In photoreceptor (PR) synaptic terminals, Cav1.4 voltage-gated Ca2+ channels mediate Ca2+ signals required for the release of glutamate at the depolarized PR membrane potential in darkness. Light-dependent hyperpolarization causes closure of Cav1.4 channels, which depolarizes ON bipolar neurons through relief of glutamatergic inhibition of a non-selective cation conductance. Mutations in the CACNA1F gene encoding Cav1.4 cause vision disorders including X-linked incomplete stationary night blindness.Citation1 Most of these mutations are expected to cause a loss of channel function, i.e., reduced Ca2+ influx in darkness. However, one mutation (I745T) causes a large negative shift in the voltage-dependence of activation.Citation2 In mice bearing the mutation (Cav1.4-IT), presynaptic Ca2+ influx is elevated in PRs.Citation3 However, PR synapses are abnormal and there is significant degeneration of cones.Citation3,4 How these defects may lead to the visual phenotypes seen in human carriers of the I745T mutation is unknown.
Knoflach et al. attempt to fill this gap by analyzing the signals retina sends to the brain in Cav1.4-IT mice.Citation5 The authors use multi-electrode arrays (MEAs) to record the responses of the ganglion cells (GCs) to light-dark changes in the retina. Unlike in wild-type (WT) mice, ˜20% of the GCs in Cav1.4-IT mice showed a higher firing rate in darkness and took 3 times longer to respond to light than in WT retina. In addition, only ~60% (compared to 99% in wild-type) of PRs responded to light. Why so many GCs do not respond to light in Cav1.4-IT mice is not fully clear, but could be due PR degeneration and/or the defects in PR synapse structure that are prevalent in these mice.Citation3-6 Contrast sensitivity was also reduced in Cav1.4-IT mice. The mechanism could involve impaired input from PRs to horizontal cells that mediate inhibitory surround of bipolar cells, which is essential for contrast enhancement. Interestingly, decreased visual acuity associated with contrast detection has been reported in patients with CACNA1F mutations.Citation7
The findings of Knoflach et al. help explain how gain-of function in Cav1.4 may lead to the visual defects associated with the I745T mutation in humans (). Because of their negative activation range, Cav1.4-IT channels would exhibit increased activity in darkness and close less efficiently during light stimuli compared to WT channels. This would decrease the sensitivity and dynamic range of the PR response to light. Since they sum the input from a large number of PRs, GCs would exhibit delayed changes in firing rate, and overall reduced responses to light. Decreased contrast sensitivity and lower temporal resolution of GCs may contribute to the poor visual acuity reportedly due to the I745T mutation in humans, which is more severe than with other CACNA1F mutations.Citation8 Defects in PR synapse morphology and molecular composition may produce variable PR responses to uniform illumination, which could also contribute to the abnormal GC response in Cav1.4-IT mice. Potentially, aberrant firing of GCs may also explain the nystagmus and strabismus phenotypes in these patients: in an attempt to improve the distorted visual signal coming from the GCs, the brain compensates with the pathological eye movements. However, confirmation of the underlying mechanism requires further study.
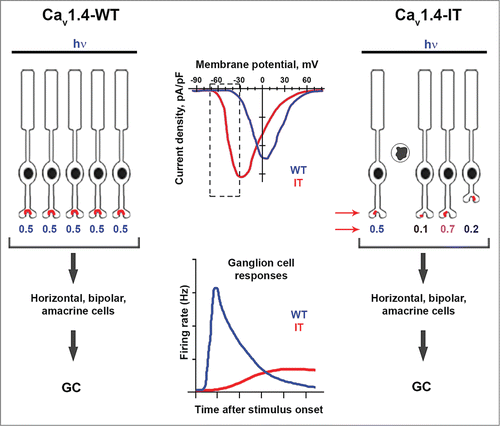
Figure 1. Retinal organization and ganglion cell (GC) signaling in wild-type (Cav1.4-WT) and Cav1.4-IT mice. Compared to WT channels, Cav1.4-IT channels activate at more negative voltages, causing greater Ca2+ influx within the physiological range of PR membrane voltages (dotted rectangle, top graph). In Cav1.4-WT retina (left), temporally precise and uniform PR responses to light rely on normal synapse structure and proper kinetics of channel activation and deactivation upon dark-light transitions (hypothetical numerical values for PR responses are shown). The result is a rapid and strong increase in GC firing in response to light stimuli (lower graph). In Cav1.4-IT retina, PR degeneration, disrupted PR synapses, and retraction of axons into the outer nuclear layer (arrows) leads to variable PR signaling. As a consequence, GCs respond to a light stimulus more sluggishly and less robustly compared to in Cav1.4-WT retina.
An important unanswered question is how the heightened Ca2+ signaling of Cav1.4-IT channels causes the morphological abnormalities in PR synapses. These include spherical rather than elongated ribbons, sprouting of processes from bipolar cells and horizontal cells, and mislocalization of pre- and post-synaptic signaling molecules.Citation3-6 These defects may result from excessive Ca2+ influx causing PR degeneration in Cav1.4-IT mice that is even more severe than in mice completely lacking Cav1.4 channels.Citation3 Alternatively, dysregulated Ca2+ signaling in Cav1.4-IT mice could lead to local disassembly of PR synapses in advance of degenerative influences. Further studies of Cav1.4-IT mice should be informative in this regard.
References
- Bech-Hansen NT, et al. Nature Genet. 1998 19: 264-67; http://dx.doi.org/10.1038/947
- Hemara-Wahanui A, et al. Proc Natl Acad Sci U S A 2005 102: 7553-58; PMID:15897456; http://dx.doi.org/10.1073/pnas.0501907102
- Regus-Leidig H, et al. PLoS One 2014 9: e86769; PMID:24466230; http://dx.doi.org/10.1371/journal.pone.0086769
- Knoflach D, et al. Channels 2013 7: 503-13; PMID:24051672; http://dx.doi.org/10.4161/chan.26368
- Knoflach D, et al. Channels 2015 9(5):298–306; PMID:26274509; http://dx.doi.org/10.1080/19336950.2015.1078040
- Liu X, et al. Channels 2013 7: 514-23; PMID:24064553; http://dx.doi.org/10.4161/chan.26376
- Bijveld MM, et al. Ophthalmology 2013 120: 2072-81; PMID:23714322; http://dx.doi.org/10.1016/j.ophtha.2013.03.002
- Hope CI, et al. Clinical & experimental ophthalmology 2005 33: 129-36; PMID:15807819; http://dx.doi.org/10.1111/j.1442-9071.2005.00987.x
