thus, the cAMP/PKA pathway leads to the development of inflammatory hyperalgesia induced by pro-inflammatory regulators, including prostaglandin E2 (PGE2).Citation99,100 In a variety of cell systems, including DRG neurons, accumulating evidence indicates that TRPV1 is modulated by PKA.Citation33,101Citation102 For instance, activated PKA increases TRPV1 phosphorylation and channel sensitivity.Citation103 Furthermore, PKA-mediated phosphorylation was demonstrated both to contribute to thermal-activated TRPV1 currentsCitation102 and to counteract Ca2+dependent desensitization.Citation91
Previous experiments have shown that stimulation with capsaicin can reduce TRPV1 phosphorylation, and that PKA can re-phosphorylate and subsequently re-sensitize TRPV1.Citation33 Law et al. reported that MOR-mediated a decrease in intracellular cAMP levels,Citation28 controlling PKA activity and decreasing TRPV1 channel activity. In accordance with this conclusion, pretreatment of DRG neurons with the potent cell-permeable cAMP analog 8-Br-cAMP reversed opioid mediated inhibition at TRPV1.Citation49 Thus, opioid induced modulation of TRPV1 responses may occur in inflamed tissues where cAMP levels are elevatedCitation100 through inhibition of AC and the subsequent inhibition of TRPV1 responses via Gi/o proteins by cAMP-dependent PKA.Citation92,93 TRPV1 can also be inhibited via MOR-mediated inhibition of AC activity and decreased cAMP levels.Citation29
Morphine can inhibit capsaicin responses when the cAMP pathway is activated. This occurs through opioid-modulation of adenylate cyclase and, therefore, indirectly through PKA-mediated TRPV1 sensitization. As TRPV1 is expressed peripherallyCitation39,40 and PKA-mediated sensitization occurs in these peripheral nociceptors via inflammatory mediators,Citation99 non-central opioid receptor targeting under inflammatory conditions may prevent peripheral sensitization and contribute to analgesia.
MAPK
The MAPK family transduces a diverse group of extracellular stimuli into a wide variety of intracellular responses by inducing transcriptional, translational and post-translational modifications of target proteins.Citation104-106 The MAPK family includes extracellular signal-regulated protein kinase (ERK), P38-mitogen activated protein kinase (P38 MAPK) and c-Jun N-terminal kinase (JNK),Citation107,108 MAPK is a key regulator of cell proliferation, differentiation, survival, learning and memory, and evidence indicates that MAPK may be a key factor in pain hypersensitivity.Citation104-106 It has been reported that treatment with multiple MAPK inhibitors reduces inflammatory and neuropathic pain without affecting the subject's perception of basal pain.Citation38,94,95,104,106,109-113 The involvement of similar cellular and molecular mechanisms has been demonstrated in the development of morphine tolerance and pathological pain.Citation114,115 Because the same treatments that block pathological pain also attenuate opioid tolerance, the abnormal pain associated with the prolonged opioid usage is a key element affecting the behavioral symptoms characteristic of opioid tolerance.Citation116,117
In DRG neurons, chronic morphine administration leads to increased p38, ERK and JNK phosphorylation.Citation21,118 Intrathecal selective MAPK inhibitor injections inhibit MAPK phosphorylation in DRG neurons by opposing p38, ERK and JNK phosphorylation. Chronic morphine administration has been reported to activate MAPK and lead to morphine tolerance. Hyperalgesia associated TRPV1 is considered a target of this mechanism.Citation21
ERK/MAPK
Significant recent advances indicate that morphine modulates ERK phosphorylation in cultured neuronal cells and in vivo.Citation119 Morphine induced ERK activation was first described in recombinant Chinese hamster ovary (CHO) cells that were stably transfected with MOR.Citation120 ERK activation was observed at 4 minutes and then, after 8 minutes, activation levels gradually decreased, recovering to basal activity levels after one hour of morphine treatment.Citation120 In C6 glioma cells stably expressing MOR and COS-7 cells transiently transfected with MOR, the ERK cascade can be strongly activated by the application of the MOR agonist DAMGO.Citation121 Human neuroblastoma SK-N-SH cells endogenously express MOR, and rapid ERK phosphorylation was observed in these cells after acute morphine incubation.Citation122,123 It is particularly interesting that prolonged morphine usage attenuated ERK phosphorylation.Citation124 In SH-SY5Y cells, morphine withdrawal also attenuated ERK phosphorylation.Citation124 Accordingly, following either acute or chronic morphine incubation, MOR activation modulated ERK activity.Citation124 It was demonstrated in vivo that long-term morphine administration in mice caused p-ERK elevation in the frontal cortex, hippocampus and striatum.Citation125 In contrast, chronic morphine treatment led to decreased p-ERK levels in various tissues, including mouseCitation126 and ratCitation127 nucleus accumbens, mouse central amygdalaCitation126 and the cerebral cortex, median eminence and hypothalamic nuclei of humans and rats.Citation122,128
Additionally, morphine withdrawal was attenuated and withdrawal-induced allodynia was decreased after antisense oligonucleotide knockdown of spinal ERK and phosphorylation reduction using intrathecal MAPK kinase inhibitor U0126.Citation129 Phosphatidylinositol 3-kinase (PI3K) and ERK can be activated in DRG neurons by intradermal injections of capsaicin and nerve growth factor (NGF).Citation130 In primary sensory neurons, PI3K acts through TRPV1 sensitization to activate ERK and mediate inflammatory heat hyperalgesia.Citation130 In light of these findings, it seems likely that PI3K induced heat hyperalgesia is regulated by TRPV1 activity in an extracellular ERK-dependent manner.Citation130
P38 and JNK
Compared with ERK, fewer studies investigating the roles of p38 and JNK in morphine-induced tolerance and dependence at the supra spinal level have been conducted. It has been suggested that NGF increases TRPV1 in inflamed skin and DRG neurons through MAPK activation.Citation46 Intrathecal administration of SB203580, a p38 specific inhibitor, significantly attenuated morphine analgesia tolerance.Citation131 Additionally, it has been reported that p-p38immunoreactive cells increased significantly in rats receiving intrathecal administration of 15 μg morphine.Citation131
Few studies have reported the role of JNK in morphine-induced anti-nociception and tolerance. c-Jun is downstream of JNK. It has been reported that during morphine withdrawal, increasing c-Jun levels affect some of the morphine withdrawal symptoms in the rat locus coeruleusCitation132 and cortex.Citation133 Additionally, 9 days of subcutaneous morphine injections (10 mg/kg) resulted in elevated levels of the JNK family member JNK3 in the rat frontal cortex. However, this treatment did not result in increased JNK3 in the thalamus, hippocampus or locus coeruleus.Citation134 Accumulating in vivo and in vitro evidence indicates that increases in phosphorylated JNK (p-JNK) are induced by repeated morphine treatment in rat DRG neurons.Citation21,118
SP and CGRP
Recent evidence suggests that long-term morphine exposure may contribute to morphine induced tolerance and dependence by regulating downstream targets of TRPV1, such as SP and CGRP. A combination of increased SP and CGRP expression in the sensory primary afferents and increased capsaicin-evoked release of SP and CGRP in the spinal dorsal horn have been described in both inflammation and morphine induced hyperalgesia.Citation6,8,135
Chronic morphine exposure also causes physical dependence that manifests as withdrawal symptoms. SP and CGRP may influence morphine withdrawal symptoms, and high levels of SP and CGRP have been reported in animals exhibiting opioid withdrawal symptoms.Citation136-138 Acute intrathecal treatment with SP or CGRP antagonists attenuates morphine withdrawal symptoms.Citation137 CGRP-deficient mice show reduced withdrawal-associated jumping,Citation139 and SP knockout mice have decreased morphine reward and withdrawal.Citation140
TRPV1 receptors co-localize with substance P (SP) and Calcitonin gene-related peptide (CGRP) in the primary sensory neurons, spinal cord, and DRG neurons.Citation141 Capsaicin induces the release of SP and CGRP, whereas a TRPV1 antagonist capsazepine reverses these activities.Citation141-143 Capsaicin causes SP and CGRP releases, whereas capsazepine reverses these activities. Activation of TRPV1 has been demonstrated to induce glutamate release,Citation144 and glutamatergic synaptic transmission can be inhibited in rats by the TRPV1 antagonist SB366791 in the spinal dorsal horn after peripheral inflammation.Citation145 Additionally, TRPV1 activation induces the central and peripheral endings of neurons associated with the neurogenic inflammatory response and nociceptive transmission to release peptide neurotransmitters, including CGRP and SP.Citation146-148 However, it has been reported that capsaicin-induced SP release is reduced by opioids.Citation149,150 Additionally, it has been reported that opioids inhibit neurogenic inflammation by reducing SP released from peripheral afferent terminals.Citation149,150 Prolonged morphine usage enhances the release of CGRP induced by capsaicin.Citation135 Neurotransmitter modulation through chronic opioid exposure activates TRPV1 and causes opioid associated tolerance and thermal hyperalgesia.Citation21 In vitro and in vivo experiments in DRG neurons suggest that chronic morphine induced increases in CGRP and SP are the result of increased MAPK and cAMP response element-binding protein (CREB) phosphorylation.Citation118 Chronic morphine exposure also provokes the manifestation of physical dependence and the resultant withdrawal symptoms. Extensive evidence indicates that neuropeptides may play an essential role in the manifestation of morphine withdrawal symptoms. Animals with opioid withdrawal symptoms exhibit elevation of both the neuropeptides SPCitation136,138,151 and CGRP.Citation136,137 Furthermore, acute intrathecal administration of SP or CGRP antagonists reduces the symptoms of morphine withdrawal. SP knockout mice exhibit diminished morphine reward and withdrawal responses,Citation140 and CGRP-knockdown mice exhibit reduced withdrawal-induced jumping.Citation139 Therefore, systemic TRPV1 receptors blockade by capsazepine may reduce withdrawal symptoms by preventing SP and CGRP releases in morphine-dependent mice. Chronic morphine activates TRPV1. In turn, TRPV1 modulates neurotransmitters such as glutamate, CGRP, and SP, and contributes to morphine tolerance and the associated thermal hyperalgesia.
Conclusions
Morphine is primarily used to treat patients experiencing moderate or severe pain. Unfortunately, the adverse effects of morphine limit its use. These detrimental effects, which include the development of analgesic tolerance and physical dependence, develop rapidly in response to chronic morphine usage in laboratory animals and human patients. Subjects also develop physical morphine dependence and exhibit withdrawal symptoms after the cessation of treatment.Citation3,4,152
In this review, we describe a compelling body of evidence from a number of laboratories indicating that TRPV1 activation in the central and peripheral nervous system is partly responsible for morphine induced anti-nociception, and the development of morphine tolerance and dependence. TRPV1 is crucial for the transduction of noxious chemical and thermal stimuli and its activity can be modulated by numerous mediators, including growth factors, neurotransmitters, peptides, small proteins, lipids, chemokines, and cytokines. It has been suggested that the expression of nerve growth factor leads to increased TRPV1 levels in DRG neurons and inflamed skin through the activation of p38 MAPK. Furthermore, MAPK may regulate TRPV1 activity in an extracellular signal-regulated protein kinase (ERK)-dependent manner. These studies imply that TRPV1 is not solely a thermoreceptor; instead, its activity appears to be modulated by various molecules that act through distinct pathways. A number of interrelated signaling pathways, such as cAMP/PKA and MAPK, are involved in this process and research on these pathways is helping to define the mechanisms governing analgesic tolerance and opioid dependence. Growing evidence indicates that, if used in conjunction with morphine treatment, TRPV1 antagonists could improve management of chronic and severe morphine-resistant pain by reducing morphine induced tolerance and physical dependence. TRPV1 antagonists might also be useful for managing and reducing morphine withdrawal syndromes. Further investigation of TRPV1 antagonists might uncover therapeutic-targets for the treatment of morphine-induced antinociception, tolerance and dependence.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The current work was partially supported by the National Natural Science Foundation Project of China (no. 81273718 and no. 81302961).
Reference
- Gamse R, Holzer P, Lembeck F. Indirect evidence for presynaptic location of opiate receptors on chemosensitive primary sensory neurones. Naunyn-Schmiedeberg's Arch Pharmacol 1979; 308:281-5; PMID:228212; http://dx.doi.org/10.1007/BF00501394
- Yoshimura M, North RA. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature 1983; 305:529-30; PMID:6621700; http://dx.doi.org/10.1038/305529a0
- Yaksh TL, Noueihed R. The physiology and pharmacology of spinal opiates. Ann Rev Pharmacol Toxicol 1985; 25:433-62; PMID:2988422; http://dx.doi.org/10.1146/annurev.pa.25.040185.002245
- Chen SR, Pan HL. Blocking mu opioid receptors in the spinal cord prevents the analgesic action by subsequent systemic opioids. Brain Res 2006; 1081:119-25; PMID:16499888; http://dx.doi.org/10.1016/j.brainres.2006.01.053
- Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun 2008; 22:1178-89; PMID:18599265; http://dx.doi.org/10.1016/j.bbi.2008.05.004
- King T, Gardell LR, Wang R, Vardanyan A, Ossipov MH, Malan TP, Jr., Vanderah TW, Hunt SP, Hruby VJ, Lai J, et al. Role of NK-1 neurotransmission in opioid-induced hyperalgesia. Pain 2005; 116:276-88; PMID:15964684; http://dx.doi.org/10.1016/j.pain.2005.04.014
- Yue X, Tumati S, Navratilova E, Strop D, St John PA, Vanderah TW, Roeske WR, Yamamura HI, Varga EV. Sustained morphine treatment augments basal CGRP release from cultured primary sensory neurons in a Raf-1 dependent manner. Eur J Pharmacol 2008; 584:272-7; PMID:18328477; http://dx.doi.org/10.1016/j.ejphar.2008.02.013
- Powell KJ, Ma W, Sutak M, Doods H, Quirion R, Jhamandas K. Blockade and reversal of spinal morphine tolerance by peptide and non-peptide calcitonin gene-related peptide receptor antagonists. Br J Pharmacol 2000; 131:875-84; PMID:11053206; http://dx.doi.org/10.1038/sj.bjp.0703655
- Ossipov MH, Lai J, King T, Vanderah TW, Malan TP, Jr., Hruby VJ, Porreca F. Antinociceptive and nociceptive actions of opioids. J Neurobiol 2004; 61:126-48; PMID:15362157; http://dx.doi.org/10.1002/neu.20091
- Vardanyan A, Wang R, Vanderah TW, Ossipov MH, Lai J, Porreca F, King T. TRPV1 receptor in expression of opioid-induced hyperalgesia. J Pain 2009; 10:243-52; PMID:18774343; http://dx.doi.org/10.1016/j.jpain.2008.07.004
- Por ED, Bierbower SM, Berg KA, Gomez R, Akopian AN, Wetsel WC, Jeske NA. beta-Arrestin-2 desensitizes the transient receptor potential vanilloid 1 (TRPV1) channel. J Biol Chem 2012; 287:37552-63; PMID:22952227; http://dx.doi.org/10.1074/jbc.M112.391847
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997; 389:816-24; PMID:9349813; http://dx.doi.org/10.1038/39807
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998; 21:531-43; PMID:9768840; http://dx.doi.org/10.1016/S0896-6273(00)80564-4
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000; 288:306-13; PMID:10764638; http://dx.doi.org/10.1126/science.288.5464.306
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999; 400:452-7; PMID:10440374; http://dx.doi.org/10.1038/22761
- Szallasi A, Nilsson S, Farkas-Szallasi T, Blumberg PM, Hokfelt T, Lundberg JM. Vanilloid (capsaicin) receptors in the rat: distribution in the brain, regional differences in the spinal cord, axonal transport to the periphery, and depletion by systemic vanilloid treatment. Brain Res 1995; 703:175-83; PMID:8719630; http://dx.doi.org/10.1016/0006-8993(95)01094-7
- Tominaga M, Julius D. Capsaicin receptor in the pain pathway. Jpn J Pharmacol 2000; 83:20-4; PMID:10887936; http://dx.doi.org/10.1254/jjp.83.20
- Szallasi A, Cruz F, Geppetti P. TRPV1: a therapeutic target for novel analgesic drugs? Trends Mol Med 2006; 12:545-54; PMID:16996800; http://dx.doi.org/10.1016/j.molmed.2006.09.001
- Levine JD, Alessandri-Haber N. TRP channels: targets for the relief of pain. Biochimica et biophysica acta 2007; 1772:989-1003; PMID:17321113; http://dx.doi.org/10.1016/j.bbadis.2007.01.008
- Roberts JC, Davis JB, Benham CD.; 3HResiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res 2004; 995:176-83; PMID:14672807; http://dx.doi.org/10.1016/j.brainres.2003.10.001
- Chen Y, Geis C, Sommer C. Activation of TRPV1 contributes to morphine tolerance: involvement of the mitogen-activated protein kinase signaling pathway. J Neurosci 2008; 28:5836-45; PMID:18509045; http://dx.doi.org/10.1523/JNEUROSCI.4170-07.2008
- Niiyama Y, Kawamata T, Yamamoto J, Omote K, Namiki A. Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons. Neuroscience 2007; 148:560-72; PMID:17656027; http://dx.doi.org/10.1016/j.neuroscience.2007.05.049
- Chen SR, Pan HL. Loss of TRPV1-expressing sensory neurons reduces spinal mu opioid receptors but paradoxically potentiates opioid analgesia. J Neurophysiol 2006; 95:3086-96; PMID:16467418; http://dx.doi.org/10.1152/jn.01343.2005
- Nguyen TL, Nam YS, Lee SY, Kim HC, Jang CG. Effects of capsazepine, a transient receptor potential vanilloid type 1 antagonist, on morphine-induced antinociception, tolerance, and dependence in mice. Br J Anaesth 2010; 105:668-74; PMID:20719804; http://dx.doi.org/10.1093/bja/aeq212
- Akins PT, McCleskey EW. Characterization of potassium currents in adult rat sensory neurons and modulation by opioids and cyclic AMP. Neuroscience 1993; 56:759-69; PMID:8255432; http://dx.doi.org/10.1016/0306-4522(93)90372-M
- Borgland SL, Connor M, Christie MJ. Nociceptin inhibits calcium channel currents in a subpopulation of small nociceptive trigeminal ganglion neurons in mouse. J Physiol 2001; 536:35-47; PMID:11579155; http://dx.doi.org/10.1111/j.1469-7793.2001.t01-1-00035.x
- Irnaten M, Aicher SA, Wang J, Venkatesan P, Evans C, Baxi S, Mendelowitz D. Mu-opioid receptors are located postsynaptically and endomorphin-1 inhibits voltage-gated calcium currents in premotor cardiac parasympathetic neurons in the rat nucleus ambiguus. Neuroscience 2003; 116:573-82; PMID:12559112; http://dx.doi.org/10.1016/S0306-4522(02)00657-7
- Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Ann Rev Pharmacol Toxicol 2000; 40:389-430; PMID:10836142; http://dx.doi.org/10.1146/annurev.pharmtox.40.1.389
- Spahn V, Fischer O, Endres-Becker J, Schafer M, Stein C, Zollner C. Opioid withdrawal increases transient receptor potential vanilloid 1 activity in a protein kinase A-dependent manner. Pain 2013; 154:598-608; PMID:23398938; http://dx.doi.org/10.1016/j.pain.2012.12.026
- Rowan MP, Bierbower SM, Eskander MA, Szteyn K, Por ED, Gomez R, Veldhuis N, Bunnett NW, Jeske NA. Activation of mu opioid receptors sensitizes transient receptor potential vanilloid type 1 (TRPV1) via beta-arrestin-2-mediated cross-talk. PloS one 2014; 9:e93688; PMID:24695785; http://dx.doi.org/10.1371/journal.pone.0093688
- Sasamura T, Sasaki M, Tohda C, Kuraishi Y. Existence of capsaicin-sensitive glutamatergic terminals in rat hypothalamus. Neuroreport 1998; 9:2045-8; PMID:9674591; http://dx.doi.org/10.1097/00001756-199806220-00025
- Koulchitsky SV, Azev OA, Gourine AV, Kulchitsky VA. Capsaicin-sensitive area in the ventral surface of the rat medulla. Neurosci Lett 1994; 182:129-32; PMID:7536311; http://dx.doi.org/10.1016/0304-3940(94)90780-3
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWF. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 2002; 35:721-31; PMID:12194871; http://dx.doi.org/10.1016/S0896-6273(02)00802-4
- Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW 4th. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc Natl Acad Sci U S A 2003; 100:12480-5; PMID:14523239; http://dx.doi.org/10.1073/pnas.2032100100
- Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem 2004; 279:7048-54; PMID:14630912; http://dx.doi.org/10.1074/jbc.M311448200
- Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci 1997; 17:3525-37; PMID:9133377
- Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem 2005; 280:13424-32; PMID:15691846; http://dx.doi.org/10.1074/jbc.M410917200
- Ma W, Quirion R. The ERK/MAPK pathway, as a target for the treatment of neuropathic pain. Exp Opin ther Targets 2005; 9:699-713; PMID:16083338; http://dx.doi.org/10.1517/14728222.9.4.699
- Tohda C, Sasaki M, Konemura T, Sasamura T, Itoh M, Kuraishi Y. Axonal transport of VR1 capsaicin receptor mRNA in primary afferents and its participation in inflammation-induced increase in capsaicin sensitivity. J Neurochem 2001; 76:1628-35; PMID:11259480; http://dx.doi.org/10.1046/j.1471-4159.2001.00193.x
- Amaya F, Oh-hashi K, Naruse Y, Iijima N, Ueda M, Shimosato G, Tominaga M, Tanaka Y, Tanaka M. Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res 2003; 963:190-6; PMID:12560124; http://dx.doi.org/10.1016/S0006-8993(02)03972-0
- Pomonis JD, Harrison JE, Mark L, Bristol DR, Valenzano KJ, Walker K. N-(4-Tertiarybutylphenyl)-4-(3-cholorphyridin-2-yl)tetrahydropyrazine -1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: II. in vivo characterization in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther 2003; 306:387-93; PMID:12721336; http://dx.doi.org/10.1124/jpet.102.046268
- Jancso G, Jancso-Gabor A. Effect of capsaicin on morphine analgesia-possible involvement of hypothalamic structures. Naunyn-Schmiedeberg's Arch pharmacol 1980; 311:285-8; PMID:7393343; http://dx.doi.org/10.1007/BF00569408
- Bevan S, Szolcsanyi J. Sensory neuron-specific actions of capsaicin: mechanisms and applications. Trends Pharmacol Sci 1990; 11:330-3; PMID:2203194; http://dx.doi.org/10.1016/0165-6147(90)90237-3
- Ko MC, Johnson MD, Butelman ER, Willmont KJ, Mosberg HI, Woods JH. Intracisternal nor-binaltorphimine distinguishes central and peripheral kappa-opioid antinociception in rhesus monkeys. J Pharmacol Exp Ther 1999; 291:1113-20; PMID:10565831
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Ann Rev Neurosci 2001; 24:487-517; PMID:11283319; http://dx.doi.org/10.1146/annurev.neuro.24.1.487
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002; 36:57-68; PMID:12367506; http://dx.doi.org/10.1016/S0896-6273(02)00908-X
- Amaya F, Shimosato G, Nagano M, Ueda M, Hashimoto S, Tanaka Y, Suzuki H, Tanaka M. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur J Neurosci 2004; 20:2303-10; PMID:15525272; http://dx.doi.org/10.1111/j.1460-9568.2004.03701.x
- Breese NM, George AC, Pauers LE, Stucky CL. Peripheral inflammation selectively increases TRPV1 function in IB4-positive sensory neurons from adult mouse. Pain 2005; 115:37-49; PMID:15836968; http://dx.doi.org/10.1016/j.pain.2005.02.010
- Endres-Becker J, Heppenstall PA, Mousa SA, Labuz D, Oksche A, Schafer M, Stein C, Zöllner C. Mu-opioid receptor activation modulates transient receptor potential vanilloid 1 (TRPV1) currents in sensory neurons in a model of inflammatory pain. Mol Pharmacol 2007; 71:12-8; PMID:17005903; http://dx.doi.org/10.1124/mol.106.026740
- Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, McIntyre P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J Pharmacol Exp Ther 2003; 304:56-62; PMID:12490575; http://dx.doi.org/10.1124/jpet.102.042010
- Fields HL, Emson PC, Leigh BK, Gilbert RF, Iversen LL. Multiple opiate receptor sites on primary afferent fibres. Nature 1980; 284:351-3; PMID:6244504; http://dx.doi.org/10.1038/284351a0
- Kohno T, Kumamoto E, Higashi H, Shimoji K, Yoshimura M. Actions of opioids on excitatory and inhibitory transmission in substantia gelatinosa of adult rat spinal cord. J Physiol 1999; 518 (Pt 3):803-13; PMID:10420016; http://dx.doi.org/10.1111/j.1469-7793.1999.0803p.x
- Abbadie C, Lombard MC, Besson JM, Trafton JA, Basbaum AI. Mu and delta opioid receptor-like immunoreactivity in the cervical spinal cord of the rat after dorsal rhizotomy or neonatal capsaicin: an analysis of pre- and postsynaptic receptor distributions. Brain Res 2002; 930:150-62; PMID:11879805; http://dx.doi.org/10.1016/S0006-8993(02)02242-4
- Light AR, Willcockson HH. Spinal laminae I-II neurons in rat recorded in vivo in whole cell, tight seal configuration: properties and opioid responses. Journal of Neurophysiol 1999; 82:3316-26; PMID:10601463
- Marker CL, Lujan R, Colon J, Wickman K. Distinct populations of spinal cord lamina II interneurons expressing G-protein-gated potassium channels. J Neurosci 2006; 26:12251-9; PMID:17122050; http://dx.doi.org/10.1523/JNEUROSCI.3693-06.2006
- Magnuson DS, Dickenson AH. Lamina-specific effects of morphine and naloxone in dorsal horn of rat spinal cord in vitro. J Neurophysiol 1991; 66:1941-50; PMID:1812227
- Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat Med 2003; 9:1003-8; PMID:12894165; http://dx.doi.org/10.1038/nm908
- Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, Chandran P, Gomtsyan A, Brown B, Bayburt EK, et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci 2006; 26:9385-93; PMID:16971522; http://dx.doi.org/10.1523/JNEUROSCI.1246-06.2006
- Chen SR, Prunean A, Pan HM, Welker KL, Pan HL. Resistance to morphine analgesic tolerance in rats with deleted transient receptor potential vanilloid type 1-expressing sensory neurons. Neuroscience 2007; 145:676-85; PMID:17239544; http://dx.doi.org/10.1016/j.neuroscience.2006.12.016
- Zhou HY, Chen SR, Chen H, Pan HL. Sustained inhibition of neurotransmitter release from nontransient receptor potential vanilloid type 1-expressing primary afferents by mu-opioid receptor activation-enkephalin in the spinal cord. J Pharmacol Exp Ther 2008; 327:375-82; PMID:18669865; http://dx.doi.org/10.1124/jpet.108.141226
- Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain 2008; 138:604-16; PMID:18381233; http://dx.doi.org/10.1016/j.pain.2008.02.022
- Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci 2008; 28:4904-17; PMID:18463244; http://dx.doi.org/10.1523/JNEUROSCI.0233-08.2008
- Zhang X, Li L, McNaughton PA. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 2008; 59:450-61; PMID:18701070; http://dx.doi.org/10.1016/j.neuron.2008.05.015
- Jeske NA, Patwardhan AM, Ruparel NB, Akopian AN, Shapiro MS, Henry MA. A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1. Pain 2009; 146:301-7; PMID:19767149; http://dx.doi.org/10.1016/j.pain.2009.08.002
- Faux MC, Scott JD. Molecular glue: kinase anchoring and scaffold proteins. Cell 1996; 85:9-12; PMID:8620541; http://dx.doi.org/10.1016/S0092-8674(00)81075-2
- Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science 1997; 278:2075-80; PMID:9405336; http://dx.doi.org/10.1126/science.278.5346.2075
- DeFea KA. Beta-arrestins as regulators of signal termination and transduction: how do they determine what to scaffold? Cell Signal 2011; 23:621-9; PMID:20946952; http://dx.doi.org/10.1016/j.cellsig.2010.10.004
- Houslay MD, Baillie GS. Beta-arrestin-recruited phosphodiesterase-4 desensitizes the AKAP79/PKA-mediated switching of beta2-adrenoceptor signalling to activation of ERK. Biochem Soc Trans 2005; 33:1333-6; PMID:16246112; http://dx.doi.org/10.1042/BST20051333
- Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, Chellappan S. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Investig 2006; 116:2208-17; PMID:16862215; http://dx.doi.org/10.1172/JCI28164
- Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, Yates JR 3rd, Lefkowitz RJ. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci U S A 2007; 104:12011-6; PMID:17620599; http://dx.doi.org/10.1073/pnas.0704849104
- Li X, Baillie GS, Houslay MD. Mdm2 directs the ubiquitination of beta-arrestin-sequestered cAMP phosphodiesterase-4D5. J Biol Chem 2009; 284:16170-82; PMID:19372219; http://dx.doi.org/10.1074/jbc.M109.008078
- Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, et al. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science 2002; 298:834-6; PMID:12399592; http://dx.doi.org/10.1126/science.1074683
- Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci U S A 2003; 100:940-5; PMID:12552097; http://dx.doi.org/10.1073/pnas.262787199
- Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, Lefkowitz RJ. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem 1998; 273:685-8; PMID:9422717; http://dx.doi.org/10.1074/jbc.273.2.685
- Vogler O, Nolte B, Voss M, Schmidt M, Jakobs KH, van Koppen CJ. Regulation of muscarinic acetylcholine receptor sequestration and function by beta-arrestin. J Biol Chem 1999; 274:12333-8; PMID:10212203; http://dx.doi.org/10.1074/jbc.274.18.12333
- Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab 2006; 17:159-65; PMID:16595179; http://dx.doi.org/10.1016/j.tem.2006.03.008
- Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell 2006; 24:643-52; PMID:17157248; http://dx.doi.org/10.1016/j.molcel.2006.11.007
- Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ. Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell 2009; 17:443-58; PMID:19853559; http://dx.doi.org/10.1016/j.devcel.2009.09.011
- Shukla AK, Kim J, Ahn S, Xiao K, Shenoy SK, Liedtke W, Lefkowitz RJ. Arresting a transient receptor potential (TRP) channel: beta-arrestin 1 mediates ubiquitination and functional down-regulation of TRPV4. J Biol Chem 2010; 285:30115-25; PMID:20650893; http://dx.doi.org/10.1074/jbc.M110.141549
- Bohn LM, Lefkowitz RJ, Caron MG. Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. J Neurosci 2002; 22:10494-500; PMID:12451149
- Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology 2011; 60:58-65; PMID:20713067; http://dx.doi.org/10.1016/j.neuropharm.2010.08.003
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 1999; 286:2495-8; PMID:10617462; http://dx.doi.org/10.1126/science.286.5449.2495
- Gavalas A, Victoratos P, Yiangou M, Hadjipetrou-Kourounakis L, Rekka E, Kourounakis P. The anti-inflammatory effect of opioids. Int J Neurosci 1994; 74:259-64; PMID:7928110; http://dx.doi.org/10.3109/00207459408987244
- Hong Y, Abbott FV. Peripheral opioid modulation of pain and inflammation in the formalin test. Eur J Pharmacol 1995; 277:21-8; PMID:7635169; http://dx.doi.org/10.1016/0014-2999(95)00045-M
- Stein A, Yassouridis A, Szopko C, Helmke K, Stein C. Intraarticular morphine versus dexamethasone in chronic arthritis. Pain 1999; 83:525-32; PMID:10568861; http://dx.doi.org/10.1016/S0304-3959(99)00156-6
- Stein C, Machelska H, Schafer M. Peripheral analgesic and antiinflammatory effects of opioids. Zeitschrift fur Rheumatologie 2001; 60:416-24; PMID:11826735; http://dx.doi.org/10.1007/s003930170004
- Ko MC, Tuchman JE, Johnson MD, Wiesenauer K, Woods JH. Local administration of mu or kappa opioid agonists attenuates capsaicin-induced thermal hyperalgesia via peripheral opioid receptors in rats. Psychopharmacology 2000; 148:180-5; PMID:10663433; http://dx.doi.org/10.1007/s002130050040
- Barrett AC, Smith ES, Picker MJ. Capsaicin-induced hyperalgesia and mu-opioid-induced antihyperalgesia in male and female Fischer 344 rats. J Pharmacol Exp Ther 2003; 307:237-45; PMID:12954802; http://dx.doi.org/10.1124/jpet.103.054478
- Wenk HN, Nannenga MN, Honda CN. Effect of morphine sulphate eye drops on hyperalgesia in the rat cornea. Pain 2003; 105:455-65; PMID:14527706; http://dx.doi.org/10.1016/S0304-3959(03)00260-4
- Sharma SK, Klee WA, Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci U S A 1975; 72:3092-6; PMID:1059094; http://dx.doi.org/10.1073/pnas.72.8.3092
- Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem 2003; 278:50080-90; PMID:14506258; http://dx.doi.org/10.1074/jbc.M306619200
- Vetter I, Cheng W, Peiris M, Wyse BD, Roberts-Thomson SJ, Zheng J, Monteith GR, Cabot PJ. Rapid, opioid-sensitive mechanisms involved in transient receptor potential vanilloid 1 sensitization. J Biol Chem 2008; 283:19540-50; PMID:18482991; http://dx.doi.org/10.1074/jbc.M707865200
- Vetter I, Wyse BD, Monteith GR, Roberts-Thomson SJ, Cabot PJ. The mu opioid agonist morphine modulates potentiation of capsaicin-evoked TRPV1 responses through a cyclic AMP-dependent protein kinase A pathway. Mol Pain 2006; 2:22; PMID:16842630; http://dx.doi.org/10.1186/1744-8069-2-22
- Bao Y, Gao Y, Yang L, Kong X, Zheng H, Hou W, Hua B. New insights into protease-activated receptor 4 signaling pathways in the pathogenesis of inflammation and neuropathic pain: a literature review. Channels 2015; 9:5-13; PMID:25664811; http://dx.doi.org/10.4161/19336950.2014.995001
- Hua B, Gao Y, Kong X, Yang L, Hou W, Bao Y. New insights of nociceptor sensitization in bone cancer pain. Exp Opin Ther Targets 2015; 19:227-43; PMID:25547644; http://dx.doi.org/10.1517/14728222.2014.980815
- Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J Neurosci 1998; 18:6081-92; PMID:9698303
- De Petrocellis L, Harrison S, Bisogno T, Tognetto M, Brandi I, Smith GD, Creminon C, Davis JB, Geppetti P, Di Marzo V. The vanilloid receptor (VR1)-mediated effects of anandamide are potently enhanced by the cAMP-dependent protein kinase. J Neurochem 2001; 77:1660-3; PMID:11413249; http://dx.doi.org/10.1046/j.1471-4159.2001.00406.x
- Elattar TM, Lin HS. The relationship between inflammation and cAMP level in human gingiva. J Dent Res 1981; 60:674-6; PMID:6259227; http://dx.doi.org/10.1177/00220345810600030101
- Malmberg AB, Brandon EP, Idzerda RL, Liu H, McKnight GS, Basbaum AI. Diminished inflammation and nociceptive pain with preservation of neuropathic pain in mice with a targeted mutation of the type I regulatory subunit of cAMP-dependent protein kinase. J Neurosci 1997; 17:7462-70; PMID:9295392
- Naik SR. Increased cyclic AMP-phosphodiesterase activity during inflammation and its inhibition by anti-inflammatory drugs. Eur J Pharmacol 1984; 104:253-9; PMID:6094215; http://dx.doi.org/10.1016/0014-2999(84)90400-X
- Hu HJ, Bhave G, Gereau RWT. Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J Neurosci 2002; 22:7444-52; PMID:12196566
- Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M. PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci 2002; 22:4740-5; PMID:12040081
- Lee SY, Lee JH, Kang KK, Hwang SY, Choi KD, Oh U. Sensitization of vanilloid receptor involves an increase in the phosphorylated form of the channel. Arch Pharm Res 2005; 28:405-12; PMID:15918513; http://dx.doi.org/10.1007/BF02977669
- Ji RR. Mitogen-activated protein kinases as potential targets for pain killers. Curr Opin Investig Drugs 2004; 5:71-5; PMID:14983977
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 2004; 5:173-83; PMID:14976517; http://dx.doi.org/10.1038/nrn1346
- Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Zhang YQ. Protein kinases as potential targets for the treatment of pathological pain. Handb Exp Pharmacol 2007:359-89; PMID:17087130
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J 1995; 9:726-35; PMID:7601337
- Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev 1999; 79:143-80; PMID:9922370
- Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life sciences 2004; 74:2643-53; PMID:15041446; http://dx.doi.org/10.1016/j.lfs.2004.01.007
- Bao Y, Hou W, Hua B. Protease-activated receptor 2 signalling pathways: a role in pain processing. Expert Opin Ther Targets 2014; 18:15-27; PMID:24147628; http://dx.doi.org/10.1517/14728222.2014.844792
- Bao Y, Hou W, Liu R, Gao Y, Kong X, Yang L, Shi Z, Li W, Zheng H, Jiang S, et al. PAR2-mediated upregulation of BDNF contributes to central sensitization in bone cancer pain. Mol Pain 2014; 10:28; PMID:24886294; http://dx.doi.org/10.1186/1744-8069-10-28
- Bao Y, Hou W, Yang L, Liu R, Gao Y, Kong X, Shi Z, Li W, Zheng H, Jiang S, et al. Increased expression of protease-activated receptor 2 and 4 within dorsal root Ganglia in a rat model of bone cancer pain. J Mol Neurosci 2015; 55:706-14; PMID:25344153; http://dx.doi.org/10.1007/s12031-014-0409-1
- Bao Y, Hua B, Hou W, Shi Z, Li W, Li C, Chen C, Liu R, Qin Y. Involvement of protease-activated receptor 2 in nociceptive behavior in a rat model of bone cancer. J Mol Neurosci 2014; 52:566-76; PMID:24057889; http://dx.doi.org/10.1007/s12031-013-0112-7
- Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain 1995; 62:259-74; PMID:8657426; http://dx.doi.org/10.1016/0304-3959(95)00073-2
- Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A 1999; 96:7731-6; PMID:10393889; http://dx.doi.org/10.1073/pnas.96.14.7731
- King T, Ossipov MH, Vanderah TW, Porreca F, Lai J. Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance? Neuro-Signals 2005; 14:194-205; PMID:16215302; http://dx.doi.org/10.1159/000087658
- Ossipov MH, Lai J, King T, Vanderah TW, Porreca F. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers 2005; 80:319-24; PMID:15795927; http://dx.doi.org/10.1002/bip.20254
- Ma W, Zheng WH, Powell K, Jhamandas K, Quirion R. Chronic morphine exposure increases the phosphorylation of MAP kinases and the transcription factor CREB in dorsal root ganglion neurons: an in vitro and in vivo study. Eur J Neurosci 2001; 14:1091-104; PMID:11683901; http://dx.doi.org/10.1046/j.0953-816x.2001.01731.x
- Chen Y, Sommer C. The role of mitogen-activated protein kinase (MAPK) in morphine tolerance and dependence. Mol Neurobiol 2009; 40:101-7; PMID:19468867; http://dx.doi.org/10.1007/s12035-009-8074-z
- Li LY, Chang KJ. The stimulatory effect of opioids on mitogen-activated protein kinase in Chinese hamster ovary cells transfected to express mu-opioid receptors. Mol Pharmacol 1996; 50:599-602; PMID:8794899
- Gutstein HB, Rubie EA, Mansour A, Akil H, Woodgett JR. Opioid effects on mitogen-activated protein kinase signaling cascades. Anesthesiology 1997; 87:1118-26; PMID:9366464; http://dx.doi.org/10.1097/00000542-199711000-00016
- Ferrer-Alcon M, Garcia-Fuster MJ, La Harpe R, Garcia-Sevilla JA. Long-term regulation of signalling components of adenylyl cyclase and mitogen-activated protein kinase in the pre-frontal cortex of human opiate addicts. J Neurochem 2004; 90:220-30; PMID:15198681; http://dx.doi.org/10.1111/j.1471-4159.2004.02473.x
- Trapaidze N, Gomes I, Cvejic S, Bansinath M, Devi LA. Opioid receptor endocytosis and activation of MAP kinase pathway. Brain Res Mol Brain Res 2000; 76:220-8; PMID:10762697; http://dx.doi.org/10.1016/S0169-328X(00)00002-4
- Bilecki W, Zapart G, Ligeza A, Wawrzczak-Bargiela A, Urbanski MJ, Przewlocki R. Regulation of the extracellular signal-regulated kinases following acute and chronic opioid treatment. Cell Mol Life Sci 2005; 62:2369-75; PMID:16158186; http://dx.doi.org/10.1007/s00018-005-5277-y
- Li SX, Wang ZR, Li J, Peng ZG, Zhou W, Zhou M, Lu L. Inhibition of Period1 gene attenuates the morphine-induced ERK-CREB activation in frontal cortex, hippocampus, and striatum in mice. Am J Drug Alcohol abuse 2008; 34:673-82; PMID:18850497; http://dx.doi.org/10.1080/00952990802308197
- Eitan S, Bryant CD, Saliminejad N, Yang YC, Vojdani E, Keith D, Jr., Polakiewicz R, Evans CJ. Brain region-specific mechanisms for acute morphine-induced mitogen-activated protein kinase modulation and distinct patterns of activation during analgesic tolerance and locomotor sensitization. J Neurosci 2003; 23:8360-9; PMID:12967998
- Muller DL, Unterwald EM. In vivo regulation of extracellular signal-regulated protein kinase (ERK) and protein kinase B (Akt) phosphorylation by acute and chronic morphine. J Pharmacol Exp Ther 2004; 310:774-82; PMID:15056728; http://dx.doi.org/10.1124/jpet.104.066548
- Schulz S, Hollt V. Opioid withdrawal activates MAP kinase in locus coeruleus neurons in morphine-dependent rats in vivo. Eur J Neurosci 1998; 10:1196-201; PMID:9753188; http://dx.doi.org/10.1046/j.1460-9568.1998.00103.x
- Cao JL, He JH, Ding HL, Zeng YM. Activation of the spinal ERK signaling pathway contributes naloxone-precipitated withdrawal in morphine-dependent rats. Pain 2005; 118:336-49; PMID:16289800; http://dx.doi.org/10.1016/j.pain.2005.09.006
- Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci 2004; 24:8300-9; PMID:15385613; http://dx.doi.org/10.1523/JNEUROSCI.2893-04.2004
- Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res 2006; 1069:235-43; PMID:16403466; http://dx.doi.org/10.1016/j.brainres.2005.11.066
- Hayward MD, Duman RS, Nestler EJ. Induction of the c-fos proto-oncogene during opiate withdrawal in the locus coeruleus and other regions of rat brain. Brain Res 1990; 525:256-66; PMID:1701330; http://dx.doi.org/10.1016/0006-8993(90)90872-9
- Couceyro P, Douglass J. Precipitated morphine withdrawal stimulates multiple activator protein-1 signaling pathways in rat brain. Mol Pharmacol 1995; 47:29-39; PMID:7838131
- Fan XL, Zhang JS, Zhang XQ, Ma L. Chronic morphine treatment and withdrawal induce up-regulation of c-Jun N-terminal kinase 3 gene expression in rat brain. Neuroscience 2003; 122:997-1002; PMID:14643766; http://dx.doi.org/10.1016/j.neuroscience.2003.08.062
- Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP, Jr., Lai J, Porreca F. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci 2002; 22:6747-55; PMID:12151554
- Tiong GK, Pierce TL, Olley JE. Sub-chronic exposure to opiates in the rat: effects on brain levels of substance P and calcitonin gene-related peptide during dependence and withdrawal. J Neurosci Res 1992; 32:569-75; PMID:1382137; http://dx.doi.org/10.1002/jnr.490320412
- Welch SP, Bass PP, Olson KG, Pugh G. Morphine-induced modulation of calcitonin gene-related peptide levels. Pharmacol Biochem Behavior 1992; 43:1107-16; PMID:1335576; http://dx.doi.org/10.1016/0091-3057(92)90489-3
- Gu G, Kondo I, Hua XY, Yaksh TL. Resting and evoked spinal substance P release during chronic intrathecal morphine infusion: parallels with tolerance and dependence. J Pharmacol Exp Ther 2005; 314:1362-9; PMID:15908510; http://dx.doi.org/10.1124/jpet.105.087718
- Salmon AM, Damaj MI, Marubio LM, Epping-Jordan MP, Merlo-Pich E, Changeux JP. Altered neuroadaptation in opiate dependence and neurogenic inflammatory nociception in alpha CGRP-deficient mice. Nat Neurosci 2001; 4:357-8; PMID:11276224; http://dx.doi.org/10.1038/86001
- Murtra P, Sheasby AM, Hunt SP, De Felipe C. Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature 2000; 405:180-3; PMID:10821273; http://dx.doi.org/10.1038/35012069
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2×3 purinoceptor and IB4 binding sites. Eur J neuroscience 1999; 11:946-58; PMID:10103088; http://dx.doi.org/10.1046/j.1460-9568.1999.00503.x
- Tang HB, Li YS, Miyano K, Nakata Y. Phosphorylation of TRPV1 by neurokinin-1 receptor agonist exaggerates the capsaicin-mediated substance P release from cultured rat dorsal root ganglion neurons. Neuropharmacology 2008; 55:1405-11; PMID:18809416; http://dx.doi.org/10.1016/j.neuropharm.2008.08.037
- Tang HB, Nakata Y. The activation of transient receptor potential vanilloid receptor subtype 1 by capsaicin without extracellular Ca2+ is involved in the mechanism of distinct substance P release in cultured rat dorsal root ganglion neurons. Naunyn-Schmiedeberg's Arch pharmacol 2008; 377:325-32; PMID:18034335; http://dx.doi.org/10.1007/s00210-007-0211-5
- Li DP, Chen SR, Pan HL. VR1 receptor activation induces glutamate release and postsynaptic firing in the paraventricular nucleus. J Neurophysiol 2004; 92:1807-16; PMID:15115794; http://dx.doi.org/10.1152/jn.00171.2004
- Lappin SC, Randall AD, Gunthorpe MJ, Morisset V. TRPV1 antagonist, SB-366791, inhibits glutamatergic synaptic transmission in rat spinal dorsal horn following peripheral inflammation. Eur J Pharmacol 2006; 540:73-81; PMID:16737693; http://dx.doi.org/10.1016/j.ejphar.2006.04.046
- Otsuka M, Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev 1993; 73:229-308; PMID:7682720
- Schicho R, Donnerer J, Liebmann I, Lippe IT. Nociceptive transmitter release in the dorsal spinal cord by capsaicin-sensitive fibers after noxious gastric stimulation. Brain Res 2005; 1039:108-15; PMID:15781052; http://dx.doi.org/10.1016/j.brainres.2005.01.050
- Rigoni M, Trevisani M, Gazzieri D, Nadaletto R, Tognetto M, Creminon C, Davis JB, Campi B, Amadesi S, Geppetti P, et al. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin. Br J Pharmacol 2003; 138:977-85; PMID:12642400; http://dx.doi.org/10.1038/sj.bjp.0705110
- Aimone LD, Yaksh TL. Opioid modulation of capsaicin-evoked release of substance P from rat spinal cord in vivo. Peptides 1989; 10:1127-31; PMID:2482963; http://dx.doi.org/10.1016/0196-9781(89)90003-X
- Jin S, Lei L, Wang Y, Da D, Zhao Z. Endomorphin-1 reduces carrageenan-induced fos expression in the rat spinal dorsal horn. Neuropeptides 1999; 33:281-4; PMID:10657505; http://dx.doi.org/10.1054/npep.1999.0040
- Zhou Q, Karlsson K, Liu Z, Johansson P, Le Greves M, Kiuru A, Nyberg F. Substance P endopeptidase-like activity is altered in various regions of the rat central nervous system during morphine tolerance and withdrawal. Neuropharmacology 2001; 41:246-53; PMID:11489461; http://dx.doi.org/10.1016/S0028-3908(01)00055-7
- Dickenson AH. Spinal cord pharmacology of pain. Br J anaesthesia 1995; 75:193-200; PMID:7577253; http://dx.doi.org/10.1093/bja/75.2.193
