The Na+/H+ exchanger isoform 1 (NHE1) is a ubiquitous plasma membrane protein that regulates intracellular pH in isolated cardiomyocytes and in other mammalian cells. The N-terminal domain of approximately 500 amino acids is responsible for removal of one intracellular proton in exchange for one extracellular sodium. The intracellular C-terminal of 315 amino acids regulates the membrane domain and is post translationally modified by protein kinase mediated phosphorylation (). NHE1 is intimately involved in heart disease. It contributes to ischemia reperfusion mediated injury. During ischemia, acid load increases and the resultant increase in NHE1 activity contributes to increased intracellular sodium. This elevated sodium leads to reversal of activity of the Na+/Ca2+ exchanger and results in an increase in intracellular calcium, triggering deleterious pathways that lead to cell damage and death. Elevated NHE1 activity also contributes to cardiac hypertrophy and its inhibition can prevent cardiac hypertrophy.Citation1
Figure 1. Hypothetical model of NHE1 activation in cardiomyocytes by growth factors (GF) or hypertrophic stimuli (HS). Either form of stimulation act through activation of receptor tyrosine kinases which work through multistep pathways to activate a MAPK interactome. This complex may bind through an unknown docking or scaffolding protein (?) to the NHE1 C-terminus and activate NHE1 through phosphorylation.
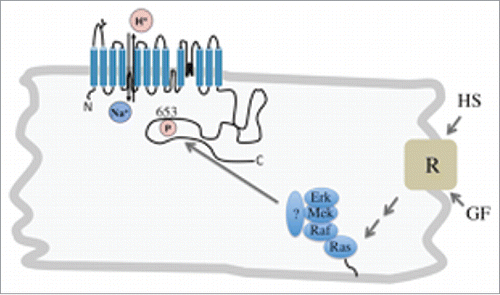
NHE1 is regulated by protein kinase mediated phosphorylation through the mitogen-activated protein kinase (MAPK) signaling pathway.Citation1 This pathway of Ras-Raf-MEK-ERK/MAPK () is conserved and controls a variety of cellular processes including proliferation and metabolism in different cell types. Raf, has three isoforms A-Raf, β−Raf and Raf-12. The Ser/Thr kinase β-Raf, has mutations in very high frequency in melanomas and in lower frequencies in other types of cancer. The V600E mutation is the most prominent and found in most patients with a β-Raf mutation.Citation3 The NHE1 protein shares some of the physiological roles of β-Raf being involved in cellular proliferation and promoting tumourigenesis.Citation4 This led us to examine the potential role of β-Raf in regulation of NHE1 and intracellular pH in malignant melanoma cells with the β-RafV600E mutation. We demonstrated that melanoma cells with the β-RafV600E mutation had elevated resting intracellular pH that was dependent on NHE1. Also, inhibition or knock down of β-Raf decreased NHE1 activity.Citation5 This report confirmed that β-Raf is capable of regulation of NHE1 in malignant melanoma cells, but how does this occur and is it common in other cell types?
In that study we also demonstrated that β-Raf binds to the cytosolic regulatory domain. β-Raf immunoprecipitated with NHE1 in both HeLa and HEK (human embryonic kidney) cells. Another observation was that in a screen for protein kinases from the heart that bind to the NHE1 tail, the strongest signal observed was an interaction between the NHE1-C terminus and β-Raf.Citation5 This suggested to us that there may be a regulatory role for β-Raf in the myocardium. It is notable that β-Raf has also been implicated in cardiac hypertrophy in addition to NHE16. It thus occurred to us that there may be a link between β-Raf and NHE1 that is responsible. Our follow up workCitation7 therefore examined whether β-Raf can regulate NHE1 in myocardial cells. In isolated cardiomyocytes, inhibition or knockdown of β-Raf reduced NHE1 activity, confirming that β-Raf plays a significant role in modulation NHE1 in the myocardium. Cell extracts from isolated cardiomyocytes contained β-Raf that bound to NHE1 and NHE1 was also co-immunoprecipitated with β-Raf, confirming an association of these proteins. In vitro phosphorylation of an expressed and purified NHE1 protein was followed by mass spectrometry. This identified amino acid Thr653 as the residue phosphorylated by β-Raf. Mutation of this residue to Ala reduced NHE1 activity in fibroblast cells confirming that this amino acid can influence NHE1 activity.
What then is the physiological and pathological role of β-Raf binding and its putative phosphorylation of NHE1? We believe that β-Raf binding to NHE1 in the myocardium occurs as part of a regulatory complex that binds to NHE1. This would constitute an interactome (). While we have demonstrated that B-Raf of cell lysates binds to NHE1 in heart cell extracts and other tissues, we have been unable to demonstrate significant binding of pure β-Raf to pure NHE1 C-terminal protein (unpublished observations). This suggests the binding is indirect.
NHE1 has earlier been identified as possessing a MAP kinase interactome that may mediate MAPK signaling.Citation8 Together with our results, this suggests that there is such a regulatory MAPK interactome present in the myocardium regulating NHE1 activity. β-Raf indirectly binds to the NHE1 regulatory tail as part of this complex (). Its binding is mediated through some other unknown protein. As β-Raf has been implicated in cardiac hypertrophy,Citation6 and the activity of NHE1 is also elevated in cardiac hypertrophy,Citation1 it seems plausible that NHE1 activity in hypertrophy is being stimulated through the action of a MAPK protein complex with elevated activity of β-Raf. Future experiments should involve characterization of the MAPK interactome of the NHE1 protein, with an emphasis on the changes in the interactome that occur in the disease state.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Odunewu-Aderibigbe A, Fliegel L. IUBMB Life 2014; 66:679-85; PMID:25354428; https://doi.org/10.1002/iub.1323
- Kolch W. Nat Rev Mol Cell Biol 2005; 6:827-37; PMID:16227978; https://doi.org/10.1038/nrm1743
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417:949-54; PMID:12068308; https://doi.org/10.1038/nature00766
- Amith SR, Fong S, Baksh S, Fliegel L. Na+/H+ exchange in the tumour microenvironment: does NHE1 drive breast cancer carcinogenesis? Int J Dev Biol 2015; 59:367-77; PMID:26679950; https://doi.org/10.1387/ijdb.140336lf
- Karki P, Li X, Schrama D, Fliegel L. B-Raf Associates with and Activates the NHE1 Isoform of the Na+/H+ Exchanger. J Biol Chem 2011; 286:13096-105; PMID:21345796; https://doi.org/10.1074/jbc.M110.165134
- Lorenz K, Schmitt JP, Vidal M, Lohse MJ. Cardiac hypertrophy: Targeting Raf/MEK/ERK1/2-signaling. Int J Biochem Cell Biol 2009; 41:2351-5; PMID:19666137; https://doi.org/10.1016/j.biocel.2009.08.002
- Li X, Augustine A, Sun D, Li L, Fliegel L. Activation of the Na+/H+ exchanger in isolated cardiomyocytes through β-Raf dependent pathways. Role of Thr653 of the cytosolic tail. J Mol Cell Cardiol 2016; 99:65-75; PMID:27555478; https://doi.org/10.1016/j.yjmcc.2016.08.014
- Bandyopadhyay S, Chiang C-Y, Srivastava J, Gersten M, White S, Bell R, Kurschner C, Martin CH, Smoot M, Sahasrabudhe S, et al. A human MAP kinase interactome. Nat Methods 2010; 7:801-5; PMID:20936779; https://doi.org/10.1038/nmeth.1506
