Protein oligomerization is a biological relevant event that may provide functional advantages to biological systems.Citation1 The association of aquaporin (AQP) protomers to form hetero-oligomeric assemblies is a current challenging area of research. PIP1 and PIP2, members of the plant AQP subfamily named PIP (for plant plasma membrane intrinsic proteins), have been intensively studied in the recent years particularly due to their ability to hetero-oligomerize.
All AQPs share a common tetrameric structure, where each protomer has 6 membrane-spanning α helices (from 1 to 6) connected by 5 loops (from A to D), and loops B and E fold as half helices inserted in the membrane to form an individual pore; in this way each AQP protomer in the tetramer has a single active pore.Citation2 Members of PIP1 and PIP2 subgroups not only have the conserved tridimensional structure of the AQP family but also display a high degree of amino acid similarity between them. However, while most PIP1 protein is retained in the endoplasmic reticulum unless co-expressed with PIP2, PIP2 is effectively transported to the plasma membrane.Citation3-6 The routing modification of PIP1 from the endoplasmatic reticulum to the plasma membrane after its co-expression with PIP2 is due to PIP1-PIP2 physical interactionCitation3,5 and although this interaction is well supported by experimental evidences, the biophysical aspects and the molecular mechanisms of this event have been identified only recently.Citation7,8
We investigated the full characterization of the biological and biophysical properties of the different hetero-oligomeric configurations formed by red beet PIP1 and PIP2 subunits. Our results showed that PIP1 and PIP2 protomers have the ability to assemble with multiple stoichiometries, giving rise to 3:1, 1:3 or 2:2 heterotetramers, and all these tetramers are localized at the plasma membrane.Citation7 A variable stoichiometry has also been proposed for strawberry PIPsCitation4 and, recently, a similar result was confirmed for maize PIPsCitation8. In the case of red beet PIPs, we showed that although all stoichiometric assemblies can be formed, which one prevails depends on PIP1 and PIP2 relative expression. If one PIP monomer (PIP1 or PIP2) outnumbers the other, the assembly of 1:3 (or 3:1) heterotetramers is favored and even homotetramers of PIP1 or PIP2 can be the predominant species in the cell.
It must be stressed that even though the association of PIP protomers occurs with random stoichiometry, their precise interactions are controlled by specific protomer contacts. The absence of these contacts can affect their biological activityCitation8 and even preclude their packing within heterotetramers.Citation6 In this regard, there are many lines of evidence that minor but critical changes in loop A, loop E and transmembrane domains, i.e. single amino acid mutations, are sufficient to produce changes in protomer interaction and, as a consequence of structural rearrangements, modifications in the functional properties of the PIP tetramers occur.
Interestingly, in the case of red beet PIPs, we found that the water permeability behavior of all PIP1-PIP2 heterotetrameric species is the same. Nevertheless, PIP1-PIP2 heterotetramers display major differences compared to homotetramer activity: i- the water transport of heterotetramers is twice the contribution of PIP2 homotetramers, while the contribution of the PIP1 homotetramer is null as they are not able to reach the plasma membrane, ii- all PIP1-PIP2 heterotetrameric species show the same Pf (osmotic water permeability coefficient) inhibition by intracellular pH, but a clear differential regulation in comparison with PIP2 homotetramers, as the closure of PIP2 homotetramers occurs at more acidic values. The only characteristic shared between PIP1-PIP2 heterotetramers and PIP2 homotetramers is the degree of cooperativity for proton sensing: all red beet PIP tetramers display not only a positive cooperativity response, but also a sigmoidal behavior for pH induced Pf block with a similar extent of cooperativity ().
Figure 1. Biological properties of all possible PIP stoichiometric tetramers. Arrows represent the expression level of PIP1/PIP2; θ, PIP tetramer stoichiometry; Ω (10−4 cm s−1 ng−1), Pf contribution of each tetrameric species at the plasma membrane; pH0.5, pH at which the Pf change is half maximal; n, Hill coefficient; and α, cellular localization of tetramers. PM corresponds to plasma membrane and IN indicates intracellular localization.
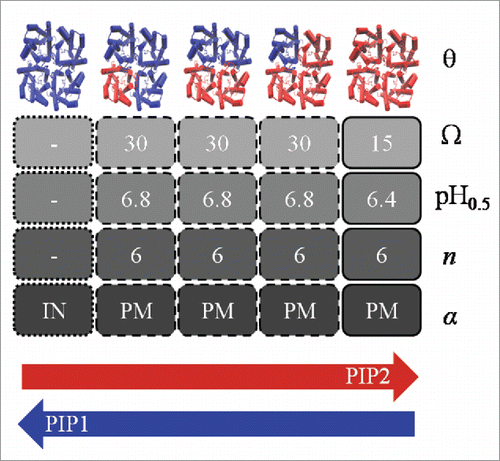
The results obtained for PIP1 and PIP2 interactions show that the mixture in plasma membrane of different amounts of PIP2 homotetramers and/or PIP1-PIP2 heterotetramers allow variable water permeability and different apparent cooperativity for the opening and closing of these channels. Remarkably, only 2 PIP aquaporins can give rise to a wide spectrum of water transport modes. In this way, the membrane water permeability can be modulated by both the relative amounts of each kind of PIP tetramer confined in the membrane and the intracellular proton concentration. These findings represent a novel regulatory mechanism to adjust water transport across the plant plasma membrane. Moreover it confirms that the biophysical studies of intersubunit protein interactions may shed light on how quaternary structures endow the protomers with specific biological properties in oligomeric channels.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grant from Universidad de Buenos Aires to K.A.: UBACYT grant no. 0159-2013.
References
- Griffin MD, Gerrard JA. The relationship between oligomeric state and protein function. Adv Exp Med Biol 2012;747:74-90; PMID:22949112; http://dx.doi.org/10.1007/978-1-4614-3229-6_5
- Kosinska Eriksson U, Fischer G, Friemann R, Enkavi G, Tajkhorshid E, Neutze R. Subangstrom Resolution X-Ray Structure Details Aquaporin-Water Interactions. Science 2013; 340:1346-9; PMID:23766328; http://dx.doi.org/10.1126/science.1234306
- Fetter K, Van Wilder V, Moshelion M, Chaumont F. Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 2004; 16:215-28PMID:14671024; http://dx.doi.org/10.1105/tpc.017194
- Yaneff A, Sigaut L, Marquez M, Alleva K, Pietrasanta LI, Amodeo G. Heteromerization of PIP aquaporins affects their intrinsic permeability. Proc Natl Acad Sci U S A 2014; 111:231-6; PMID:24367080; http://dx.doi.org/10.1073/pnas.1316537111
- Zelazny E, Borst JW, Muylaert M, Batoko H, Hemminga MA, Chaumont F. FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proc Natl Acad Sci U S A 2007; 104:12359-64; PMID:17636130; http://dx.doi.org/10.1073/pnas.0701180104
- Jozefkowicz C, Rosi P, Sigaut L, Soto G, Pietrasanta LI, Amodeo G, Alleva K. Loop A is critical for the functional interaction of two Beta vulgaris PIP aquaporins. PLoS One 2013; 8:e57993; PMID:23483963; http://dx.doi.org/10.1371/journal.pone.0057993
- Jozefkowicz C, Sigaut L, Scochera F, Soto G, Ayub N, Pietrasanta LI, Amodeo G, González Flecha FL, Alleva K. PIP water transport and its pH dependence are regulated by tetramer stoichiometry. Biophys J 2016; 110:1312-21; PMID:27028641; http://dx.doi.org/10.1016/j.bpj.2016.01.026
- Berny MC, Gilis D, Rooman M, Chaumont F. Single mutations in the transmembrane domains of maize plasma membrane aquaporins affect the activity of the monomers within a heterotetramer. Mol Plant 2016; S1674-2052:30028-4; PMID:27109604; http://dx.doi.org/10.1016/j.molp.2016.04.006.
