ABSTRACT
LRRC8 proteins have been shown to underlie the ubiquitous volume regulated anion channel (VRAC). VRAC channels are composed of the LRRC8A subunit and at least one among the LRRC8B-E subunits. In addition to their role in volume regulation, LRRC8 proteins have been implicated in the uptake of chemotherapeutic agents. We had found that LRRC8 channels can be conveniently expressed in Xenopus oocytes, a system without endogenous VRAC activity. The fusion with fluorescent proteins yielded constitutive activity for A/C, A/D and A/E heteromers. Here we tested the effect of the anticancer drug cisplatin on LRRC8A-VFP/8E-mCherry and LRRC8A-VFP/8D-mCherry co-expressing oocytes. Incubation with cisplatin dramatically activated currents for both subunit combinations, confirming that VRAC channels provide an uptake pathway for cisplatin and that intracellular cisplatin accumulation strongly activates the channels. Thus, specific activators of LRRC8 proteins might be useful tools to counteract chemotherapeutic drug resistance.
Introduction
The ability to regulate cell volume is essential for normal cell functions including proliferation, migration, signaling, and apoptosis. Cellular volume regulation has been studied for decades, highlighting the importance of the so-called volume regulated anion channel, VRAC.Citation1 This ubiquitously expressed anion channel mediates swelling-activated Cl− currents in practically all cell types studied.Citation1,2 The parallel activation of a potassium and a chloride conductance is believed to underlie the process of regulatory volume decrease (RVD), mediated by an efflux of KCl from the cells followed by water efflux. The VRAC channel is practically inactive under resting conditions, but opens within minutes upon hypotonic swelling. VRAC is also permeable to organic osmolytes such as taurine and excitatory amino acids.Citation2 In fact, further physiologic functions of VRAC are probably related to its ability to release glutamateCitation3 and ATP.Citation4 VRAC is also linked to the cell cycle and to proliferation.Citation5 Cell shrinkage due to VRAC activation is essential for apoptosis, in a process called apoptotic volume decrease (AVD).
The molecular identity of VRAC type channels was revealed in 2014 when two studies identified the leucine-rich repeat containing protein 8A (LRRC8A) as an essential component of VRAC,Citation6,7 and 4 closely related homologs (LRRC8B to -E) as complementary VRAC subunits.Citation6 VRAC channels are heteromers composed of at least one LRRC8A subunit and one among the LRRC8B-E group.Citation6 LRRC8 proteins are characterized by 4 transmembrane segments and up to 17 leucine-rich repeats (LRRs) in their cytoplasmic C-terminus.Citation8
In addition to the physiologic functions of VRAC it was found that downregulation of LRRC8 proteins or low activity of VRAC in cells is associated with resistance to chemotherapeutics (e.g. cisplatin).Citation9-13 Additionally, patients with a low LRRC8D gene expression in their ovarian cancers displayed reduced survival.Citation10 Similarly, LRRC8D expression was shown to be essential for the cytotoxic effect of the antibiotic blasticidin S.Citation14
Detailed reductionist studies require heterologous expression. However, standard systems like HEK or CHO cells endogenously express VRAC, requiring LRRC8 gene-knockout cell lines for mechanistic investigationsCitation6,15 We have recently found that Xenopus oocytes represent a convenient expression system for LRRC8 proteins.Citation16 Un-injected oocytes, or oocytes injected with single LRRC8 subunits had no detectable VRAC currents, whereas co-injection of LRRC8A and 8C, 8D or 8E (but not 8B) RNA yielded typical VRAC currents that slowly activated in hypotonic conditions. Furthermore, we discovered that the addition of fluorescent proteins to the C-terminus dramatically increased the activity: heteromeric LRRC8A-VFP/8C-mCherry, LRRC8A-VFP/8D-mCherry and LRRC8A-VFP/8E-mCherry exhibited considerable currents even in isotonic conditions, which were further strongly stimulated by hypotonicity.Citation16 We performed a detailed electrophysiological characterization of these currents (ion selectivity, single channel analysis, radiotracer fluxes, blocker sensitivity).Citation16
Triggered by the proposed role of VRAC channels for the uptake of anticancer drugs, in the present addendum we exploited the oocyte expression system to test acute and chronic effects of the chemotherapeutic agent cisplatin. We found that overnight incubation with cisplatin dramatically increases currents in oocytes that express heteromeric LRRC8A-VFP/8D-mCherry or LRRC8A-VFP/8E-mCherry.
Results
Triggered by the findings that LRRC8 protein downregulation increases cisplatin resistanceCitation10-13 and that LRRC8D is essential for blasticidin uptakeCitation14 we tested cisplatin and blasticidin effects on oocytes expressing fluorescently tagged constitutively active 8A-VFP/8E-mCh and 8A-VFP/8D-mCh channels.
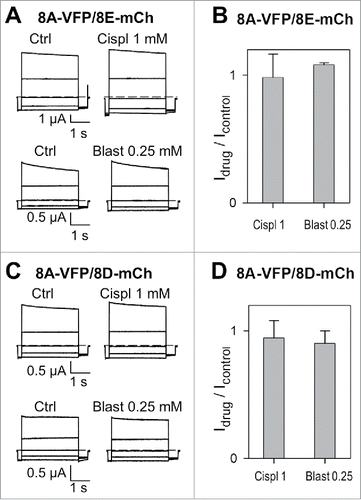
These compounds cannot be applied at high enough concentrations to estimate changes of the reversal potential directly related to their permeability through LRRC8 heteromers. A priori, since they are of relatively large molecular dimension, it might be expected that even at relatively low concentrations these drugs could impede Cl− ion flow by obstruction of the pore. However, acute application of 1 mM cisplatin or 0.25 mM blasticidin had no effect on currents mediated by 8A-VFP/8E-mCh (). Similarly 1 mM cisplatin, in contrast to previous results,Citation10 or 0.25 mM blasticidin did not affect 8A-VFP/8D-mCh mediated anion currents ().
Figure 1. Acute application of cisplatin and blasticidin does not affect currents mediated by 8A-VFP/8E-mCh or 8A-VFP/8D-mCh. (A) Typical current traces of 8A-VFP/8E-mCh expressing oocytes evoked by the “IV-pulse protocol” (see Materials and Methods) in control conditions and after perfusion of 1 mM cisplatin (top) or 0.25 mM blasticidin (bottom). (B) 8A-VFP/8E-mCh currents measured at 60 mV in presence of 1mM cisplatin or 0.25 mM blasticidin were normalized to the value measured before drug application (n = 5 for cisplatin; n = 3 for blasticidin). (C-D) Acute effect of cisplatin and blasticidin on 8A-VFP/8D-mCh. (C) Voltage clamp traces of oocytes expressing 8A-VFP/8D-mCh before and after perfusion of 1 mM cisplatin (top) or 0.25 mM blasticidin (bottom). (D) 8A-VFP/8D-mCh currents recorded at 60 mV were normalized to the value measured before drug application (n = 3). Error bars indicate SD.
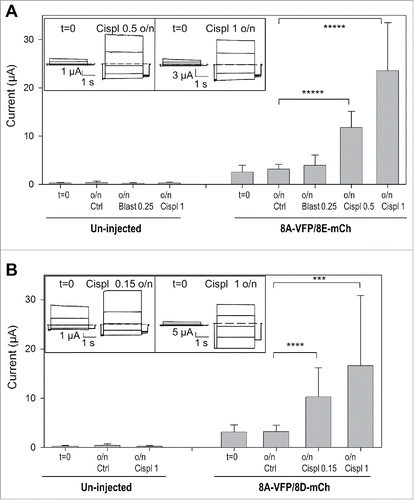
We next subjected 8A-VFP/8E-mCh and 8A-VFP/8D-mCh expressing oocytes to overnight incubation in various conditions. For these experiments we measured currents in all oocytes before and after incubation. 8A-VFP/8E-mCh mediated currents were strongly activated by 1 mM, or even 0.5 mM, cisplatin (). In contrast, incubation with 0.25 mM blasticidin did not lead to a significant current activation in 8A-VFP/8E-mCh expressing oocytes (), showing that the effect of cisplatin is drug specific. Similarly incubation in 1 mM and even in 0.15 mM cisplatin increased currents of 8A-VFP/8D-mCh injected oocytes more than 3-fold (). Importantly, these treatments did not induce any currents in un-injected oocytes ().
Figure 2. Cisplatin incubation strongly increases currents of oocytes expressing 8A-VFP/8E-mCh or 8A-VFP/8D-mCh. (A) Mean currents from un-injected or from 8A-VFP/8E-mCh injected oocytes measured before (t = 0; un-injected n = 29; 8A-VFP/8E-mCh n = 60) and after overnight incubation in “Maintaining” solution (Ctrl; un-injected n = 11; 8A-VFP/8E-mCh n = 29), 0.25 mM blasticidin (Blast 0.25; un-injected n = 4; 8A-VFP/8E-mCh n = 9), 0.5 mM cisplatin (Cispl 0.5; 8A-VFP/8E-mCh n = 7) or 1 mM cisplatin (Cispl 1; un-injected n = 14; 8A-VFP/8E-mCh n = 15; *****P < 10−9). The insets show 8A-VFP/8E-mCh traces from one oocyte before and after overnight incubation in 0.5 mM (left) or 1 mM cisplatin (right). (B) Bars represent currents of un-injected or 8A-VFP/8D-mCh injected oocytes recorded before (t = 0; un-injected n = 19; 8A-VFP/8D-mCh n = 39) and after overnight incubation in “Maintaining” solution (Ctrl; un-injected n = 8; 8A-VFP/8D-mCh n = 21), 0.15 mM cisplatin (Cispl 0.15; 8A-VFP/8D-mCh n = 6) or 1 mM cisplatin (Cispl 1; un-injected n = 11; 8A-VFP/8D-mCh n = 12; ***P < 0.001; ****P < 0.0001). The insets show typical 8A-VFP/8D-mCh traces from one oocyte before and after overnight incubation in 0.15 mM (left) or 1 mM cisplatin (right). Error bars indicate SD.
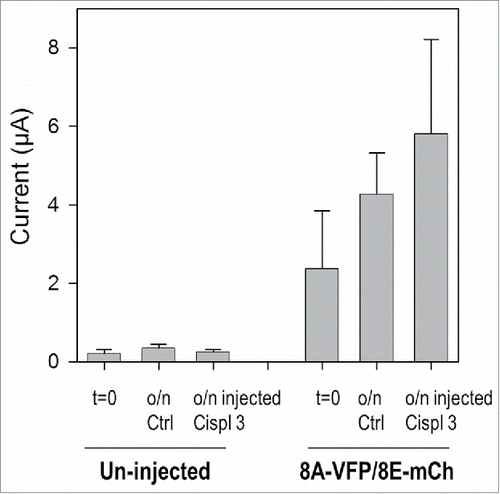
These results suggest that cisplatin is taken up by the oocytes through LRRC8 channels and in turn further activates the channels. To test for a direct effect of intracellularly applied cisplatin, we injected 50 nl of a 3 mM cisplatin solution in un-injected oocytes and in oocytes expressing 8A-VFP/8E-mCh channels and incubated them overnight. However, this maneuver did not significantly activate 8A-VFP/8E-mCh mediated currents ().
Figure 3. Cisplatin injection does not modify 8A-VFP/8E-mCh currents. Bars represent mean currents of un-injected or 8A-VFP/8E-mCh injected oocytes measured before (t = 0; un-injected n = 11; 8A-VFP/8E-mCh n = 15) and after overnight incubation in “Maintaining” solution. After recording currents in control conditions, some oocytes were just incubated (o/n Ctrl; un-injected n = 4; 8A-VFP/8E-mCh n = 8), others were injected with 50 nl of a 3 mM cisplatin solution and then incubated overnight (o/n inj Cispl 3; un-injected n = 7; 8A-VFP/8E-mCh n = 7; P > 0.05). Error bars indicate SD.
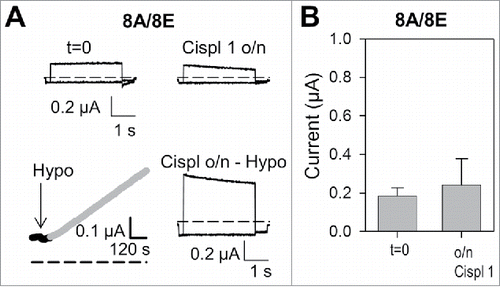
We next asked if cisplatin incubation also leads to the activation of WT, i.e. untagged, channels. A typical experiment is shown in : currents mediated by 8A-8E were measured before (t = 0) and after overnight incubation in 1 mM cisplatin. Since no significant activation of constitutive currents in these conditions was observed, we finally exposed the oocyte to a hypotonic stimulus, leading to a normal sized increase of currents ().Citation16 Thus, overnight incubation of WT 8A/8E expressing oocytes in 1 mM cisplatin neither induced constitutive currents, nor increased hypotonicity activated currents (). Overnight incubation of WT 8A-8E expressing oocytes in a hypotonic solution in the presence of 1 mM cisplatin led to severe deterioration of the oocytes not allowing any assessment of a possible current increase (data not shown).
Figure 4. Cisplatin incubation does not alter currents of oocytes expressing WT 8A/8E. (A) Top: typical current traces measured in isotonic conditions of an 8A/8E expressing oocyte before and after overnight cisplatin incubation in the “maintaining” solution. Bottom: stimulation of the same oocyte after o/n incubation with hypotonic solution activates normal sized anion currents (left: current measured at 60 mV during hypotonic stimulation, right: voltage-clamp traces after hypotonic activation). (B) Mean values of 8A/8E currents in isotonic conditions before and after o/n cisplatin incubation (n = 7; P > 0.05). Error bars indicate SD.
Discussion
The volume-regulated anion channel, VRAC, also called VSOAC (volume-sensitive organic osmolyte-anion channel) or VSOR (volume-sensitive outwardly rectifying anion channel),Citation17 has been studied for decades and appears to be present in practically all mammalian cell types.Citation2 The activation of the channel by cell swelling suggests that its primary physiologic role is to aid in regulatory volume decrease allowing the efflux of anionic osmolytes including Cl− ions. However, many more physiologic roles have been attributed to VRAC, for example release of glutamate in the brain that could serve a signaling function.Citation2,17
The present study focuses on the potential role of VRAC/LRRC8 in the resistance to one of the most potent antitumor agents, cisplatin. It has been used alone or in combination with other drugs to treat various types of human cancers. However its action can be attenuated and cisplatin resistance represents a major limitation of its use.Citation18,19 Early studies showed that reducing VRAC activity by anion channel blockers conferred resistance to cisplatin in human epidermoid cancer cells.Citation20 The same group found that the cisplatin resistant KCP-4 epidermoid cancer cell line almost completely lacks VRAC activity, but that partial restoration of VRAC decreased cisplatin resistance.Citation9 These studies pointed to a role of VRAC in cisplatin uptake. Recently, the identification of LRRC8 proteins as the molecular constituents of VRAC channels, composed of the obligatory LRRC8A subunit and at least one subunit among LRRC8B-E,Citation6,7 opened the door for a detailed molecular investigation of this channel. In fact, in agreement with the reports of VRAC involvement in cisplatin resistance,Citation20,9 Planells-Cases et al discovered in an unbiased genetic screen that the inactivation of LRRC8A or of LRRC8D conferred partial cisplatin resistance in haploid KBM7 cells.Citation10 In addition, using a similar gene trap screen, Lee et al found that inactivation of LRRC8D conferred resistance to the antibiotic blasticidin.Citation14
The ubiquitous presence of VRAC in practically all mammalian cell types used for heterologous expression hinders the study of these proteins. To overcome this problem, Voss et al. developed knockout cell lines in which all LRRC8 genes were inactivated.Citation6 Xenopus oocytes were recently identified by us as an additional convenient expression system for detailed biophysical and pharmacological studies.Citation16 Oocytes possess a negligible background of volume-stimulated currents, and co-expression of LRRC8A with LRRC8C, LRRC8D, or LRRC8E induces hypotonicity activated currents with properties that are similar to those described in cellular systems.Citation6,16 In addition, we discovered that adding fluorescent proteins to the C-terminus of LRRC8 proteins induced constitutive anion currents that were further stimulated by hypotonicity, resulting in potentially very large currents that can be easily investigated.Citation16 Taking advantage of this expression system, we studied here the effect of the drugs cisplatin and blasticidin on LRRC8 induced currents.
Essentially we found that incubation of oocytes expressing the constitutively active tagged subunits 8A-VFP/8E-mCh or 8A-VFP/8D-mCh with cisplatin leads to a strong increase of currents. No effect was instead observed upon incubation with cisplatin for WT 8A-8E expressing channels. These results strongly suggest that cisplatin enters the oocytes through the constitutively active LRCC8 channels and that the compound somehow further stimulates the channel activity. Even though we could not determine cisplatin or blasticidin permeability directly, e.g., by a shift of the reversal potential or by induction of channel block, the permeability of these relatively polar molecules is in line with the relatively large permeability of LRRC8 channels for various organic molecules, including ATP.Citation16 Planells-Cases et al. reported an acute blocking effect of 8A-8D channels by cisplatin when the substance was dissolved in the presence of DMSO.Citation10 We could not see this in oocytes expressing constitutively active 8A-VFP/8D-mCh channels (data not shown). We hypothesize that intracellular cisplatin but not blasticidin may activate signal transduction cascades activating LRRC8 proteins. Blasticidin seems to kill cells by acting as a translational inhibitor,Citation21 whereas cisplatin is chemically reactive, crosslinking for example DNA and inducing apoptosis.Citation18,19
The cisplatin induced increase of currents could be caused by a direct activation of the exogenously expressed LRRC8 proteins or, it could occur indirectly, by the activation of unidentified endogenous channels. However, the outwardly rectifying phenotype and the characteristic kinetics of the currents measurable in oocytes with intermediate current amplitudes strongly suggested that the currents were mediated by the exogenous LRRC8 channels. This is in line with the activation of VRAC currents found in cisplatin treated cells.Citation20 If intracellular cisplatin directly or indirectly activates LRRC8 channels, it might be expected that currents should increase after cisplatin injection. Yet, we could not detect any significant effect on currents after cisplatin injection. However, it should be kept in mind that, due to the reactivity of cisplatin, the single injection bolus is probably chemically inactivated by reaction with the large amounts of intracellular proteins and lipids in the oocyte cytosol. Thus, the effective concentration present some time after injection is most likely much lower than the concentration that can be achieved by steady replenishment from the extracellular solution under conditions of incubation, provided a significant membrane permeability is present.
The widely used chemotherapeutic agent cisplatin has many and complex effects in cells and its main cytotoxic activity is thought to be the ability to crosslink DNA and, consequently, to activate signal transduction pathways that lead to apoptosis.Citation18,19 Our results point to a potential additional mechanism that could be involved in cisplatin toxicity: considerable activation of VRAC, induced by accumulated cisplatin, will inevitably lead to the loss of small cytosolic molecules including ATP, eventually resulting in cell death due to starving. Such a potential dual role of VRAC in cisplatin uptake and cisplatin effect would lead to a positive feedback, making LRRC8 proteins highly critical for cisplatin effectiveness.
In any case, even though we are aware that the role of LRRC8 proteins as cisplatin effectors remains to be validated, our study supports the importance of LRRC8 proteins in cisplatin uptake across the plasma membrane, and suggests that LRRC8 proteins might be interesting candidates as drug targets for combatting cisplatin resistance.
Materials and methods
Molecular biology
Plasmids used have been described in detail in Gaitán-Peñas, et al.Citation16 For some experiments we used WT LRRC8A, LRRC8D, or LRRC8E. In addition we used constructs in which LRRC8A was fused at the C-terminal to VFP (short: 8A-VFP), while LRRC8D and LRRC8E were fused at the C-terminal to mCherry (short: 8D-mCh, 8E-mCh, respectively).
For expression in Xenopus oocytes, after linearization by NotI, cRNA was transcribed using the mMessage mMachine SP6 kit (Ambion).
Oocyte preparation and injection
Oocytes were prepared and injected as described in ref Citation16. Animal protocols were approved by the Ethics Committee for Animal Experimentation of the Biophysics Institute. 50 nl cRNA, containing ∼12 ng of each subunit, was injected with a microinjector. After injection, oocytes were maintained at 18°C in a “Maintaining” solution containing (in mM): 90 NaCl, 2 KCl, 1 MgCl2, 1 CaCl2, 10 Hepes at pH 7.5.
Electrophysiology
One to 3 d after injection, voltage clamp measurements were performed with the custom acquisition program GePulse and a Turbo TEC-03X amplifier (npi electronics, Tamm, Germany). For the measurements on untagged construct, 8A/8E, the standard extracellular hypoosmotic solution (“Hypo”) was (in mM): 48 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 10 Hepes at pH 7.3 (osmolarity: 120 mOsm); “Hypo” was supplemented with mannitol to obtain the “Iso” solution (osmolarity: 200 mOsm). The standard bath solution used for the measurements on tagged constructs, 8A-VFP/8E-mCh and 8A-VFP/8D-mCh, contained (in mM): 100 NaCl, 5 MgSO4, 10 Hepes at pH 7.3 (osmolarity: 215 mOsm).
To estimate LRRC8-mediated currents at different potentials, the “IV-pulse protocol” was applied: a prepulse to −100 mV for 200 msec was followed by voltages ranging from −100 to 60 mV with 40 mV increments for 3000 msec. Pulses ended with a tail to −70 mV for 500 msec.
Cisplatin, blasticidin S
To test the acute effect of cisplatin and blasticidin S (for short called henceforth blasticidin), the drugs were dissolved in the standard bath solution at the desired concentration: cisplatin at 1 mM, blasticidin at 0.25 mM (both cisplatin and blasticidin were purchased from Sigma Aldrich (Milan, Italy)). Under continuous perfusion, the current was monitored by repetitive 200 ms pulses to 60 mV once every 5 seconds. Currents measured under perfusion were normalized to those in standard bath solution. To estimate the direct intracellular effect of cisplatin, oocytes that expressed 8A-VFP/8D-mCh or 8A-VFP/8E-mCh were injected with 50 nl of cisplatin at 3 mM in distilled water and overnight incubated in “Maintaining” solution. Assuming an oocyte volume of 1 µl, an effective concentration in the cytosol of 0.15 mM is estimated. Currents were measured before and after incubation. To study the chronic effect on oocytes injected with un-tagged and tagged LRRC8 constructs cisplatin was dissolved in “Maintaining” solution at 1 mM, 0.5 mM and 0.15 mM, blasticidin at 0.25 mM. The currents were measured before and after overnight incubation (indicated in figures o/n and corresponding to 12–16 h).
To rule out unspecific effects, un-injected oocytes underwent the same treatments of injected oocytes. Additionally, all experiments that required incubation were combined with parallel measurements in control solution without drug. Error bars indicate SD. The unpaired Student's t test was used to compare the mean currents.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by a grant from the Fondazione Compagnia di San Paolo, Torino (2013.0922 to A. Boccaccio). In addition, this work was supported in part by the European Leukodystrophies Association (ELA) Research Foundation (ELA2012-014C2B) to RE, Ministerio de Ciencia e Innovación (MICINN) SAF2015-70377 to RE, Generalitat de Catalunya SGR2014-1178 to RE) and Instituto de Salud Carlos III ERARE to RE. RE is a recipient of an ICREA Academia prize.
References
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol 1997; 68:69-119; PMID:9481145; https://doi.org/10.1016/S0079-6107(97)00021-7
- Jentsch TJ. VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat Rev Mol Cell Biol 2016; 17(5):293-307; PMID:27033257; https://doi.org/10.1038/nrm.2016.29
- Okada Y, Sato K, Numata T. Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J Physiol 2009; 587:2141-9; PMID:19171657
- Hisadome K, Koyama T, Kimura C, Droogmans G, Ito Y, Oike M. Volume-regulated anion channels serve as an auto/paracrine nucleotide release pathway in aortic endothelial cells. J Gen Physiol 2002; 119:511-20; PMID:12034759; https://doi.org/10.1085/jgp.20028540
- Eggermont J, Trouet D, Carton I, Nilius B. Cellular function and control of volume-regulated anion channels. Cell Biochem Biophys 2001; 35:263-74; PMID:11894846; https://doi.org/10.1385/CBB:35:3:263
- Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 2014; 344:634-8; PMID:24790029; https://doi.org/10.1126/science.1252826
- Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 2014; 157:447-58; PMID:24725410; https://doi.org/10.1016/j.cell.2014.03.024
- Abascal F, Zardoya R. LRRC8 proteins share a common ancestor with pannexins, and may form hexameric channels involved in cell-cell communication. Bioessays 2012; 34:551-60; PMID:22532330; https://doi.org/10.1002/bies.201100173
- Lee EL, Shimizu T, Ise T, Numata T, Kohno K, Okada Y. Impaired activity of volume-sensitive Cl- channel is involved in cisplatin resistance of cancer cells. J Cell Physiol 2007; 211:513-21; PMID:17186499; https://doi.org/10.1002/jcp.20961
- Planells-Cases R, Lutter D, Guyader C, Gerhards NM, Ullrich F, Elger DA, Kucukosmanoglu A, Xu G, Voss FK, Reincke SM, et al. Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. Embo J 2015; 34:2993-3008;PMID:26530471;https://doi.org/10.15252/embj.201592409
- Sorensen BH, Dam CS, Sturup S, Lambert IH. Dual role of LRRC8A-containing transporters on cisplatin resistance in human ovarian cancer cells. J Inorg Biochem 2016; 160:287-95; PMID:27112899; https://doi.org/10.1016/j.jinorgbio.2016.04.004
- Sorensen BH, Nielsen D, Thorsteinsdottir UA, Hoffmann EK, Lambert IH. Down-regulation of LRRC8A protects Human Ovarian and Alveolar Carcinoma cells against Cisplatin-induced expression of p53, MDM2, p21 and Caspase-9/-3 activation. Am J Physiol Cell Physiol 2016:310:C857-73; https://doi.org/10.1152/ajpcell.00256.2015
- Sorensen BH, Thorsteinsdottir UA, Lambert IH. Acquired cisplatin resistance in human ovarian A2780 cancer cells correlates with shift in taurine homeostasis and ability to volume regulate. Am J Physiol Cell Physiol 2014; 307:C1071-80; PMID:25252947; https://doi.org/10.1152/ajpcell.00274.2014
- Lee CC, Freinkman E, Sabatini DM, Ploegh HL. The protein synthesis inhibitor blasticidin s enters mammalian cells via leucine-rich repeat-containing protein 8D. J Biol Chem 2014; 289:17124-31; PMID:24782309; https://doi.org/10.1074/jbc.M114.571257
- Ullrich F, Reincke SM, Voss FK, Stauber T, Jentsch TJ. Inactivation and anion selectivity of volume-regulated VRAC channels depend on carboxy-terminal residues of the first extracellular loop. J Biol Chem 2016; 291:17040-8; PMID:27325695; https://doi.org/10.1074/jbc.M116.739342
- Gaitán-Peñas H, Gradogna A, Laparra-Cuervo L, Solsona C, Fernández-Dueñas V, Barrallo-Gimeno A, Ciruela F, Lakadamyali M, Pusch M, Estévez R. Investigation of LRRC8-mediated volume-regulated anion currents in Xenopus oocytes. Biophys J 2016; 111:1429-43; PMID:27705766; https://doi.org/10.1016/j.bpj.2016.08.030
- Pedersen SF, Okada Y, Nilius B. Biophysics and physiology of the volume-regulated anion channel (VRAC)/volume-sensitive outwardly rectifying anion channel (VSOR). Pflugers Arch 2016; 468:371-83; PMID:26739710; https://doi.org/10.1007/s00424-015-1781-6
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 2003; 22:7265-79;PMID:14576837;https://doi.org/10.1038/sj.onc.1206933
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 2014; 740:364-78; PMID:25058905; https://doi.org/10.1016/j.ejphar.2014.07.025
- Ise T, Shimizu T, Lee EL, Inoue H, Kohno K, Okada Y. Roles of volume-sensitive Cl- channel in cisplatin-induced apoptosis in human epidermoid cancer cells. J Membr Biol 2005; 205:139-45; PMID:16362502; https://doi.org/10.1007/s00232-005-0779-y
- Svidritskiy E, Ling C, Ermolenko DN, Korostelev AA. Blasticidin S inhibits translation by trapping deformed tRNA on the ribosome. Proc Natl Acad Sci U S A 2013; 110:12283-8; PMID:23824292; https://doi.org/10.1073/pnas.1304922110
