ABSTRACT
This study aimed to investigate the expression and function of BKCa channels in the Sphincter of Oddi (SO) in a rabbit model of hypercholesterolemia (HC). New Zealand white rabbits were randomly divided into 2 groups: the control group was fed standard chow (n = 18) whereas the high-cholesterol group was fed cholesterol-enriched chow containing 1.5% cholesterol (n = 18). The serum cholesterol level was significantly greater in the HC groups than in the control group, but there was no significant difference in body weight between the control and HC groups. Although the total protein expression of BKCa α- and β1-subunit was not significantly different between the control and HC groups, the Tyr-phosphorylation of BKCa α-subunit was significantly decreased in the HC group than in the control group. In addition, hypercholesterolemia significantly increased Acetylcholine (ACh)-induced contraction of the SO rings. Pretreatment with 30 μM NS1619, a BKCa channel agonist, significantly reduced ACh-induced contraction of the SO rings in HC rabbits. Moreover, pretreatment with 100 μM Na3OV4, a protein tyrosine phosphatase inhibitor, significantly reduced ACh-induced contraction of the SO rings in HC rabbits, whereas it significantly increased upon pretreating with 10 μM Genistein, a tyrosine kinase inhibitor. Whole-cell patch clamp recordings showed that BKCa current density was significantly lower in SOSMCs from HC group than that from control group. Our findings suggest that hypercholesterolemia-induced downregulation of BKCa channel, and Tyr-phosphorylation of BKCa α-subunit may contribute to the hyperresponsiveness of the SO ring in HC rabbits.
Introduction
Cholelithiasis is a common disease, caused by increased intake of high cholesterol diets, which could seriously damage human health. Many factors have been found to contribute to cholelithiasis formation, while cholestasis is considered to be an important contributing factor for cholelithiasis formation.Citation1 The sphincter of Oddi (SO) plays an important role in preventing the reflux of duodenal fluid, and regulating the bile duct pressure.Citation2 An increase in the bile duct pressure can lead to dysfunction of SO contractility, which is associated with cholestasis.
Hypercholesterolemia (HC) is known to be a risk factor for inducing SO dysfunction and lithogenesis. Szilvassy et al.,Citation3 have reported that hypercholesterolemia impairs the relaxation of the SO in both experimental animals and clinical patients. It has been reported that hypercholesterolemia decreases the contractile amplitude of SO, leading to SO dysfunction (SOD).Citation1 In addition, hypercholesterolemia can induce the accumulation of intracellular free Ca2+ in SO cells in rabbits.Citation4 It is well known that the large conductance Ca2+-activated K+ channel (BKCa) is involved in the excitability of smooth muscle cells (SMCs) for regulating SMC contraction.Citation5 However, the underlying mechanism of the BKCa in hypercholesterolemia-induced dysfunction of SO motility remains unclear.
The BKCa channel regulates cell membrane excitability in a wide variety of cellsCitation6-8 and plays an important role in the regulation of smooth muscle tone.Citation9,10 The BKCa channel is composed of a structural subunit (α-subunit) and a regulatory subunit (β-subunit). The α subunit is the pore-forming subunit that contains the voltage- and Ca2+-sensitive domains, whereas the β-subunit regulates the voltage and Ca2+-sensitivity of the BKCa. α-subunit and β-subunit are assembled via the binding domain in the S0 segmentCitation11,12 It has been established that increasing intracellular Ca2+ concentration ([Ca2+]i) results in activation of BKCa channels to evoke membrane hyperpolarization. Acetylcholine (ACh) can stimulate smooth muscle by increasing [Ca2+]i through binding to muscarinic ACh receptors, including M2 and M3-type G-protein-coupled receptors which influence smooth muscle contraction via distinct signaling pathway.Citation13 It is generally recognized M2 receptors interact with Go and Gi to suppress adenylyl cyclase and Ca2+-activated K+ channels and to potentiate a Ca2+-dependent, nonselective cation conductance. In contrast, M3 receptors interact with Gq to trigger phosphoinositide hydrolysis, Ca2+ mobilization and to induce a direct contractile response.Citation14 Subsequently, activation of BKCa channels by increasing Ca2+ can induce membrane hyperpolarization, which, in turn, closes L-type voltage-dependent calcium channels (L-VDCC) and induces a decrease in [Ca2+]i and relaxation of SMCs. Thus, the BKCa channels play a key role in the regulation of vascular tone and blood pressure.Citation11 Furthermore, Toth et al.Citation15 have suggested that BKCa channels contribute to the negative feedback regulation of vascular smooth muscle tone in cerebral arteries. Moreover, activation of BKCa channels has been found to alter contractility of smooth muscle cells (SMCs), such as uterine arteries vascular SMCs and detrusor SMCs.Citation16-18 It has been reported that BKCa channel is a key regulator of the smooth muscle motility in the bladder.Citation19 However, it remains unclear how the BKCa channel regulates SO motility under hypercholesterolemia.
In this study, we investigated the expression and function of BKCa channels in the SO in a rabbit model of hypercholesterolemia. We found that HC downregulated the tyrosine phosphorylation (Tyr-phosphorylation) of BKCa α-subunits, and reduced BKCa channel currents, accompanied with increased ACh-induced contraction of the SO rings. Our finding suggests that Tyr-phosphorylation of BKCa α-subunits may be a key factor for the activity of BKCa channels which may contribute to increased SO contractility in rabbits.
Results
Hypercholesterol diet increased the serum concentration of cholesterol in rabbits
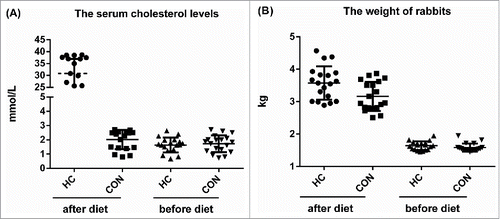
The serum cholesterol level and the body weight of the rabbit were measured before and after normal or HC diet for 12 weeks. The serum cholesterol level of all experimental rabbits was under 3 mmol/L before giving their diets. After 12 weeks, rabbits with HC diet showed serum cholesterol levels of equal or greater than 10 mmol/L, confirming that the high cholesterol chow diet had a measurable impact on circulating cholesterol levels. The serum cholesterol levels were significantly greater in the HC groups than those in the control group (, p < 0.01). There was no significant difference in body weight between the control and HC groups ().
BKCa currents were reduced in SOSMCs in HC rabbits
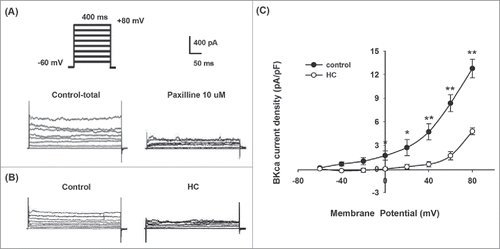
We performed whole-cell patch clamp recordings to record the current of BKCa channels on SOSMCs from the control and HC groups (). BKCa current density at 0 mV to +80 mV was significantly lower in SOSMCs in the HC group rabbits than that in the control group (p < 0.05 or p < 0.01, ). These results indicated that the BKCa channel activity was downregulated in SOSMCs from the HC group compared with the control group.
Figure 2. BKCa channel currents recorded on SOSMCS in rabbits from the control and HC groups. (A) Representative potassium currents recorded in SOSMCs from control group in the presence or absence of 10 μM paxilline. (B) BKCa currents recorded in primarily cultured SOSMCs from rabbits in the control group and HC group (the voltage protocol shown above). (C) I-V relationships of BKCa current density in SOSMCs from the control group (n = 8) and HC group (n = 8). *p < 0.05, ** p < 0.01.
Hypercholesterolemia induced downregulation of phosphorylated BKCa α-subunit in SOSMC cells
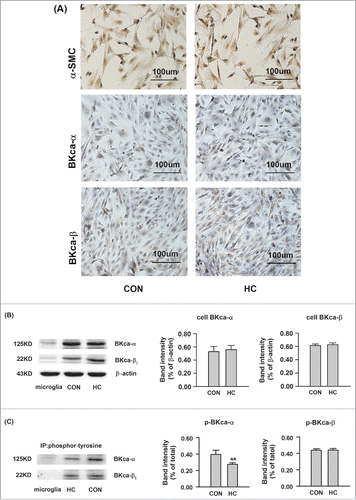
The protein expression of the α- and β1-subunits of the BKCa channel in SOSMC cells was examined using immunohistochemistry and Western blot. There were no differences in the protein expression of BKCa α-subunits and β1-subunits between the control and HC groups ( and ). However, the Tyr-phosphorylation of BKCa α-subunits were less abundant in the HC group ().
Figure 3. The protein expression of BKCa channels in SOSMCs in the control and HC groups. (A) Representative immunohistochemically staining of α-SMC, BKCa α- subunits, and BKCa β1-subunits in SOSMCs. Magnification, ×200. (B) Western blot analysis showing the total protein expression of BKCa α-. and β1-.subunits. (C) The protein expression of Tyr-phosphorylated BKCa α-. and β1-.subunits. The proteins were immunoprecipitated with antibodies against phosphorylated BKCa α- and β1-subunits and detected with Western blot. CON: SOSMCs from control group rabbits; HC: SOSMCs from hypercholesterolemia group rabbits; Microglial cells used for negative control. n = 5. **p < 0.01, HC vs control.
Effects of NS1619, Na3OV4 and Genistein on ACh-induced contraction of the SO rings in HC rabbits
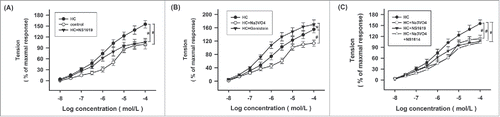
The contraction of the SO rings induced by ACh was increased in a dose-dependent manner. Hypercholesterolemia significantly increased ACh-induced contraction of the SO rings (). Pretreatment with 30 μM NS1619, a BKCa channel agonist, significantly reduced ACh-induced contraction of the SO rings in the HC rabbits (). In addition, pretreatment with 100 μM Na3OV4, a protein tyrosine phosphatase inhibitor, significantly reduced ACh-induced contraction of the SO rings in HC rabbits, whereas it significantly increased upon pretreating with 10 μM Genistein, a tyrosine kinase inhibitor (). Furthermore, pretreatment with both NS1619 and Na3OV4 did not significantly reduce ACh-induced contraction of the SO rings in HC rabbits compared with pretreatment with NS1619 or Na3OV4 alone ().
Figure 4. Effects of NS1619, Na3VO4, and Genistein on ACh-induced contraction of the SO rings in HC rabbits. (A) The concentration-response curve of ACh-induced contraction of the SO rings in the control, HC and HC+NS1619 groups. SO rings were pretreated with NS1619 (30 μM) for 15minutes. (B) The concentration-response curve of ACh-induced contraction of the SO rings in the HC, HC + Na3VO4, and HC + Genistein groups. SO rings were pretreated with Na3VO4 (100 μM) for 30 min, or Genistein (10 μM) for 30 min. (C) The concentration-response curve of ACh-induced contraction of the SO rings in the HC, HC + Na3VO4, HC + NS1619, and HC + Na3VO4 + NS1619 groups. SO rings were pretreated with Na3VO4 (100 μM) for 30 min, or/and NS1619 (30 μM) for 30 min. n = 8. *p < 0.05, # p < 0.01.
Discussion
In this study, we investigated the expression and function of the BKCa channel in a rabbit model of hypercholesterolemia. We found that the BKCa current density was significantly reduced, and the Tyr-phosphorylation of BKCa α-subunit was significantly downregulated in the SOSMCs in HC rabbits compared with the controls. Furthermore, hypercholesterolemia significantly increased ACh-induced contraction of the SO rings. Pretreatment with the BKCa channel agonist NS1619 or the protein tyrosine phosphatase inhibitor Na3OV4 significantly reduced the hyperresponsiveness of SO rings, whereas pretreatment with the tyrosine kinase inhibitor Genistein significantly increased ACh-induced contraction of the SO rings in HC rabbits. Our findings suggest that Tyr-phosphorylation of BKCa α-subunit is important for the inhibition of the SO ring contraction in rabbits, and hypercholesterolemia-induced downregulated Tyr-phosphorylation of BKCa α-subunit may contribute to the hyperresponsiveness of the SO ring in HC rabbits.
Previously, Wei et al. reported that the SO was under spasmodic status in HC rabbits [1], their finding is consistent with our findings showing an increase in the ACh-induced contraction of the SO rings in HC rabbits. It has been reported that BKCa channels play an important role in regulating arterial smooth muscle contractility.Citation20 BKCa channels play a negative feedback inhibition of muscular tone in VSMCs (vascular smooth muscle cells)Citation21 and maintain myogenic tone in SMCs of the superior epigastric arteries.Citation22 Furthermore, a similar negative feedback regulatory mechanism of BKCa channels was found in cerebral arteries.Citation15 Our findings showed that hypercholesterolemia increased contraction of the SO and downregulated Tyr-phosphorylation of BKCa α-subunits suggested that BKCa channels may also contribute to negative feedback inhibition on the contraction of SOSMCs in HC rabbits, and the tyrosine phosphorylation of BKCa α-subunit may be a critical factor in functional regulation of SO muscle relaxation. Our findings are consistent with previous reports by Toth et al.,Citation15 and Park et al.Citation22
It has been reported that the function of BKCa channels changes under certain physiologic and pathophysiological conditions such as pregnancy, hypertrophy, hypertension and atherosclerosis.Citation11 However, it remains unclear whether hypercholesterolemia alters the function of BKCa channels and whether BKCa channels contribute to muscle relaxation in SOSMCs in HC rabbits. In the present study, we found that hypercholesterolemia impaired the function of BKCa channels and increased SO muscle tone. Many studies have found that hypercholesterolemia induces endothelium-dependent relaxation of SMCs.Citation23-25 It has been reported that nitric oxide (NO) may be the transmitter of the terminal neuron in SOSMCs, and HC impairs endothelium-dependent and NO-mediated relaxation of SMCs.Citation26 However, Wei et al.Citation1 reported that NO was not the key factor in regulating the function of SOSMCs with hypercholesterolemia. It is possible that endothelium-dependent and NO-mediated regulatory mechanisms are specific to vascular smooth muscles that are covered by endothelial cells. Since the smooth muscle in the SO is not covered by endothelial cells, our findings support that BKCa channels play an important role in the regulation of SO muscular tone. The decreased Tyr-phosphorylation of BKCa α-subunit with hypercholesterolemia, which is closely associated with BKCa channels activity and may contribute to increasing muscle tone of SO in HC rabbits.
The expression of BKCa channels has been found to be affected by many factors such as diet and age. It has been found that the α- and β1-subunits of the BKCa channels are differentially expressed in different arteries of rats with diet-induced obesity.Citation27 In addition, there are aging-associated regional differences in the expression and function of BKCa channel.Citation28 In this study, we found that HC did not alter the total expression of BKCa α- and β1-subunits, but significantly downregulated the Tyr-phosphorylation of BKCa α-subunit, suggesting that the Tyr-phosphorylation of BKCa α-subunit may be important for the regulation of SO muscle tone. Interestingly, the decreased BKCa current in HC rabbits was accompanied with the decreased Tyr-phosphorylation of BKCa α-subunit. Not only that, both activation of BKCa channels by NS1619 and increasing Tyr-phosphorylation of BKCa α-subunit by Na3VO4 could facilitate the SO motility and reduce SO muscle tone. Thereby it is reasonable to speculate that Tyr-phosphorylation of BKCa α-subunits play a key role in regulating the BKCa channel activity which is closely associated with SO motility in HC rabbits. It is well known that the tyrosine phosphorylation is directly regulated by protein tyrosine kinase (PTK) which had been revealed contributing to the regulation of BKCa channel activity. Our study is consistent with previous ones in that tyrosine phosphorylation depending on some subtypes of PTK have an important effect on BKCa channel activity. Ling et al. has reported that Src, one member of non-receptor tyrosine kinase family, can phosphorylates BKCa channel subunits to regulate the channel activity.Citation29 And Pyk2, one member of non-receptor tyrosine kinase Fak family, also has been confirmed to involment in the regulation of BKCa channel.Citation30 However, whether the decreased Tyr-phosphorylation of BKCa α-subunit was induced directly by ones of PTK and how the hypercholesterolima resluted in the change of Tyr-phosphorylation of BKCa α-subunit in SOSMCs are needed our further researches.
Remarkably, Cholesterol has been found to inhibit the tyrosine phosphorylation of high affinity IgE receptor signal pathway via Lyn, a Src family kinase in mast cells and basophilsCitation31 In addition, although cholesterol has been reported to increase protein tyrosine phosphorylation through a cAMP-dependent pathway during mouse sperm capacitationCitation32 there is report that cholesterol contributes to cholelithic forming through increasing phosphorylation of protein kinase C, α.33 Those studies suggest that high cholesterol contributes to the protein phosphorylation through different signaling pathways involved in various physiologic function of cells, but there is none report about inhibition of tyrosine phosphorylation induced by high cholesterol and their effects on BKCa channel activity and SO motility. Fortunately, the present study demonstrated that hypercholesterolemia may be a critical factor for BKCa channel activity and SO motility via regulating the Tyr-phosphorylation of BKCa α-subunit level in SOSMCs. However, the underlying mechanism for this specific tyrosine phosphorylation change with hypercholesterolemia remains unclear, further investigation on this issue is needed.
Hypercholesterolemia has been known to increase the risk of gallstone.Citation34 Statins are widely used for lowering serum cholesterol. However, it has been reported that statin therapy, which is taken as a class of most effective drugs for lipid-lower therapy, is not significantly associated with either an increased or decreased risk of gallstone.Citation35 Therefore, statins may not be effective to reverse hypercholesterolemia-induced changes in gallstone formation. In the present study, we found that hypercholesterolemia reduced Tyr-phosphorylation of BKCa α-subunit, which may play a key role in downregulated BKCa channel activity and SO motility. Therefore, BKCa channel may represent a new therapeutic target that could be effective in treating hypercholesterolemia-induced gallstone formation.
In conclusion, the regulation of BKCa channel depending on phosphorylation through different pathway mediated by cholesterol may be a key factor for many disease, such as SOD even gallstone. Hence, to clarify the molecular mechanism contributes to proposing more effective therapeutic method. In this study, we found that HC reduced the tyrosine phosphorylation of BKCa α-subunits, accompanied by a reduction in BKCa channel currents and an abnormal contraction in SO smooth muscle cells. Our findings suggest that the tyrosine phosphorylation of BKCa α-subunit may be important for the regulation of BKCa activity and SO motility. However, the molecular mechanism of BKCa channels in SOSMCs excitability by hypercholesterolemia needs further investigation. Nevertheless, our results will help to supply the new research orientation for therapeutic schedule of hypercholesterolemia, SOD and gallstone.
Materials and methods
The animal model of hypercholesterolemia
New Zealand white rabbits (1.5 kg∼2.0 kg, 2 months old) were purchased from the Experimental Animal Center of the Fourth Military Medical University, China. Rabbits were randomly divided into 2 groups as follows: the control group was fed standard chow (CON, n = 18) and the high-cholesterol group which was fed cholesterol-enriched chow containing 1.5% cholesterol (Biosharp, http://www.biosharp.cn/catalog/item/BS046C) (HC, n = 18). Every week, the animals in the HC group were fed cholesterol-enriched chow for 6 d then standard chow at the seventh day. They were housed individually in cages under an artificial 12-h light/dark cycle at room temperature (22 ± 2°C) and relative humidity of 65%. They had free access to food and water. All procedures were performed in accordance with the Guidelines for Animal Care and Use of the Fourth Military Medical University (Xi'an, People's Republic of China).
Tissue preparations and primary cell culture
The SO segments were removed from the rabbits in the control and HC groups, and bathed in precooled 10% Penicillin-Streptomycin PBS (Phosphate Buffered Saline, pH 7.4) for 5 minutes. The SO tissues were then removed and rinsed twice and they were cut into pieces and digested by 0.1% collagenase IV together with 0.25% trypsin (4:1) at 37°C, for 1 h. The primary culture of smooth muscle cells of the SO (SOSMCs) was performed as previously reported.Citation36
Immunohistochemical staining
Immunohistochemistry was performed to observe the cell morphology and location of BKCa channels. Briefly, the SOSMCs were placed on glass sheets, and incubated with primary antibodies against α-actin (Santa Cruz Biotechnology, http://www.scbt.com/catalog/item/sc-32251), BKCa-α (Santa Cruz Biotechnology, http://www.scbt.com/catalog/item/sc-374142) and BKCa-β1 (Santa Cruz Biotechnology, http://www.scbt.com/catalog/item/sc-377023). Then, immunostaining was performed using rabbit SP immunohistochemical kit.
Western blot and immunoprecipitation
After the fat and connective tissues were removed, the SO tissues were homogenized in RIPA buffer using Selecta Sonopuls (SCIENTZ-IID, Ningbo Scientz Biotechnology CO,.LTD), and total lysates were prepared as described previously.Citation37 The total protein expression of BKCa α and β1-subunits was examined by Western blot. After protein quantification, proteins were resolved by 9% SDS-PAGE and transferred onto PVDF membrane using electroblotting. The membrane was incubated with 5% skim milk for 1 h at room temperature, and then incubated with primary antibodies against BKCa α- and β1 subunits at 4°C overnight. The next day, membranes were incubated with secondary antibodies for 1 h at room temperature. The protein bands were visualized using a chemiluminescence detection system.
Immunoprecipitation was used to detect the expression of tyrosine phosphorylated BKCa α- and β1-subunits. Briefly, cell lysates were incubated with protein A and G Magnetic Beads (Merck Millipore Corporation, http://www.merckmillipore.com/catague/item/LSKMAGAG02), which were coated with antibodies against protein tyrosine phosphorylation (Cell Signaling Technology, http://www.cellsignal.com/catague/item/#9411) at 4°C overnight. After centrifugation, the beads were collected and were analyzed using Western blot as above.
Muscle tension measurement
The SO segments were isolated from animals in the control and HC groups. After the surrounding fat and connective tissues were carefully removed, the proximal part of the SO segments was cut into muscle rings with a diameter of 4 mm. The SO rings were mounted on 2 small L-shaped glass hooks. One hook was connected to a muscle tension transducer (JZ-BK, Beca company of China) for measurement and recording tension in response to acetylcholine (ACh), and the other hook was used to fix the SO ring. The tension of SO muscle rings was converted to electrical signals via the transducer and recorded by desktop automatic balance recorder (XWTD464, Shanghai Dahua Instrument and Meter Plant). The experiments were performed in an organ bath containing Krebs-Ringer bicarbonate solution (mM: NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, glucose 10 and NaHCO3 25, pH 7.4 at 37°C). The SO rings with changes in the amplitude of less than 10% between 2 stimulations using 60 mM KCl were selected to be used in the experiment. The SO rings were pretreated with NS1619 (30μM) for 15 minutes, Genistein (10 μM) for 30 minutes, or Na3OV4 (100 μM) for 30 minutes. Subsequently, ACh was cumulatively added to the organ bath to record the concentration-response curve. The maximum response of the SO ring was defined as the amplitude activated by 1 × 10−4mol/L ACh in the control group and was set as 100%. The tension of the SO rings was presented as the percentage of the maximum response.
Whole-cell patch-clamp for measurement of BKCa outward currents
The primarily cultured SO cells were used for whole-cell patch clamp recording. SOSMCs from the control and HC groups were plated on glass coverslips and placed at 37°C incubator with 5% CO2. Then, the coverslips were transferred and bathed in a recording chamber filled with bath solution on the Olympus microscope stage. The bath solution contains the followings: NaCl 124 mM, NaHCO3 25 mM, KCl 2.5 mM, KH2PO4 1 mM, CaCl2 2 mM, MgSO4 2 mM and glucose 10 mM. The pipettes solution consisted of: potassium gluconate 145 mM, NaCl 5 mM, MgCl2 1 mM, EGTA 0.2 mM, Hepes 10 mM, Mg-ATP 2 mM and Na3-GTP 0.1 mM, adjusted to pH 7.2 with KOH. Potassium currents were performed,Citation38 using an Axon 200B amplifier (Axon Instruments, CA, USA). Cells were voltage clamped with 400-ms voltage steps from −60 to +80 mV in 20-mV increments from a holding potential of −60 mV. BKca currents were identified by the use of a BKCa inhibitor, 10 μM paxilline (Sigma-Aldrich, www.sigmaaldrich.com/ catague/item/P2928).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Prof. Wei JG for the manuscript revision and critical discussion, and thank Prof. Wu YM and Dr. Huang YF for providing technical guidance.
Funding
The research is supported by National Natural Science Foundation of China (No. 81270534).
Reference
- Wei JG, Wang YC, Du F, Yu HJ. Dynamic and ultrastructural study of sphincter of Oddi in early-stage cholelithiasis in rabbits with hypercholesterolemia. World J Gastroenterol 2000; 6(1):102-106; PMID:11819533; https://doi.org/10.3748/wjg.v6.i1.102
- Wei JG, Wang YC, Liang GM, Wang W, Chen BY, Xu JK, Song LJ. The study between the dynamics and the X-ray anatomy and regularizing effect of gallbladder on bile duct sphincter of the dog. World J Gastroenterol 2003; 9(5):1014-1019; PMID:12717848; https://doi.org/10.3748/wjg.v9.i5.1014
- Szilvassy Z, Nagy I, Szilvassy J, Jakab I, Csati S, Lonovics J. Impaired nitrergic relaxation of the sphincter of Oddi of hyperlipidaemic rabbits. Eur J Pharmacol 1996; 301(1–3):R17-18; PMID:8773472; https://doi.org/10.1016/0014-2999(96)00159-8
- Wang XJ, Wei JG, Wang CM, Wang YC, Wu QZ, Xu JK, Yang XX. Effect of cholesterol liposomes on calcium mobilization in muscle cells from the rabbit sphincter of Oddi. World J Gastroenterol 2002; 8(1):144-149; PMID:11833091; https://doi.org/10.3748/wjg.v8.i1.144
- Wallner M, Meera P, Toro L. Determinant for beta-subunit regulation in high-conductance voltage-activated and Ca(2+)-sensitive K+ channels: an additional transmembrane region at the N terminus. Proc Natl Acad Sci U S A 1996; 93(25):14922-14927; PMID:8962157; https://doi.org/10.1073/pnas.93.25.14922
- Yazejian B, Sun XP, Grinnell AD. Tracking presynaptic Ca2+ dynamics during neurotransmitter release with Ca2+-activated K+ channels. Nat Neurosci 2000; 3(6):566-571; PMID:10816312; https://doi.org/10.1038/75737
- Soloviev A, Zholos A, Ivanova I, Novokhatska T, Tishkin S, Raevska A, Stroyuk A, Yefanov V. Plasmonic gold nanoparticles possess the ability to open potassium channels in rat thoracic aorta smooth muscles in a remote control manner. Vascul Pharmacol 2015; 72:190-196; PMID:26044181; https://doi.org/10.1016/j.vph.2015.05.016
- Lorca RA, Prabagaran M, England SK. Functional insights into modulation of BKCa channel activity to alter myometrial contractility. Front Physiol 2014; 5:289; PMID:25132821; https://doi.org/10.3389/fphys.2014.00289
- Hill MA, Yang Y, Ella SR, Davis MJ, Braun AP. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett 2010; 584(10):2033-2042; PMID:20178789; https://doi.org/10.1016/j.febslet.2010.02.045
- Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006; 21:69-78; PMID:16443824; https://doi.org/10.1152/physiol.00040.2005
- Hu XQ, Zhang L. Function and regulation of large conductance Ca(2+)-activated K+ channel in vascular smooth muscle cells. Drug Discov Today 2012; 17(17–18):974-987; PMID:22521666; https://doi.org/10.1016/j.drudis.2012.04.002
- Ghatta S, Nimmagadda D, Xu X, O'Rourke ST. Large-conductance, calcium-activated potassium channels: structural and functional implications. Pharmacol Ther 2006; 110(1):103-116; PMID:16356551; https://doi.org/10.1016/j.pharmthera.2005.10.007
- Bhattacharya S, Mahavadi S, Al-Shboul O, Rajagopal S, Grider JR, Murthy KS. Differential regulation of muscarinic M2 and M3 receptor signaling in gastrointestinal smooth muscle by caveolin-1. Am J Physiol Cell Physiol 2013; 305(3):C334-347; PMID:23784544; https://doi.org/10.1152/ajpcell.00334.2012
- Ehlert FJ. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal, airway and urinary bladder smooth muscle. Life Sci 2003; 74(2–3):355-366; PMID:14607264; https://doi.org/10.1016/j.lfs.2003.09.023
- Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, Ungvari Z. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol 2013; 305(12):H1698-1708; PMID:24097425; https://doi.org/10.1152/ajpheart.00377.2013
- Pinterova M, Kunes J, Zicha J. Altered neural and vascular mechanisms in hypertension. Physiol Res 2011; 60(3):381-402; PMID:21615201
- Rosenfeld CR, Word RA, DeSpain K, Liu XT. Large conductance Ca2+-activated K+ channels contribute to vascular function in nonpregnant human uterine arteries. Reprod Sci 2008; 15(7):651-660; PMID:18836130; https://doi.org/10.1177/1933719108319160
- Provence A, Hristov KL, Parajuli SP, Petkov GV. Regulation of Guinea Pig Detrusor Smooth Muscle Excitability by 17beta-Estradiol: The role of the large conductance Voltage- and Ca2+-Activated K+ Channels. PLoS One 2015; 10(11):e0141950; PMID:26536038; https://doi.org/10.1371/journal.pone.0141950
- Vahabi B, Lawson K, McKay NG, Sellers DJ. Phasic activity of urinary bladder smooth muscle in the streptozotocin-induced diabetic rat: effect of potassium channel modulators. Eur J Pharmacol 2011; 660(2–3):431-437; PMID:21497590; https://doi.org/10.1016/j.ejphar.2011.03.053
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 1995; 270(5236):633-637; PMID:7570021; https://doi.org/10.1126/science.270.5236.633
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol 1995; 268(4 Pt 1):C799-822; PMID:7733230
- Park WS, Heo SC, Jeon ES, Hong da H, Son YK, Ko JH, Kim HK, Lee SY, Kim JH, Han J. Functional expression of smooth muscle-specific ion channels in TGF-beta(1)-treated human adipose-derived mesenchymal stem cells. Am J Physiol Cell Physiol 2013; 305(4):C377-391; PMID:23761629; https://doi.org/10.1152/ajpcell.00404.2012
- Shimokawa H, Vanhoutte PM. Impaired endothelium-dependent relaxation to aggregating platelets and related vasoactive substances in porcine coronary arteries in hypercholesterolemia and atherosclerosis. Circ Res 1989; 64(5):900-914; PMID:2495869; https://doi.org/10.1161/01.RES.64.5.900
- Zeiher AM, Drexler H, Wollschlager H, Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation 1991; 83(2):391-401; PMID:1991363; https://doi.org/10.1161/01.CIR.83.2.391
- Fleischhacker E, Esenabhalu VE, Holzmann S, Skrabal F, Koidl B, Kostner GM, Graier WF. In human hypercholesterolemia increased reactivity of vascular smooth muscle cells is due to altered subcellular Ca(2+) distribution. Atherosclerosis 2000; 149(1):33-42; PMID:10704612; https://doi.org/10.1016/S0021-9150(99)00290-7
- Thune A, Delbro DS, Nilsson B, Friman S, Svanvik J. Role of nitric oxide in motility and secretion of the feline hepatobiliary tract. Scand J Gastroenterol 1995; 30(7):715-720; PMID:7481537; https://doi.org/10.3109/00365529509096318
- Howitt L, Sandow SL, Grayson TH, Ellis ZE, Morris MJ, Murphy TV. Differential effects of diet-induced obesity on BKCa {beta}1-subunit expression and function in rat skeletal muscle arterioles and small cerebral arteries. Am J Physiol Heart Circ Physiol 2011; 301(1):H29-40; PMID:21536854; https://doi.org/10.1152/ajpheart.00134.2011
- Hayoz S, Bradley V, Boerman EM, Nourian Z, Segal SS, Jackson WF. Aging increases capacitance and spontaneous transient outward current amplitude of smooth muscle cells from murine superior epigastric arteries. Am J Physiol Heart Circ Physiol 2014; 306(11):H1512-1524; PMID:24705555; https://doi.org/10.1152/ajpheart.00492.2013
- Ling S, Woronuk G, Sy L, Lev S, Braun AP. Enhanced activity of a large conductance, calcium-sensitive K+ channel in the presence of Src tyrosine kinase. J Biol Chem 2000; 275(39):30683-30689; PMID:10893418; https://doi.org/10.1074/jbc.M004292200
- Ling S, Sheng JZ, Braun AP. The calcium-dependent activity of large-conductance, calcium-activated K+ channels is enhanced by Pyk2- and Hck-induced tyrosine phosphorylation. Am J Physiol Cell Physiol 2004; 287(3):C698-706; PMID:15128501; https://doi.org/10.1152/ajpcell.00030.2004
- Sheets ED, Holowka D, Baird B. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcepsilonRI and their association with detergent-resistant membranes. J Cell Biol 1999; 145(4):877-887; PMID:10330413; https://doi.org/10.1083/jcb.145.4.877
- Visconti PE, Ning X, Fornes MW, Alvarez JG, Stein P, Connors SA, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol 1999; 214(2):429-443; PMID:10525345; https://doi.org/10.1006/dbio.1999.9428
- Kong J, Liu BB, Wu SD, Wang Y, Jiang QQ, Guo EL. Enhancement of interaction of BSEP and HAX-1 on the canalicular membrane of hepatocytes in a mouse model of cholesterol cholelithiasis. Int J Clin Exp Pathol 2014; 7(4):1644-1650; PMID:24817961
- Taheri H, Ghemian N, Taghavi Y, Shokry-Shirani J. Biochemical profile of bile fluid in patients with malignant cholestasis in comparison with cholestasis due to gall stone. Caspian J Intern Med 2015; 6(3):165-169; PMID:26644885
- Martin D, Schmidt R, Mortensen EM, Mansi I. Association of Statin Therapy and Risks of Cholelithiasis, Biliary Tract Diseases, and Gallbladder Procedures: Retrospective Cohort Analysis of a US Population. Ann Pharmacother 2016; 50(3):161-171; PMID:26706861; https://doi.org/10.1177/1060028015622649
- Simula ME, Brookes SJ, Meedeniya AC, Toouli J, Saccone GT. Distribution of nitric oxide synthase and vasoactive intestinal polypeptide immunoreactivity in the sphincter of Oddi and duodenum of the possum. Cell Tissue Res 2001; 304(1):31-41; PMID:11383884; https://doi.org/10.1007/s004410100357
- Benham CD, Bolton TB, Lang RJ, Takewaki T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+ -channels in arterial and intestinal smooth muscle cell membranes. Pflugers Arch 1985; 403(2):120-127; PMID:2580269; https://doi.org/10.1007/BF00584088
- Yang Y, Li PY, Cheng J, Mao L, Wen J, Tan XQ, Liu ZF, Zeng XR. Function of BKCa channels is reduced in human vascular smooth muscle cells from Han Chinese patients with hypertension. Hypertension 2013; 61(2):519-525; PMID:23232643; https://doi.org/10.1161/HYPERTENSIONAHA.111.00211