Small-molecule modulators of inward rectifier potassium (Kir) channels must cross virtual oceans of fluid and phospholipid to reach the rich binding terrain of the channel protein. With the exception of anti-diabetic medications (e.g. glibenclamide) targeting ATP-regulated Kir channels, the molecular pharmacology of the Kir channels is still very rudimentary. Commonly used Kir channel inhibitors include the inorganic cations barium and cesium, and clinically used drugs that exhibit relatively weak off-target activity toward Kir channels.Citation1 A major hurdle to realizing the therapeutic value of some Kir channels for treating disorders such as hypertension, pain, and metabolic diseases, is developing subtype-specific Kir channel modulators. Over the last several years, our group has taken a 2-pronged approach to developing what is in some cases first generation Kir channel inhibitors: 1) Discovery and optimization of completely novel small-molecule inhibitors using high-throughput screening (HTS) of large compound libraries and medicinal chemistry; 2) Characterizing small-molecule binding sites using comparative homology modeling, site-directed mutagenesis, and patch clamp electrophysiology. As discussed below, these studies are revealing how synthetic inhibitors can co-opt binding sites for naturally occurring pore blockers such as polyamines and magnesium to inhibit Kir channel activity.
VU5902 was the first publicly disclosed small-molecule inhibitor of Kir1.1 (IC50 = 0.2 µM), an emerging diuretic target,Citation3 and Kir7.1 (IC50 = 6 µM), a putative drug target for postpartum hemorrhageCitation4 and central regulation of energy homeostasis.Citation5 VU590 was discovered in a HTS of approximately 225,000 compounds for small-molecule modulators of Kir1.1.Citation2 A first clue that the VU590 binding site is located in central cavity of both Kir1.1 and Kir7.1 came from experiments showing that block was voltage dependent and sensitive to the potassium equilibrium potential difference.Citation2,6 Subsequent mutagenesis and electrophysiology experiments identified asparagine 171 (N171) in Kir1.1, and glutamate 149 (E149) and serine 150 (S150) in Kir7.1 as necessary for block by VU590 (). Kir1.1-N171 and Kir7.1-E149 are called “rectification controllers” because the side chain charge at these positions determines the strength of pore block by polyamines and magnesium. Although N171 and E149 occupy the same general location in their respective pores, VU590 interacts with Kir1.1 and Kir7.1 in mechanistically distinguishable ways. For example, mutation of N171 to a negatively charged aspartate (N171D) residue leads to a 75-fold loss of Kir1.1 sensitivity to VU590, leading us to postulate that the negatively charged E149 accounted for the reduced VU590 sensitivity of Kir7.1. Contrary to our hypothesis, however, we found that substitution of an uncharged residue (E149Q) in Kir7.1 actually reduced VU590 potency. Furthermore, comparing how modifications to the core chemical structure of VU590 affect potency toward the 2 channels reveals divergent structure-activity relationships.Citation6 Taken together, these data support the notion that the VU590-channel interface is unique in Kir1.1 and Kir7.1.
Figure 1. Residues in Kir1.1 and Kir7.1 required for VU590-dependent block. (Left) Kir1.1 requires N171 for maximal sensitivity to VU590. Mutation to aspartate (N171D) or glutamate (N171E) abolishes VU590 sensitivity. (Right) A polar barrier created by T153 hinders access of VU590 (and VU714) to a deeper binding site comprised of E149 and S150 in Kir7.1. Mutation of T153 to the corresponding residue in Kir1.1 (T153C) increases VU590 activity toward Kir7.1 by 6-fold. The reverse mutation in Kir1.1 (C175T) decreases VU590 sensitivity only in a mutant form of the channel (i.e., N171Q) that exhibits a lower sensitivity to VU590.
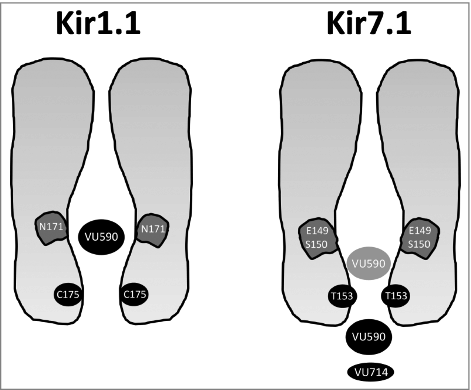
Further exploration revealed a pore polar barrier in Kir7.1 that accounts for much of the disparity in VU590 activity toward the 2 channels.Citation6 Indeed, mutation of threonine 153 (T153) to the corresponding cysteine residue in Kir1.1 (T153C; ) led to a 6-fold improvement in Kir7.1 sensitivity to VU590. By studying mutations that change polarity and/or steric hindrance at this position, we concluded that the constrained polarity of the T153 hydroxyl group creates an energetic and/or physical barrier at the inner pore mouth that restricts VU590 access to a deeper binding site comprised of E149 and S150 (). This is not unique to VU590 since the T153C mutation also increases Kir7.1 sensitivity to VU714, a 1.5 µM IC50 Kir7.1 inhibitor described previously by our group that also interacts with E149 and S1507. Interestingly, however, the potency of ML418, a higher affinity (IC50 = 310 nM) analog of VU714, is unaffected by the T153C mutation. This suggested the pore polar barrier affects binding site access of low-affinity, but not high-affinity, pore-blocking ligands. Consistent with this idea, we found that the reverse mutation in Kir1.1 (C175T) affects VU590 potency only when it is introduced into a mutated version of the channel (i.e. Kir1.1-N171Q) that is inhibited by VU590 with lower potency (IC50 = 0.7 µM).Citation6
We believe our efforts to track down the pore polar barrier in the binding landscape of the membrane channel pore have notable implications for the development of the Kir7.1 pharmacology. In a recent HTS of several thousand compounds for small-molecule modulators of Kir7.1, we identified surprisingly few tractable hits (0.2% hit rate) that can be moved forward to lead optimization.Citation7 Based on the findings described above, we speculate that the pore polar barrier might be at least in part to blame. If this is correct, performing a HTS against the Kir7.1-T153C mutant should yield more chemically tractable hits for optimization. Furthermore, and importantly, using the T153C mutant as a surrogate should not adversely affect the potency of optimized, sub-micromolar inhibitors toward the wild type channel.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Swale DR, Kharade SV, Denton JS. Cardiac and renal inward rectifier potassium channel pharmacology: emerging tools for integrative physiology and therapeutics. Curr Opin Pharmacol. 2014;15:7-15. doi:10.1016/j.coph.2013.11.002. PMID:24721648
- Lewis LM, Bhave G, Chauder BA, Banerjee S, Lornsen KA, Redha R, Fallen K, Lindsley CW, Weaver CD, Denton JS., High-throughput screening reveals a small-molecule inhibitor of the renal outer medullary potassium channel and Kir7.1. Mol Pharmacol. 2009;76(5):1094-103. doi:10.1124/mol.109.059840. PMID:19706730
- Denton JS, Pao AC, Maduke M. Novel Diuretic Targets. Am J Physiol Renal Physiol. 2013;305(7):F931-42. doi:10.1152/ajprenal.00230.2013. PMID:23863472
- McCloskey C, Rada C, Bailey E, McCavera S, van den Berg HA, Atia J, Rand DA, Shmygol A, Chan YW, Quenby S, et al. The inwardly rectifying K+ channel KIR7.1 controls uterine excitability throughout pregnancy. EMBO Mol Med. 2014;6(9):1161-74. doi:10.15252/emmm.201403944. PMID:25056913
- Ghamari-Langroudi M, Digby GJ, Sebag JA, Millhauser GL, Palomino R, Matthews R, Gillyard T, Panaro BL, Tough IR, Cox HM, et al. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature. 2015;520(7545):94-8. doi:10.1038/nature14051. PMID:25600267
- Kharade S, Sheehan J, Figueroa E, Meiler J, Denton J. Pore polarity and charge determine differential block of Kir1.1 and Kir7.1 potassium channels by the small-molecule inhibitor VU590. Mol Pharmacol. 2017;92(3):338-346. doi:10.1124/mol.117.108472. PMID:28619748
- Swale DR, Kurata H, Kharade SV, Sheehan J, Raphemot R, Voigtritter KR, Figueroa EE, Meiler J, Blobaum AL, Lindsley CW, Hopkins CR, Denton JS. ML418: The first selective, sub-micromolar pore blocker of Kir7.1 potassium channels. ACS Chem Neurosci. 2016;7(7):1013-23. doi:10.1021/acschemneuro.6b00111. PMID:27184474
