Abstract
Calcium (Ca2+) and magnesium (Mg2+) ions have been shown to play an important role in regulating various neuronal functions. In the present review we focus on the emerging role of transient potential melastatin-7 (TRPM7) channel in not only regulating Ca2+ and Mg2+ homeostasis necessary for biological functions, but also how alterations in TRPM7 function/expression could induce neurodegeneration. Although eight TRPM channels have been identified, the channel properties, mode of activation, and physiological responses of various TRPM channels are quite distinct. Among the known 8 TRPM channels only TRPM6 and TRPM7 channels are highly permeable to both Ca2+ and Mg2+; however here we will only focus on TRPM7 as unlike TRPM6, TRPM7 channels are abundantly expressed in neuronal cells. Importantly, the discrepancy in TRPM7 channel function and expression leads to various neuronal diseases such as Alzheimer disease (AD) and Parkinson disease (PD). Further, it is emerging as a key factor in anoxic neuronal death and in other neurodegenerative disorders. Thus, by understanding the precise involvement of the TRPM7 channels in different neurodegenerative diseases and by understanding the factors that regulate TRPM7 channels, we could uncover new strategies in the future that could evolve as new drug therapeutic targets for effective treatment of these neurodegenerative diseases.
Introduction
Calcium (Ca2+) acts as a ubiquitous second messenger that has achieved a well-establish role in controlling cellular functions.Citation1 All cells express various Ca2+ channels, pumps, and Ca2+ binding proteins that tightly control the intracellular free Ca2+ concentration [Ca2+]i. The [Ca2+]i is maintained at low nanomolar levels, because a small increase in [Ca2+]i will result in the activation of various cellular processes, that ranging from short-term responses such as muscle contraction, secretion, and neuronal transmission to long term modulation of cell growth and gene transcription.Citation2-6 Magnesium (Mg2+) is the second most abundant intracellular cation and is also a versatile ion, which is involved in practically every major metabolic and biochemical process within the cell.Citation7 Mg2+ is required for the production of cellular energy, cell growth and development.Citation8 Mg2+ is also an essential cofactor for ATP polyphosphates such as DNA and RNA and metabolic enzymes essential in nerve impulse transmission, and muscle contraction.Citation9 The compartmentalization of Mg2+ within the cell is a key element necessary to coordinate the regulation of pathways in which transphosphorylation reactions serves as the rate-limiting step.Citation10 Additionally, Mg2+ also has a role in the regulation of protein synthesis, which is very sensitive to small changes of intracellular Mg2+ within physiological ranges and the onset of DNA synthesis is dependent on the rate of protein synthesis.Citation11,12 Recent studies reported that Mg2+ also plays a part in intracellular signaling (as does Ca2+), regulation of neuronal development and modulation of electric synapses.Citation8,13,14
In neurons, Ca2+ and Mg2+ together plays a vital role in a variety of physiological processes, from regulating gene transcription to neuronal growth, survival and even differentiation.Citation2,15 Interestingly, Mg2+ was initially identified as a powerful Ca2+ antagonist, despite both having similar charge and chemical properties. Moreover, Mg2+ also protects the neuronal cells from Ca2+ overload.Citation16 Previous studies have also indicated a tight balance between Ca2+ and Mg2+ ions that is needed for maintaining proper physiological functions such as control of muscle movement (motor neurons).Citation17 Thus, influx of both extracellular Ca2+ and Mg2+ must be tightly maintained for proper intracellular ion homeostasis as alterations in Ca2+ and Mg2+ homeostasis will alter cellular functions and possibly lead to cell death.
Disturbances in Ca2+ homeostasis have been involved in neurodegenerative diseases such as Parkinson, Alzheimer, and Huntington's,Citation18-20 which is mainly due to the high dependence of Ca2+ signaling in modulating neuronal functions.Citation21 In contrast, brain Mg2+ levels have been shown to decline in a number of acute and chronic pathologies including neurodegeneration, traumatic brain injury, and depression.Citation15 Prominently, cation mobilization in neuronal cells is tightly regulated by different ion channels and pumps in the plasma membrane and the organelle membrane. In this regard, one potentially important ion influx pathway may be the activation of TRPM channels. TRPM7 channels are selective to Ca2+ and Mg2+ and activation of TRPM7 channels are tightly regulated by intracellular Mg2+ levels, which emerges as a key factor in various neurodegenerative disorders. The current review therefore highlights recent progress in research on neurodegenerative diseases focusing on advances of TRPM7 channel to various pathologies.
TRPM Subfamily
TRP channels constitute a family of ion channels that are divided into TRPC (Canonical/Classical), TRPV (Vanilloid), and TRPM (Melastatin) sub-families. Importantly, all members of TRP family are moderately conserved and share significant homology among them.Citation22,23 The TRPM subfamily consist of 8 members, which are sub-divided into 3 subgroups on the basis of sequence homology; TRPM1/TRPM3, TRPM4/TRPM5 and TRPM6/7, with TRPM2/TRPM8 being distinct proteins from the other groups (). TRPM channels lack the multiple N-terminal ankyrin repeats present in TRPC and TRPV channels; instead they contain a conserved N-terminal intracellular domain of unknown function and a conserved coiled-coil region in the C-terminus that is thought to facilitate subunit assembly. The modes of activation and regulation of TRPM channels are diverse which separates them from other members. In general TRPM channels are cation channels that contribute to changes in [Ca2+]i concentrations by acting as Ca2+ entry channels in the plasma membrane directly or by changing membrane potential, modulating the driving force for other Ca2+ entry channels with the exceptions of TRPM4 and TRPM5, which are only permeable to monovalent cations.Citation24,25 TRPM6 channels are highly selective to Mg2+.Citation26 Among all the TRPM channels only TRPM7 is the cation selective ion channel that is highly permeable to both Ca2+ and Mg2+ signifying its importance and thus could have unique function in neuronal cells. In addition, TRPM7 can also conduct toxic metals,Citation27 that could also lead to neurodegeneration.
Table 1. Ion selectivity of TRPM subfamily: The TRPM subfamily consists of 8 members. All TRPM channels are cation channels and ion selectivity is diverse as shown in the table.
The physiological functions and biophysical properties of TRPM1 are not as yet characterized. This is mainly due to the fact that TRPM1 is primarily localized to intracellular vesicles rather than at the plasma membrane.Citation28 However, recent effort revealed that TRPM1 is critical for a non-selective cation conductance in melanocytes and retinal bipolar cells.Citation28,29 Similarly, TRPM2 is also a divalent cation-permeable channel, which transports Ca2+ and is activated by adenosine diphosphoribose (ADPR), H2O2 and heat.Citation30 TRPM2 also contains a functional ADPR hydrolase enzyme on its C-terminus and has been suggested to play an indirect role in anoxic-mediated neurodegeneration.Citation31 However, physiological function for TRPM2 in neuronal cells has yet to be identified.
TRPM3 has multiple splice variants that differ in their ion selectivity. TRPM3 channels are, much like TRPM6 and TRPM7, but are known to be constitutively active, outwardly rectifying, and are inhibited by intracellular Mg2+ ions; however their in vivo role in neuronal cells remains unknown. TRPM4 and TRPM5 are heat-sensitive, Ca2+ activated channels that are monovalent selective. TRPM4 is thought to play an important role in regulating smooth muscle contraction,Citation32 suggesting that it may play a role in regulating cerebral blood flow. In contrast, TRPM5 is limited to cells of the gastrointestinal tract, and taste buds Citation24 and thus may not be relevant to neuronal function.
TRPM6 and TRPM7 are homologous in their protein structure, each containing an atypical α kinase domain on their C-terminus. TRPM6 expression is limited to renal and intestinal epithelium where it is thought to play a role in physiological Mg2+ homeostasis.Citation26 TRPM8 is primarily known as a thermosensor, activated by cool temperatures (comprised between 15 and 28°C), and is also gated by exogenous compounds that elicit a cooling sensation.Citation33 TRPM7 channels are also activated by oxidative stress, and/or ADPR and are highly expressed in neuronal tissues.Citation27 Notably, TRPM7 is crucial to both Ca2+ and Mg2+ homeostasis and alterations in TRPm7 function has been reported to play pathological roles in the brain especially in neurodegeneration, which will be discussed in depth in this review.
TRPM7 Channels Properties and Mode of Activation
TRPM7, formerly known as LTRPC7, TRP-PLIK and ChaK1, is a ubiquitously expressed dual-function plasma membrane protein consisting of a TRP ion channel fused to a protein kinase domain.Citation34-36 TRPM7 protein forms a nonselective cation channels with a strong outwardly rectifying current–voltage signature (PCa/PNa ∼ 0.34).Citation36 Previous studies indicated that phosphotransferase activity is not required for TRPM7 channel activity.Citation37,38 However, recently, annexin 1, a Ca2+-dependent membrane binding protein, was identified as a substrate for TRPM7 kinase.Citation39 Furthermore, phosphorylation of annexin 1 by TRPM7 kinase at Ser5 within the N-terminal α-helix is stimulated by Ca2+ influx through the channel domain and implicate an interaction between channel and kinase functions.Citation40,41 Thus, it can be suggested that cations entering through TRPM7 channel may play a crucial role in regulation of the kinase function and the subsequent activation of downstream signaling components. TRPM7 is an Mg2+ and Ca2+ permeable ion channel that maintains the cellular Mg2+ and Ca2+ homeostasis.Citation42 Intracellular free Mg2+, MgATP, pH, phosphatidylinositol 4,5-bisphosphate (PIP2), cyclic adenosine 3,5-monophosphate (cAMP) and polyvalent cations have all been reported to regulate TRPM7 channel activity.Citation44-46 Phosphorylation of TRPM6 has recently been shown to regulate TRPM7 channel activity.Citation43 There is a general consensus that TRPM7 channel is inhibited by free intracellular Mg2+,Citation47 but there is some discrepancy whether TRPM7 is activated or inhibited by phospholipase C (PLC).Citation44,48 In addition, it has also been proposed that TRPM7 channels are either activated or inhibited by cellular ATP. Early characterization of TRPM7 showed currents that were activated by low MgATP levels and were thus termed as magnesium-nucleotide-regulated metal ion current (MagNuM).Citation36,46 One potential reason for this difference could be that cytoplasmic MgATP effectively inhibits only when a weak Mg2+ chelator is present in the pipette solution. Under such conditions, MgATP acts as a source of Mg2+ rather than a source of ATP.Citation46 TRPM7 is also sensitive to changes in pH and significantly potentiated by acidic pH,Citation49 which implies that TRPM7 may play a role under acidic pathological conditions. TRPM7 has been proposed to maintain normal physiological functions, by regulating Ca2+ and Mg2+ homeostasis. This is essential for regulating cell growth and proliferation, synaptic vesicle release, detoxification, cell adhesion and cell spreading, and myosin stability. Similarly, TRPM7 has also been shown to play a role in cancer proliferation, stroke, hydrogen peroxide dependent neurodegeneration, and even heavy metal toxicity.Citation27,50,51 However, it is not clear if much of these functions are due to abnormal Ca2+ and Mg2+ homeostasis or due to the fact that TRPM7 also has an α kinase with multiple targets and could very well regulate some of these conditions by changing the phosphorylation status of proteins essential for thewe functions. Importantly, initial studies showed that TRPM7 can form fully functional channels in the absence of its C-terminal domain that lacks the kinase domain;Citation37,38 however others have shown that the kinase activity was required for TRPM7 channel function.Citation40,41
TRPM7 is a unique protein that contains an atypical kinase domain at its C-terminus, that is similar to eukaryotic elongation factor-2 kinase (eEF2K).Citation35 TRPM7 is cleaved by caspases at D1510, disassociating the C-terminal kinase domain from the pore without disrupting the phosphotransferase activity of the released kinase. The cleaved kinase fragments (M7CKs) have been shown to translocate to the nucleus and bind to the multiple components of the chromatin-remodeling complexes.Citation52 Karpivinsky et al showed that in the nucleus, the kinase domain of TRPM7 phosphorylates specific serines/threonines of histones and M7CK-dependent phosphorylation of H3Ser10 at promoters of TRPM7-dependent genes and correlates with their activity. The authors also showed that TRPM7-mediated modulation of intracellular zinc concentration couples ion-channel signaling to epigenetic chromatin covalent modifications that affect gene expression patterns.Citation53 Where else the studies by Kaitsuka et al in the same year showed that TRPM7 kinase activity does not impair its channel activity and kinase activity is not essential for regulation of mammalian Mg2+ homeostasis.Citation54 Some of these discrepancies could be explained by the differential expression of various other TRPM channels in various cells as TRPM channels have been shown to form heteromers, which could exhibit different channel properties leading to alteration in the biological function.
Biological Functions of TRPM7 in Neuronal Cells
TRPM7 is the most abundantly expressed TRPM channel in the majority of mouse organs and widely distributed in the central nervous system including hippocampus, cerebrum, cerebellum and truncus encephali.Citation55 TRPM7 also plays a key role in embryonic development. The global deletion of TRPM7 in mice is lethal and death occurs before embryonic day 7.5.Citation59 The proposed mechanism suggests that TRPM7 deletion leads to growth defects and embryonic lethality which involves a role for both channel activity and Mg2+. Deletion of TRPM7 also leads to proliferative arrest and cellular quiescence,Citation36 which could be ameliorated either by supplementation of high concentrations of Mg2+ or by co-expression of a TRPM7 mutant that are Mg2+-permeant but lack the phosphotransferase activity. Consistent with these results overexpression of other magnesium transporters particularly, the plasma-membrane Mg2+ transporters MagT1 and SLC41A2 can restore Mg2+ uptake and stimulate growth in TRPM7-deficient cells,Citation60 suggesting that these functions are truly dependent on magnesium influx.
TRPM7 has been shown to be required for increased cell proliferation and migration.Citation50 Overexpression of TRPM7 in a neuroblastoma cell line increased focal adhesion formation and cellular spreading. TRPM7 could phosphorylate myosin II and thus regulate actomyosin relaxation and myosin filament stability to alter the cellular cytoskeleton.Citation61 Furthermore, knockdown of TRPM7 in retinoblastoma cells decreased proliferation and halted cell cycle progression.Citation62 In contrast although TRPM7 channels are highly expressed in the tips of the growth cone, both knockdown of TRPM7 and blockage of the channel by a specific blocker waixenicin A enhanced axonal outgrowth in culture.Citation63 Furthermore, TRPM7 co-immunoprecipitated and colocalized with F-actin and α-actinin-1 at the growth cone, which suggested that Ca2+ influx through TRPM7 inhibits axonal outgrowth and maturation by regulating the F-actin and α-actinin-1 protein complex.Citation63 Thus, these evidences indicated TRPM7 channels function as a mechanosensitive regulator of neuronal cytoskeleton, which may affect axonal growth in a way that is different from other stimulus.
TRPM7 may also play a major role in vesicular trafficking, membrane reorganization, and neurotransmitter release. TRPM7 has been shown to be located in the membranes of acetylcholine (ACh)-secreting synaptic vesicles of sympathetic neurons. TRPM7 forms a molecular complex with proteins of the vesicular fusion machinery and is critical for stimulated neurotransmitter release.Citation58 Studies also indicated that TRPM7 is located in ACh-secreting small synaptic-like vesicles (SSLV). Knocking down the TRPM7 channel or abolishing TRPM7 channel activity by mutation, attenuated the frequency of spontaneous and voltage-stimulated SSLV fusion events without affecting large dense core vesicle secretion, thereby suggesting that the conductance of TRPM7 across the vesicle membrane is important in SSLV fusion.Citation57
TRPM7 Channels and Neurodegeneration
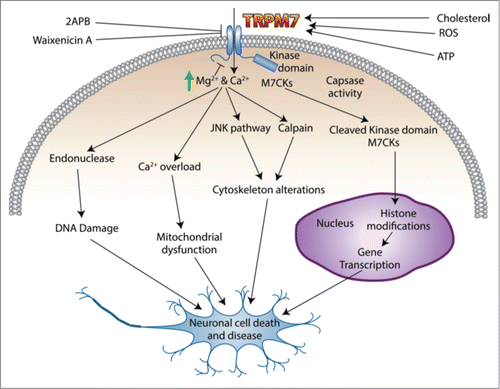
Neurodegeneration comprises the assembly of pathological events that give rise to a progressive loss of neuronal structure and function including cellular damage, diseases development, or cellular death.Citation64 In most of these processes, Ca2+ and Mg2+ play a pivotal role and as TRPM7 regulates both these ions it can be postulated that TRPM7 will play a major role in these conditions ().
Several physiological pathways are responsible for the production of reactive oxygen species (ROS) including respiration and activation of the arachidonic acid cascade.Citation65 Overproduction of ROS has been proposed to play crucial roles in the pathogenesis of neurodegenerative disorders including Alzheimer disease (AD) and Parkinson disease (PD).Citation66-68 Oxidative stress could result in cellular defects including a defect in ER Ca2+ uptake and Ca2+efflux, thereby increasing [Ca2+]i and Ca2+ influx. This increase in [Ca2+]i induces an exacerbation of oxidative stress, and activates several Ca2+−dependent enzymes including, calpain and the endonuclease pathways, which ultimately cause cytoskeleton alterations and cell death.Citation69-71 In oxidative stress, ROS activated TRPM7 channels induced an increase in [Ca2+]i concentrations and TRPM7 currents are prominently facilitated by H2O2 exposure.Citation36 In addition, application of 2-aminoethoxydiphenyl borate (2-APB), an inhibitor of TRPM7, or knockdown of TRPM7 protected the neurons from H2O2- mediated injury. In contrast, overexpressing TRPM7 channels has also shown to increase H2O2- mediated injury,Citation51 suggesting that a set-point of TRPM7 expression is vital for neuronal function and alteration in its activity and/or expression could alter this tight balance. Importantly, suppression of TRPM7 rescued anoxic neurons and permitted the survival of neurons previously destined to die from prolonged anoxia. Considering all these results, TRPM7 emerges as a key factor in anoxic neuronal death.Citation72 Interestingly, in primary cortical neurons, knockdown of TRPM7 also resulted in knockdown of TRPM2 suggesting that expression of these 2 proteins in cortical neurons is co-ordinated in some manner.Citation72 These two channels form heteromers in cortical neurons. The observed anoxia-induced current is carried by TRPM2/TRPM7 heteromers. Although interaction between TRPM2 and TRPM7 at a protein level has not yet been demonstrated, TRPM2/TRPM7 heteromeric channels may represent a critical cation channel species that are modulated by intracellular free radicals and conducts toxic levels of Ca2+ which contribute to an increased susceptibility to degenerative processes.
AD is a form of dementia in which patients show neurodegeneration, complete loss of cognitive abilities. In AD, central to the neurodegenerative process is the inability of neurons to properly regulate [Ca2+]i concentration. Increased levels of amyloid β-peptide (Aβ) induce neurotoxic factors including ROS and cytokines, which impair cellular Ca2+ homeostasis and render neurons vulnerable to apoptosis and excitotoxicity.Citation73 Familial Alzheimer disease (FAD)-associated presenilin (PS) mutations cause an imbalance in PIP2 metabolism, which is an important cellular effector whose functions include the regulation of TRPM7 channel. Modulation of cellular PIP2 also closely correlates with 42-residue amyloid β-peptide (Aβ42) levels. Importantly, a recent study indicated that TRPM7 channel underlies Ca2+ entry deficits in presenilin FAD mutant cells and the observed channel deficits are restored by the addition of PIP2.Citation74 These results suggests that TRPM7 is involved in the normal function of PS and in its regulation through a PIP2-dependent mechanism.Citation75
TRPM7 is also an important Mg2+ transporter. A critical role for Mg2+ has been implicated in AD, Huntington's disease, and mitochondrial cytopathies, although most recent studies have focused on PD.Citation15,76-78 PD is a common neurodegenerative disorder and loss of dopaminergic (DA) neurons in the substantia nigra (SN) region underlies the main motor symptoms of PD. In vitro animal PD models showed Mg2+ deficiency increased the susceptibility to methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity.Citation79 Furthermore, that Mg2+ administration significantly inhibited the toxicity of (methyl-4-phenylpyridium ion (MPP+) and prevented any decrease in the number of DA neurons and the length of DA neurites is also significantly preserved,Citation80 which is consistent with the finding that Mg2+ deficiency in rats for over a number of generations significantly showed decreased DA neurons in the SN.Citation81 Thus as, TRPM7 channel are tightly linked with Mg2+ transport, they may play a role in various neurodegenerative disease onsets under some circumstances. Consistent with these observations, TRPM7 channels are expressed in SN neurons. Recently, TRPM7 mutant Zebrafish showed defects in the production or release of dopamine which lead to hypomotile and a failure to make a dopamine-dependent developmental transition. Importantly, both of these deficits were partially rescued by the application of dopamine.Citation82 In SH-SY5Y cells, which models aspects of human DA neurons, forced expression of a channel-dead variant of TRPM7 causes cell death.Citation82 Further reports indicate that mRNA levels of TRPM7 also significantly decreased in SN area of PD patients.Citation83 Together, these suggest that DA neurons depend on the TRPM7 expression and loss of TRPM7channel function may be involved in PD.
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by the progressive degeneration of motor neurons.Citation84 Parkinsonism dementia is also related to ALS, but is a distinct, neurodegenerative disease. ALS and PD can share overlapping clinical symptoms and frequently occur together in the same families, as well as even in the same individual. Further, the basic pathogenic mechanisms underlying these disorders are similar.Citation85-89 Extensive studies conducted over the years strongly suggest that ALS and Parkinsonism Dementia are connected with low levels of Ca2+ and Mg2+, which create a condition that could affect the proper function of TRPM7 channels.Citation89-91 Importantly, a heterozygous variant of TRPM7 encoding a channel protein with a missense mutation was found in a subset of ALS/PD cases in Guam but not in the Kii peninsula of Japan.Citation89,92,93 Notably, the mutation did not affect TRPM7 kinase activity but increased the channel's sensitivity to an intracellular Mg2+ block, resulting in imbalance in Mg2+ and Ca2+ homeostasis that could affect vital cellular processes including those responding to increased oxidative stress and those causing activation of proinflammatory pathways.Citation89 TRPM7 also resides in the membrane of synaptic vesicles and interacts with snapin altering acetylcholine release. Thus, indicating that vesicular TRPM7 channel activity is critical to neurotransmitter release in sympathetic neurons.Citation57,58 Importantly, disruption and loss of neuromuscular synapse has been reported as one of earliest pathological events in ALS and occurs long before the appearance of clinical symptoms.Citation94 Thus, TRPM7 mutation may play a crucial role in neurotransmitter release in ALS.
Another neurodegenerative disease where TRPM7 can play a vital role is stroke. Stroke is a sudden loss of brain function due to an interruption of the brain's blood supply. Loss of oxygen leads to a decrease in ATP levels and as neuronal cells are dependent on glucose metabolism, inhibition of mitochondrial function could lead to neuronal cell death and loss of brain function. Additionally in strokes, cell death is triggered by membrane depolarization, Ca2+ overload, and the production of ROS. As indicated above, TRPM7 could lead to Ca2+ toxicity and are activated by free radical which could amplify neuronal degeneration during ischemia.Citation62 Consistent with this inhibition of TRPM7 activity and siRNA knockdown has proven to be neuroprotective after prolonged oxygen glucose deprivation (an in vitro model of stroke), suggesting that its activity may be responsible for the delayed cell death and Ca2+ overload that inevitably accompany stroke.Citation72 In addition, TRPM7 has also been proposed as a downstream targets since it was first implicated in the cell death that follows stroke.
Carvacrol, a pungent natural compound, has been reported to block TRPM7 current in hippocampal neuron, as well as provide neuroprotection in adult mice subjected to focal ischemia TRPM7 inhibitor.Citation95,96 Carvacrol pre-treatment protects against neonatal hypoxic-ischemic brain injury by reducing brain infarct volume, promoting pro-survival signaling and inhibiting pro-apoptotic signaling, as well as improving behavioral outcomes.Citation97 This suggess that the neuroprotective effect may be mediated by the inhibition of TRPM7 channel function and TRPM7 may be a feasible target for pharmacological intervention during stroke. Sun et al showed that suppression of TRPM7 activity in hippocampal neurons made them resistant to ischemic death and it also preserved neuronal morphology and function. Moreover, TRPM7 suppression prevented ischemia-induced deficits in long-term potentiation (LTP) and preserved the performance in fear associated and spatial-navigational memory tasks.Citation98 Recent studies by Chen et al also showed that carvacrol pre-treatment protects against neonatal hypoxic-ischemic brain injury and this neuroprotective effect may be mediated by the inhibition of TRPM7 channel function.Citation99 Both in vitro and in vivo studies indicate the involvement of TRPM7 channels in ischemic neuronal injury Citation100 but further extensive preclinical testing is obligatory to evaluate the therapeutic potential of the TRPM7 blockade in stroke.
In addition, TRPM7 also interacts with several proteins such as PLC, Fas-associated death domain protein (FADD), and eEF2K, which are all associated with neurodegenerative disease. PLC enzymes are crucial signaling elements and can convert the membrane-bound PIP2 to the second messengers Diacylglycerol (DAG).Citation101 AD related Aβ peptide significantly altered muscarinic cholinergic receptor (mChR) signaling on the level of G protein regulated PLC leading to the lower formation of inositol-1,4,5-triphosphate (IP3) and DAG.Citation102 TRPM7 Ser/Thr-kinase can interact with PLCβ1 -β2 -β3, and -γ1eEF2 controlling PLC activity.Citation44,101 eEF2K are key downstream effectors that mediate the detrimental effects of hyperactive AMPK in AD.Citation103 Levels of p-eEF2K were significantly increased and total eEF2 is significantly decreased in AD.Citation104 TRPM7, via its kinase, mediates enhanced phosphorylation of eEF2, which does not directly phosphorylate eEF2, but rather to influence the amount of eEF2s cognate kinase eEF2k to regulate eEF2.Citation105 The FAS gene plays a role in apoptosis and is associated with AD by modulating the apoptosis and neuronal loss secondary to AD neuropathology.Citation106,107 PD patients also show mitochondrial, ubiquitin-proteasome system dysfunction, associated to high expression of the Fas molecule, activation of caspase-3 and -9 and proneness to apoptosis.Citation108 A recent study indicated TRPM7 regulates endocytic compartmentalization of the Fas receptor and knockdown TRPM7 considerably reduced incidence of Fas receptor internalization after receptor stimulation,Citation52 an important process for apoptotic signaling through Fas receptors. Thus, functional interaction of TRPM7 with these proteins maybe one of the additional mechanisms of TRPM7 involved in neurodegenerative disease.
Conclusion and Future Directions
The activity of TRPM7 channel is crucial for maintenance of appropriate levels of intracellular Ca2+ and Mg2+ levels and alterations in this homeostasis will change cell function. This can lead to elevated oxidative stress reasonably which disrupt the function, differentiation, and survival of neurons. Hence, genetic variations in TRPM7 channels may influence susceptibility to neurodegenerative diseases. However, whether TRPM7 channel is linked to intracellular signal cascades via its kinase domain or associated proteins remains to be determined.Citation109 Phosphorylation by TRPM7 kinase within the N-terminal α-helix is stimulated by Ca2+ influx through the channel domain and allude to an interaction between channel and kinase functions. TRPM7 channel kinase domain is also required for embryonic development making the TRPM7 knockout mice lethal and thereby restricting the knockout studies.Citation59 Importantly, recent reports have also shown that the kinase domain of TRPM7 is essential for regulating the epigenome. Although these studies were performed in non-neuronal cells, it is exciting and opens new direction as to how TRPM7 can regulate neuronal functions. Thus, further studies will lead to fully evaluating the significant role of TRPM7 channel in neuronal functions and how loss of these vital functions may be involved in different types of neurodegeneration. In conclusion regulating TRPM7 channel functions may uncover new strategies in the future to prevent the progression of neurodegenerative diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Ginny Aachen for the valuable comments and grammar corrections.
Funding
We duly acknowledge the grant support from the National Institutes of Health (DE017102).
References
- Komuro H, Kumada T. Ca2+ transients control CNS neuronal migration. Cell Calcium 2005; 37:387-93; PMID:15820385; http://dx.doi.org/10.1016/j.ceca.2005.01.006.
- Clapham DE. Calcium signaling. Cell 2007; 131:1047-58; PMID:18083096; http://dx.doi.org/10.1016/j.cell.2007.11.028.
- Chauhan A, Sun Y, Pani B, Quenumzangbe F, Sharma J, Singh BB, Mishra BB. Helminth induced suppression of macrophage activation is correlated with inhibition of calcium channel activity. PLoS ONE 2014; 9:e101023; PMID:25013939; http://dx.doi.org/10.1371/journal.pone.0101023.
- Sun Y, Chauhan A, Sukumaran P, Sharma J, Singh BB, Mishra BB. Inhibition of store-operated calcium entry in microglia by helminth factors: implications for immune suppression in neurocysticercosis. J Neuroinflammation 2014; 11:210; PMID:25539735; http://dx.doi.org/10.1186/s12974-014-0210-7.
- Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev 1997; 77:901-30; PMID:9354808.
- Löf C, Viitanen T, Sukumaran P, Törnquist K. TRPC2: of mice but not men. Adv Exp Med Biol 2011; 704:125-34; PMID:21290292; http://dx.doi.org/10.1007/978-94-007-0265-3_6.
- de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev 2015; 95:1-46; PMID:25540137; http://dx.doi.org/10.1152/physrev.00012.2014.
- Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 2011; 475:471-6; PMID:21796205; http://dx.doi.org/10.1038/nature10246.
- Anghileri LJ. Magnesium, calcium and cancer. Magnes Res 2009; 22:247-55; PMID:20228002.
- Vidair C, Rubin H. Mg2+ as activator of uridine phosphorylation in coordination with other cellular responses to growth factors. Proc Natl Acad Sci U S A 2005; 102:662-6; PMID:15647355; http://dx.doi.org/10.1073/pnas.0409082102.
- Rubin H. The logic of the Membrane, Magnesium, Mitosis (MMM) model for the regulation of animal cell proliferation. Arch Biochem Biophys 2007; 458:16-23; PMID:16750508; http://dx.doi.org/10.1016/j.abb.2006.03.026.
- Rubin H. Magnesium: The missing element in molecular views of cell proliferation control. Bioessays 2005; 27:311-20; PMID:15714553; http://dx.doi.org/10.1002/bies.20183.
- Jin J, Wu LJ, Jun J, Cheng X, Xu H, Andrews NC, Clapham DE. The channel kinase, TRPM7, is required for early embryonic development. Proc Natl Acad Sci U S A 2012; 109:E225-233; PMID:22203997; http://dx.doi.org/10.1073/pnas.1120033109.
- Palacios-Prado N, Chapuis S, Panjkovich A, Fregeac J, Nagy JI, Bukauskas FF. Molecular determinants of magnesium-dependent synaptic plasticity at electrical synapses formed by connexin36. Nat Commun 2014; 5:4667; PMID:25135336; http://dx.doi.org/10.1038/ncomms5667.
- Vink R, Cook NL, van den Heuvel C. Magnesium in acute and chronic brain injury: an update. Magnes Res 2009; 22:158S-62S; PMID:19780402.
- Levitsky DO, Takahashi M. Interplay of Ca(2+) and Mg (2+) in sodium-calcium exchanger and in other Ca(2+)-binding proteins: magnesium, watchdog that blocks each turn if able. Adv Exp Med Biol 2013; 961:65-78; PMID:23224871; http://dx.doi.org/10.1007/978-1-4614-4756-6_7.
- Callera GE, He Y, Yogi A, Montezano AC, Paravicini T, Yao G, Touyz RM. Regulation of the novel Mg2+ transporter transient receptor potential melastatin 7 (TRPM7) cation channel by bradykinin in vascular smooth muscle cells. J Hypertens 2009; 27:155-66; PMID:19145781; http://dx.doi.org/10.1097/HJH.0b013e3283190582.
- Selvaraj S, Sun Y, Singh BB. TRPC channels and their implication in neurological diseases. CNS Neurol Disord Drug Targets 2010; 9:94-104; PMID:20201820; http://dx.doi.org/10.2174/187152710790966650.
- Yamamoto S, Wajima T, Hara Y, Nishida M, Mori Y. Transient receptor potential channels in Alzheimer disease. Biochim Biophys Acta 2007; 1772:958-67; PMID:17490865; http://dx.doi.org/10.1016/j.bbadis.2007.03.006.
- Simon F, Varela D, Cabello-Verrugio C. Oxidative stress-modulated TRPM ion channels in cell dysfunction and pathological conditions in humans. Cell Signal 2013; 25:1614-24; PMID:23602937; http://dx.doi.org/10.1016/j.cellsig.2013.03.023.
- Reboreda A, Jimenez-Diaz L, Navarro-Lopez JD. TRP channels and neural persistent activity. Adv Exp Med Biol 2011; 704:595-613; PMID:21290318; http://dx.doi.org/10.1007/978-94-007-0265-3_32.
- Pan Z, Yang H, Reinach PS. Transient receptor potential (TRP) gene superfamily encoding cation channels. Hum Genomics 2011; 5:108-16; PMID:21296744; http://dx.doi.org/10.1186/1479-7364-5-2-108.
- Sukumaran P, Lof C, Kemppainen K, Kankaanpaa P, Pulli I, Nasman J, Viitanen T, Tornquist K. Canonical transient receptor potential channel 2 (TRPC2) as a major regulator of calcium homeostasis in rat thyroid FRTL-5 cells: importance of protein kinase C delta (PKCdelta) and stromal interaction molecule 2 (STIM2). J Biol Chem 2012; 287:44345-60; PMID:23144458; http://dx.doi.org/10.1074/jbc.M112.374348.
- Liman ER. Trpm5. Handb Exp Pharmacol 2014; 222:489-502; PMID:24756718; http://dx.doi.org/10.1007/978-3-642-54215-2_19.
- Mathar I, Jacobs G, Kecskes M, Menigoz A, Philippaert K, Vennekens R. Trpm4. Handb Exp Pharmacol 2014; 222:461-87; PMID:24756717; http://dx.doi.org/10.1007/978-3-642-54215-2_18.
- Chubanov V, Gudermann T. Trpm6. Handb Exp Pharmacol 2014; 222:503-20; PMID:24756719; http://dx.doi.org/10.1007/978-3-642-54215-2_20.
- Fleig A, Chubanov V. Trpm7. Handb Exp Pharmacol 2014; 222:521-46; PMID:24756720; http://dx.doi.org/10.1007/978-3-642-54215-2_21.
- Irie S, Furukawa T. Trpm1. Handb Exp Pharmacol 2014; 222:387-402; PMID:24756714; http://dx.doi.org/10.1007/978-3-642-54215-2_15.
- Oancea E, Wicks NL. TRPM1: new trends for an old TRP. Adv Exp Med Biol 2011; 704:135-45; PMID:21290293; http://dx.doi.org/10.1007/978-94-007-0265-3_7.
- Xie YF, Macdonald JF, Jackson MF. TRPM2, calcium and neurodegenerative diseases. Int J Physiol Pathophysiol Pharmacol 2010; 2:95-103; PMID:21383889.
- Fonfria E, Mattei C, Hill K, Brown JT, Randall A, Benham CD, Skaper SD, Campbell CA, Crook B, Murdock PR, et al. TRPM2 is elevated in the tMCAO stroke model, transcriptionally regulated, and functionally expressed in C13 microglia. J Recept Signal Transduct Res 2006; 26:179-98; PMID:16777714; http://dx.doi.org/10.1080/10799890600637522.
- Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 2004; 95:922-9; PMID:15472118; http://dx.doi.org/10.1161/01.RES.0000147311.54833.03.
- Benemei S, Patacchini R, Trevisani M, Geppetti P. TRP channels. Curr Opin Pharmacol 2015; 22C:18-23; http://dx.doi.org/10.1016/j.coph.2015.02.006.
- Runnels LW. TRPM6 and TRPM7: A Mul-TRP-PLIK-cation of channel functions. Curr Pharm Biotechnol 2011; 12:42-53; PMID:20932259; http://dx.doi.org/10.2174/138920111793937880.
- Yamaguchi H, Matsushita M, Nairn AC, Kuriyan J. Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol Cell 2001; 7:1047-57; PMID:11389851; http://dx.doi.org/10.1016/S1097-2765(01)00256-8.
- Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 2001; 411:590-5; PMID:11385574; http://dx.doi.org/10.1038/35079092.
- Schmitz C, Dorovkov MV, Zhao X, Davenport BJ, Ryazanov AG, Perraud AL. The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J Biol Chem 2005; 280:37763-71; PMID:16150690; http://dx.doi.org/10.1074/jbc.M509175200.
- Matsushita M, Kozak JA, Shimizu Y, McLachlin DT, Yamaguchi H, Wei FY, Tomizawa K, Matsui H, Chait BT, Cahalan MD, et al. Channel function is dissociated from the intrinsic kinase activity and autophosphorylation of TRPM7/ChaK1. J Biol Chem 2005; 280:20793-803; PMID:15781465; http://dx.doi.org/10.1074/jbc.M413671200.
- Dorovkov MV, Ryazanov AG. Phosphorylation of annexin I by TRPM7 channel-kinase. J Biol Chem 2004; 279:50643-6; PMID:15485879; http://dx.doi.org/10.1074/jbc.C400441200.
- Dorovkov MV, Kostyukova AS, Ryazanov AG. Phosphorylation of annexin A1 by TRPM7 kinase: a switch regulating the induction of an α-helix. Biochemistry 2011; 50:2187-93; PMID:21280599; http://dx.doi.org/10.1021/bi101963h.
- Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J 2006; 25:290-301; PMID:16407977; http://dx.doi.org/10.1038/sj.emboj.7600931.
- Paravicini TM, Chubanov V, Gudermann T. TRPM7: a unique channel involved in magnesium homeostasis. Int J Biochem Cell Biol 2012; 44:1381-4; PMID:22634382; http://dx.doi.org/10.1016/j.biocel.2012.05.010.
- Brandao K, Deason-Towne F, Zhao X, Perraud AL, Schmitz C. TRPM6 kinase activity regulates TRPM7 trafficking and inhibits cellular growth under hypomagnesic conditions. Cell Mol Life Sci 2014; 71:4853-67; PMID:24858416; http://dx.doi.org/10.1007/s00018-014-1647-7.
- Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat Cell Biol 2002; 4:329-36; PMID:11941371.
- Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc Natl Acad Sci U S A 2004; 101:6009-14; PMID:15069188; http://dx.doi.org/10.1073/pnas.0307565101.
- Kozak JA, Cahalan MD. MIC channels are inhibited by internal divalent cations but not ATP. Biophys J 2003; 84:922-7; PMID:12547774; http://dx.doi.org/10.1016/S0006-3495(03)74909-1.
- Sun Y, Selvaraj S, Varma A, Derry S, Sahmoun AE, Singh BB. Increase in serum Ca2+/Mg2+ ratio promotes proliferation of prostate cancer cells by activating TRPM7 channels. J Biol Chem 2013; 288:255-63; PMID:23168410; http://dx.doi.org/10.1074/jbc.M112.393918.
- Langeslag M, Clark K, Moolenaar WH, van Leeuwen FN, Jalink K. Activation of TRPM7 channels by phospholipase C-coupled receptor agonists. J Biol Chem 2007; 282:232-9; PMID:17095511; http://dx.doi.org/10.1074/jbc.M605300200.
- Jiang J, Li M, Yue L. Potentiation of TRPM7 inward currents by protons. J Gen Physiol 2005; 126:137-50; PMID:16009728; http://dx.doi.org/10.1085/jgp.200409185.
- Sun Y, Sukumaran P, Varma A, Derry S, Sahmoun AE, Singh BB. Cholesterol-induced activation of TRPM7 regulates cell proliferation, migration, and viability of human prostate cells. Biochim Biophys Acta 2014; 1843:1839-50; PMID:24769209; http://dx.doi.org/10.1016/j.bbamcr.2014.04.019.
- Coombes E, Jiang J, Chu XP, Inoue K, Seeds J, Branigan D, Simon RP, Xiong ZG. Pathophysiologically relevant levels of hydrogen peroxide induce glutamate-independent neurodegeneration that involves activation of transient receptor potential melastatin 7 channels. Antioxid Redox Signal 2011; 14:1815-27; PMID:20812867; http://dx.doi.org/10.1089/ars.2010.3549.
- Desai BN, Krapivinsky G, Navarro B, Krapivinsky L, Carter BC, Febvay S, Delling M, Penumaka A, Ramsey IS, Manasian Y, et al. Cleavage of TRPM7 releases the kinase domain from the ion channel and regulates its participation in Fas-induced apoptosis. Dev Cell 2012; 22:1149-62; PMID:22698280; http://dx.doi.org/10.1016/j.devcel.2012.04.006.
- Krapivinsky G, Krapivinsky L, Manasian Y, Clapham DE. The TRPM7 chanzyme is cleaved to release a chromatin-modifying kinase. Cell 2014; 157:1061-72; PMID:24855944; http://dx.doi.org/10.1016/j.cell.2014.03.046.
- Kaitsuka T, Katagiri C, Beesetty P, Nakamura K, Hourani S, Tomizawa K, Kozak JA, Matsushita M. Inactivation of TRPM7 kinase activity does not impair its channel function in mice. Sci Rep 2014; 4:5718; PMID:25030553; http://dx.doi.org/10.1038/srep05718.
- Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics 2006; 7:159; PMID:16787531; http://dx.doi.org/10.1186/1471-2164-7-159.
- Chen HC, Su LT, Gonzalez-Pagan O, Overton JD, Runnels LW. A key role for Mg(2+) in TRPM7s control of ROS levels during cell stress. Biochem J 2012; 445:441-8; PMID:22587440; http://dx.doi.org/10.1042/BJ20120248.
- Brauchi S, Krapivinsky G, Krapivinsky L, Clapham DE. TRPM7 facilitates cholinergic vesicle fusion with the plasma membrane. Proc Natl Acad Sci U S A 2008; 105:8304-8; PMID:18539771; http://dx.doi.org/10.1073/pnas.0800881105.
- Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron 2006; 52:485-96; PMID:17088214; http://dx.doi.org/10.1016/j.neuron.2006.09.033.
- Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 2008; 322:756-60; PMID:18974357; http://dx.doi.org/10.1126/science.1163493.
- Deason-Towne F, Perraud AL, Schmitz C. The Mg2+ transporter MagT1 partially rescues cell growth and Mg2+ uptake in cells lacking the channel-kinase TRPM7. FEBS Lett 2011; 585:2275-8; PMID:21627970; http://dx.doi.org/10.1016/j.febslet.2011.05.052.
- Clark K, Middelbeek J, Lasonder E, Dulyaninova NG, Morrice NA, Ryazanov AG, Bresnick AR, Figdor CG, van Leeuwen FN. TRPM7 regulates myosin IIA filament stability and protein localization by heavy chain phosphorylation. J Mol Biol 2008; 378:790-803; PMID:18394644; http://dx.doi.org/10.1016/j.jmb.2008.02.057.
- Hanano T, Hara Y, Shi J, Morita H, Umebayashi C, Mori E, Sumimoto H, Ito Y, Mori Y, Inoue R. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J Pharmacol Sci 2004; 95:403-19; PMID:15286426; http://dx.doi.org/10.1254/jphs.FP0040273.
- Turlova E, Bae CY, Deurloo M, Chen W, Barszczyk A, Horgen FD, Fleig A, Feng ZP, Sun HS. TRPM7 Regulates Axonal Outgrowth and Maturation of Primary Hippocampal Neurons. Mol Neurobiol 2014; PMID:25502295; http://dx.doi.org/10.1007/s12035-014-9032-y
- Jaskova K, Pavlovicova M, Jurkovicova D. Calcium transporters and their role in the development of neuronal disease and neuronal damage. Gen Physiol Biophys 2012; 31:375-82; PMID:23255663; http://dx.doi.org/10.4149/gpb_2012_053.
- Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol 1997; 82:291-5; PMID:9129943; http://dx.doi.org/10.1113/expphysiol.1997.sp004024.
- Behl C. Amyloid β-protein toxicity and oxidative stress in Alzheimer disease. Cell Tissue Res 1997; 290:471-80; PMID:9369525; http://dx.doi.org/10.1007/s004410050955.
- Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med 2013; 62:90-101; PMID:23200807; http://dx.doi.org/10.1016/j.freeradbiomed.2012.11.014.
- Nunomura A, Moreira PI, Lee HG, Zhu X, Castellani RJ, Smith MA, Perry G. Neuronal death and survival under oxidative stress in Alzheimer and Parkinson diseases. CNS Neurol Disord Drug Targets 2007; 6:411-23; PMID:18220780; http://dx.doi.org/10.2174/187152707783399201.
- Ureshino RP, Rocha KK, Lopes GS, Bincoletto C, Smaili SS. Calcium signaling alterations, oxidative stress, and autophagy in aging. Antioxid Redox Signal 2014; 21:123-37; PMID:24512092; http://dx.doi.org/10.1089/ars.2013.5777.
- Barsukova AG, Bourdette D, Forte M. Mitochondrial calcium and its regulation in neurodegeneration induced by oxidative stress. Eur J Neurosci 2011; 34:437-47; PMID:21722208; http://dx.doi.org/10.1111/j.1460-9568.2011.07760.x.
- Ermak G, Davies KJ. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol 2002; 38:713-21; PMID:11841831; http://dx.doi.org/10.1016/S0161-5890(01)00108-0.
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell 2003; 115:863-77; PMID:14697204; http://dx.doi.org/10.1016/S0092-8674(03)01017-1.
- Park L, Wang G, Moore J, Girouard H, Zhou P, Anrather J, Iadecola C. The key role of transient receptor potential melastatin-2 channels in amyloid-β-induced neurovascular dysfunction. Nat Commun 2014; 5:5318; PMID:25351853; http://dx.doi.org/10.1038/ncomms6318.
- Landman N, Jeong SY, Shin SY, Voronov SV, Serban G, Kang MS, Park MK, Di Paolo G, Chung S, Kim TW. Presenilin mutations linked to familial Alzheimer disease cause an imbalance in phosphatidylinositol 4,5-bisphosphate metabolism. Proc Natl Acad Sci U S A 2006; 103:19524-9; PMID:17158800; http://dx.doi.org/10.1073/pnas.0604954103.
- Oh HG, Chun YS, Kim Y, Youn SH, Shin S, Park MK, Kim TW, Chung S. Modulation of transient receptor potential melastatin related 7 channel by presenilins. Dev Neurobiol 2012; 72:865-77; PMID:22102510; http://dx.doi.org/10.1002/dneu.22001.
- Andrasi E, Igaz S, Molnar Z, Mako S. Disturbances of magnesium concentrations in various brain areas in Alzheimer disease. Magnes Res 2000; 13:189-96; PMID:11008926.
- Starling AJ, Andre VM, Cepeda C, de Lima M, Chandler SH, Levine MS. Alterations in N-methyl-D-aspartate receptor sensitivity and magnesium blockade occur early in development in the R6/2 mouse model of Huntington's disease. J Neurosci Res 2005; 82:377-86; PMID:16211559; http://dx.doi.org/10.1002/jnr.20651.
- Iotti S, Malucelli E. In vivo assessment of Mg2+ in human brain and skeletal muscle by 31P-MRS. Magnes Res 2008; 21:157-62; PMID:19009818.
- Muroyama A, Inaka M, Matsushima H, Sugino H, Marunaka Y, Mitsumoto Y. Enhanced susceptibility to MPTP neurotoxicity in magnesium-deficient C57BL/6N mice. Neurosci Res 2009; 63:72-5; PMID:18977253; http://dx.doi.org/10.1016/j.neures.2008.09.009.
- Hashimoto T, Nishi K, Nagasao J, Tsuji S, Oyanagi K. Magnesium exerts both preventive and ameliorating effects in an in vitro rat Parkinson disease model involving 1-methyl-4-phenylpyridinium (MPP+) toxicity in dopaminergic neurons. Brain Res 2008; 1197:143-51; PMID:18242592.
- Oyanagi K, Kawakami E, Kikuchi-Horie K, Ohara K, Ogata K, Takahama S, Wada M, Kihira T, Yasui M. Magnesium deficiency over generations in rats with special references to the pathogenesis of the Parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Neuropathology 2006; 26:115-28; PMID:16708544; http://dx.doi.org/10.1111/j.1440-1789.2006.00672.x.
- Decker AR, McNeill MS, Lambert AM, Overton JD, Chen YC, Lorca RA, Johnson NA, Brockerhoff SE, Mohapatra DP, MacArthur H, et al. Abnormal differentiation of dopaminergic neurons in zebrafish trpm7 mutant larvae impairs development of the motor pattern. Dev Biol 2014; 386:428-39; PMID:24291744; http://dx.doi.org/10.1016/j.ydbio.2013.11.015.
- Cook NL, Heuvel Cvd, Vink R. Characterisation of TRPM channel mRNA levels in Parkinson disease. In: The 12th International Magnesium Symposium. Magnesium Research 2009; 22(3):188-9.
- Shaw PJ. Molecular and cellular pathways of neurodegeneration in motor neurone disease. J Neurol Neurosurg Psychiatry 2005; 76:1046-57; PMID:16024877; http://dx.doi.org/10.1136/jnnp.2004.048652.
- Gajdusek DC, Salazar AM. Amyotrophic lateral sclerosis and parkinsonian syndromes in high incidence among the Auyu and Jakai people of West New Guinea. Neurology 1982; 32:107-26; PMID:7198738; http://dx.doi.org/10.1212/WNL.32.2.107
- Hirano A, Malamud N, Kurland LT. Parkinsonism-dementia complex, an endemic disease on the island of Guam. II. Pathological features. Brain 1961; 84:662-79; PMID:13907610; http://dx.doi.org/10.1093/brain/84.4.662.
- Kimura K. [Studies on amyotrophic lateral sclerosis. I. Epidemiological, geomedical and genetic studies on amyotrophic lateral sclerosis and its allied diseases in Kii Peninsula, Japan]. Seishin Shinkeigaku zasshi 1963; 65:31-8.
- Garruto RM. A commentary on neuronal degeneration and cell death in Guam ALS and PD: an evolutionary process of understanding. Curr Alzheimer Res 2006; 3:397-401; PMID:17017870; http://dx.doi.org/10.2174/156720506778249425.
- Hermosura MC, Garruto RM. TRPM7 and TRPM2-Candidate susceptibility genes for Western Pacific ALS and PD? Biochim Biophys Acta 2007; 1772:822-35; PMID:17395433; http://dx.doi.org/10.1016/j.bbadis.2007.02.008.
- Yase Y. The pathogenesis of amyotrophic lateral sclerosis. Lancet 1972; 2:292-6; PMID:4115029; http://dx.doi.org/10.1016/S0140-6736(72)92903-0.
- Yanagihara R, Garruto RM, Gajdusek DC, Tomita A, Uchikawa T, Konagaya Y, Chen KM, Sobue I, Plato CC, Gibbs CJ, Jr. Calcium and vitamin D metabolism in Guamanian Chamorros with amyotrophic lateral sclerosis and parkinsonism-dementia. Ann Neurol 1984; 15:42-8; PMID:6546847; http://dx.doi.org/10.1002/ana.410150108.
- Ozoguz A, Uyan O, Birdal G, Iskender C, Kartal E, Lahut S, Omur O, Agim ZS, Eken AG, Sen NE, et al. The distinct genetic pattern of ALS in Turkey and novel mutations. Neurobiol Aging 2015; 36:1764.e9-18.
- Hara K, Kokubo Y, Ishiura H, Fukuda Y, Miyashita A, Kuwano R, Sasaki R, Goto J, Nishizawa M, Kuzuhara S, Tsuji S. TRPM7 is not associated with amyotrophic lateral sclerosis-parkinsonism dementia complex in the Kii peninsula of Japan. Am J Med Genet Part B Neuropsychiatric Genet 2010; 153B:310-3; PMID:19405049.
- Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci 2000; 20:2534-42; PMID:10729333.
- Parnas M, Peters M, Dadon D, Lev S, Vertkin I, Slutsky I, Minke B. Carvacrol is a novel inhibitor of Drosophila TRPL and mammalian TRPM7 channels. Cell Calcium 2009; 45:300-9; PMID:19135721; http://dx.doi.org/10.1016/j.ceca.2008.11.009.
- Yu H, Zhang ZL, Chen J, Pei A, Hua F, Qian X, He J, Liu CF, Xu X. Carvacrol, a food-additive, provides neuroprotection on focal cerebral ischemia/reperfusion injury in mice. PloS one 2012; 7:e33584; PMID:22438954; http://dx.doi.org/10.1371/journal.pone.0033584.
- Chen W, Xu B, Xiao A, Liu L, Fang X, Liu R, Turlova E, Barszczyk A, Zhong X, Sun CL, Britto LR, Feng ZP, Sun HS. TRPM7 inhibitor carvacrol protects brain from neonatal hypoxic-ischemic injury. Mol Brain 2015; 8:11; PMID:25761704; http://dx.doi.org/10.1186/s13041-015-0102-5.
- Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, Kiyonaka S, Mori Y, Jones M, Forder JP, et al. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci 2009; 12:1300-7; PMID:19734892; http://dx.doi.org/10.1038/nn.2395.
- Chen W, Xu B, Xiao A, Liu L, Fang X, Liu R, Turlova E, Barszczyk A, Zhong X, Sun CL, et al. TRPM7 inhibitor carvacrol protects brain from neonatal hypoxic-ischemic injury. Mol Brain 2015; 8:11; PMID:25761704; http://dx.doi.org/10.1186/s13041-015-0102-5.
- Bae CY, Sun HS. TRPM7 in cerebral ischemia and potential target for drug development in stroke. Acta Pharmacol Sin 2011; 32:725-33; PMID:21552293; http://dx.doi.org/10.1038/aps.2011.60.
- Deason-Towne F, Perraud AL, Schmitz C. Identification of Ser/Thr phosphorylation sites in the C2-domain of phospholipase C gamma2 (PLCgamma2) using TRPM7-kinase. Cell Signal 2012; 24:2070-5; PMID:22759789; http://dx.doi.org/10.1016/j.cellsig.2012.06.015.
- Zambrzycka A, Strosznajder RP, Strosznajder JB. Aggregated β amyloid peptide 1-40 decreases Ca2+- and cholinergic receptor-mediated phosphoinositide degradation by alteration of membrane and cytosolic phospholipase C in brain cortex. Neurochem Res 2000; 25:189-96; PMID:10786701; http://dx.doi.org/10.1023/A:1007511217525.
- Ma T, Chen Y, Vingtdeux V, Zhao H, Viollet B, Marambaud P, Klann E. Inhibition of AMP-activated protein kinase signaling alleviates impairments in hippocampal synaptic plasticity induced by amyloid β. J Neurosci 2014; 34:12230-8; PMID:25186765; http://dx.doi.org/10.1523/JNEUROSCI.1694-14.2014.
- Li X, Alafuzoff I, Soininen H, Winblad B, Pei JJ. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer disease brain. FEBS J 2005; 272:4211-20; PMID:16098202; http://dx.doi.org/10.1111/j.1742-4658.2005.04833.x.
- Perraud AL, Zhao X, Ryazanov AG, Schmitz C. The channel-kinase TRPM7 regulates phosphorylation of the translational factor eEF2 via eEF2-k. Cell Signal 2011; 23:586-93; PMID:21112387; http://dx.doi.org/10.1016/j.cellsig.2010.11.011.
- Erten-Lyons D, Jacobson A, Kramer P, Grupe A, Kaye J. The FAS gene, brain volume, and disease progression in Alzheimer disease. Alzheimer Dement 2010; 6:118-24; http://dx.doi.org/10.1016/j.jalz.2009.05.663.
- Cheng XL, Li MK. Effect of topiramate on apoptosis-related protein expression of hippocampus in model rats with Alzheimers disease. Eur Rev Med Pharmacol Sci 2014; 18:761-8; PMID:24706297.
- Macchi B, Di Paola R, Marino-Merlo F, Felice MR, Cuzzocrea S, Mastino A. Inflammatory and cell death pathways in brain and peripheral blood in Parkinson disease. CNS Neurol Disord Drug Targets 2015; 14:313-24; PMID:25714978; http://dx.doi.org/10.2174/1871527314666150225124928.
- Aarts MM, Tymianski M. TRPMs and neuronal cell death. Pflugers Archiv 2005; 451:243-9; PMID:16044308; http://dx.doi.org/10.1007/s00424-005-1439-x.
- Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham DE. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal 2009; 2:ra21; PMID:19436059.
- Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem 2003; 278:21493-501; PMID:12672799; http://dx.doi.org/10.1074/jbc.M300945200.
- Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol 2006; 127:525-537; PMID:16636202; http://dx.doi.org/10.1085/jgp.200609502.
- Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol 2003; 121:49-60; PMID:12508053; http://dx.doi.org/10.1085/jgp.20028740.
- Heiner I, Eisfeld J, Halaszovich CR, Wehage E, Jungling E, Zitt C, Luckhoff A. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem J 2003; 371:1045-53; PMID:12564954; http://dx.doi.org/10.1042/BJ20021975.
- Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 2001; 411:595-9; PMID:11385575; http://dx.doi.org/10.1038/35079100.
- Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science 2001; 293:1327-30; PMID:11509734; http://dx.doi.org/10.1126/science.1062473.
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002; 416:52-8; PMID:11882888; http://dx.doi.org/10.1038/nature719.
- Hui K, Guo Y, Feng ZP. Biophysical properties of menthol-activated cold receptor TRPM8 channels. Biochem Biophys Res Commun 2005; 333:374-82; PMID:15950184; http://dx.doi.org/10.1016/j.bbrc.2005.05.123.
- Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet JP. TRPM4 regulates calcium oscillations after T cell activation. Science 2004; 306:1374-7; PMID:15550671; http://dx.doi.org/10.1126/science.1098845.
- Nilius B, Prenen J, Janssens A, Owsianik G, Wang C, Zhu MX, Voets T. The selectivity filter of the cation channel TRPM4. J Biol Chem 2005; 280:22899-906; PMID:15845551; http://dx.doi.org/10.1074/jbc.M501686200.
- Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr Biol 2003; 13:1153-8; PMID:12842017; http://dx.doi.org/10.1016/S0960-9822(03)00431-7.