ABSTRACT
To explore the research status, hotspots, and trends in research on nicotinic acetylcholine receptor (nAChR) channel. The Web of Science core collection database from 2000 to 2020 was used as the data source. The visual analysis software VOSviewer1.6.16 and Citespace5.7 R3 were used to visualize the studies of the nAChR channel. The national/institutional distribution, journal distribution, authors, and related research were discussed. A total of 5,794 articles were obtained. The USA and the Utah System of Higher Education were the most productive country and institution for nAChR channel research. Journal of Biological Chemistry was the most productive journal (212) and the most productive researcher was McIntosh, J. Michael. The first highly co-cited article was “Refined structure of the nicotinic acetylcholine receptor at 4A resolution.” The most researched area was neurosciences neurology. The hot spots of nAChR channel research were “subunit and structure of nAChR,” “activation/agonist of nAChR channel,” and “Changes in nAChRs With Alzheimer’s Disease.” The top three research frontiers of nAChR channel research were “neuropathic pain,” “neuroinflammation,” and “α7 nACHR.” The study provides a perspective to visualize and analyze hotspots and emerging trends in the nAChR channel.
There are two types of acetylcholine receptors (AChR): nicotinic AChR (nAChR) and muscarinic AChR. nAChRs are fast cationic channels, initially discovered in muscles and autonomic ganglia function, while muscarinic AChRs are class I heptahelical G-protein-coupled receptors with a slower signaling pace [Citation1,Citation2].
nAChRs are members of the Cys-loop ligand-gated ion channel superfamily, which also comprises the γ-aminobutyric acid type A (GABAA), glycine (Gly), and serotonin type 3 (5-HT3) receptors. nAChR is a pentamer with five homomeric or heteromeric subunits. The diversity of nAChR subunit combinations determines their ligand-binding sites, which regulate various physiological processes. For example, nAChRs can affect the cation permeability, from monovalent Na+and K+ ions to divalent Ca2+ ions. In turn, Ca2+ ions influence signal transduction, which may affect their modulation by external Ca2+ and Zn2+ cations [Citation3–5].
Aberrant expression or activation of nAChRs cause human diseases, including addiction, schizophrenia [Citation6], epilepsy [Citation7], Alzheimer’s disease [Citation8], Parkinson’s disease [Citation9], myasthenia gravis [Citation10], and neuropathic pain [Citation11], making nAChRs a major neurotherapeutic target.
Bibliometric analysis has been widely used to calculate the productivity of countries, institutions, authors, and the frequency of keywords to explore research hotspots/frontiers in specific fields [Citation12–14]. Although nAChR channels have been a hotspot of multidisciplinary research for decades, no bibliometric studies regarding the trends in nAChR channels research activity have been published. Here, we collected scientific publications on nAChR channels research in the past 21 years, then used CiteSpace and VOSviewer for data analysis and visualization to provide researchers with some direction regarding nAChR channels research [Citation15,Citation16].
Data collection
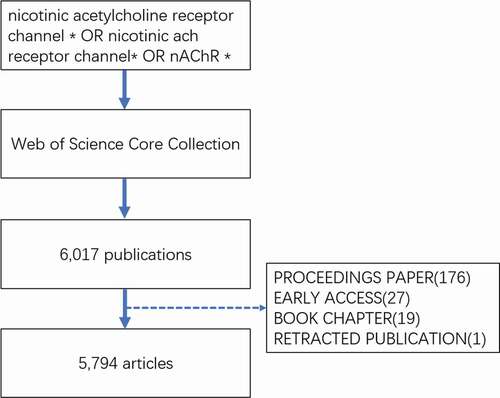
The data search was conducted on 1 December 2020. The search keywords entered into the database were as follows: TS = (nicotinic acetylcholine receptor channel * OR nicotinic ACh receptor channel* OR nAChR *), language: (English) and year range: (2000–2020). The data were extracted from the Science Citation Index Expanded (SCI-expanded) of Web of Science Core Collection (WoSCC) bibliographic database, and the data were collected within 1 day to avoid any potential deviation due to the daily updating of the database. In this study, the data were downloaded directly from the database as secondary data without further animal experiments. Therefore, no ethical approval was required. Six thousand seventeen publications were obtained, and the following documents were excluded: proceedings paper (176), early access (27), book chapter (19), and retracted publication (1). In total, 5,794 articles were analyzed. The retrieval strategy of the experiments is shown in . The VOSviewer 1.6.16 was used to identify top countries, institutions, authors, and journals. The CiteSpace 5.7 R3 was used to analyze keywords, co-cited references, and trends.
General information and annual publication output
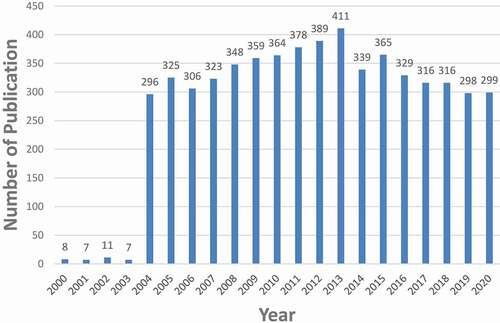
Five thousand seven hundred and ninety-four articles were obtained. To explore the trends in nAChR channel research, we showed the number of articles per year in the form of a histogram. As shown in , the number of publications on nAChR channel research increased gradually since 2004, reached a peak in 2013, and then began to decline, but it was still above 250. The average annual number of publications was 275.9.
Active countries and institutions
The co-occurrence map provides valuable information and helps researchers to identify the cooperative relationship [Citation17,Citation18]. lists the top 10 countries and institutions that contributed to publications on the nAChR channel. Countries and institutions co-occurrence maps are shown in ).
Table 1. The top 10 countries and institutions contributed to publications on nAChR channel research
Figure 3. The analysis of countries and institutions. (a). The network of countries/territories engaged in nAChR channel research; (b). The network of institutions engaged in nAChR channel research
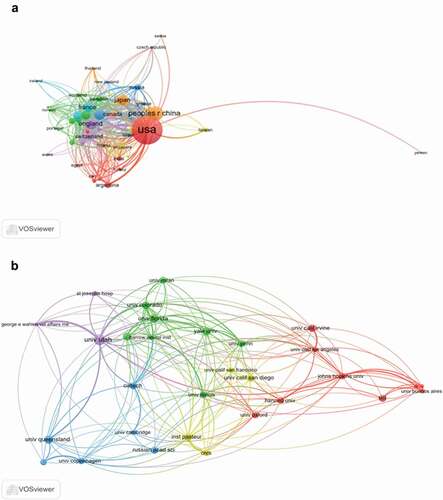
The 5,794 articles on nAChR channel research were published by more than 2,931 research institutions in 87 countries/territories. The USA, Peoples R China, England, Germany, and Japan were the top five productive countries (). The United States published the most papers (2,905 articles), followed by China (672 articles), and they were the two critical countries in nAChR channel research. ) shows that the United States attached great importance to cooperation and had close collaborations with China, Japan, Germany, England France Australia, and Italy. shows that American institutions published most of the publications. The Utah System of Higher Education (University of Utah) produced the highest number of publications on nAChR channels (417), followed by the University of California System (306). The co-occurrence map of institutions showed that scientific cooperation among institutions was greatly affected by the geographical location, and there are more cooperations among institutions in the same region ().
Active journals
The 5,794 articles were published in 1,003 journals. lists the top 10 journals that published articles on nAChR channel research. The Journal of Biological Chemistry had the highest number at 212 (3.65%) (IF2019 = 4.238), followed by Neuropharmacology published 186 papers (3.21%) (IF2019 = 4.431), and the Journal of Neuroscience ranked third at 167 articles (2.882%) (IF2019 = 5.673).
Table 2. The top 10 journals that published articles on nAChR channel research
Active authors
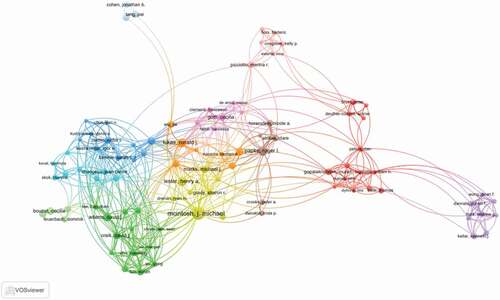
Author co-occurrence map can provide information on influential research groups and potential collaborators. It can help researchers to find potential collaborators [Citation17,Citation18]. Approximately 17,830 authors contributed 5,794 articles related to nAChR channel research. The networks shown in indicate the cooperation among authors, and the top 10 active authors are listed in . McIntosh, J. Michael ranked first in nAChR channel publication with 160 articles, who mainly focused on alpha9 nAChRs and their role in chronic pain [Citation19,Citation20]. Papke, Roger L. was the second highly published author (82 articles). His research focused on the mechanisms of nAChR ligands and signaling and contributed to addiction, pain, inflammation, and other medically important issues [Citation21–23].
Table 3. The top 10 active authors in nAChR channel research
Co-cited references
Five thousand seven hundred and ninety-four articles were visualized and analyzed using CiteSpace with a period time from 2000 to 2020, and a time slice of 1 was chosen for the analysis of the co-cited references. The network of co-cited references on nAChR channels consists of references with higher centrality and citation counts which is presented in . The highly cited references were analyzed to determine the key knowledge base in the field. The top 10 highest co-cited references are summarized in .
Table 4. The top 10 Co-cited (CR) in nAChR channel research
Figure 5. The analysis of Co-cited references: Co-citation network of references from publications on nAChR channel research
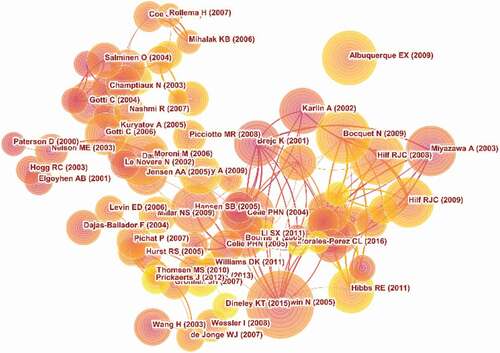
The 10 co-cited references on nAChR channel are mainly basic research from structure to function. The first highly co-cited article was “Refined structure of the nicotinic acetylcholine receptor at 4A resolution.”(460 citation rate), in which Unwin N confirmed that two ligand-binding subunits had a different extended conformation from three other subunits in the closed channel, identified several interactions on both pairs of subunit interfaces and within the subunits, and finally proposed a refined structure of the nicotinic acetylcholine Receptor at 4 A°Resolution. There were five co-cited references published in Nature: In 2001, Brejc K studied the crystal structure of the molluscan acetylcholine-binding protein (AChBP), a structural and functional homologue of the amino-terminal ligand-binding domain of a nAChR a-subunit, which assisted in the development of anti-Alzheimer’s disease and nicotine addiction drugs [Citation24]. In 2003, Miyazawa A mainly focused on the structure and gating mechanism of the acetylcholine receptor pore, present an atomic model of the closed pore to shield the inner ring from the lipids [Citation25]. In 2008, Hilf RJC reveals the first structure of pentameric ligand-gated ion channels (pLGICs) at 3.3 A°resolution and provides an important model system for the investigation of the general mechanisms of ion permeation and gating within the family [Citation26]. One year later, Bocquet N presented the X-ray structure at 2.9 A°resolution of the bacterial Gloeobacter violaceus pLGICs at pH 4.6 in an apparently open conformation [Citation27]. In 2011, Hibbs RE presented the first three-dimensional structure at 3.3 A°resolution to explain the principles of activation and permeation in an anion-selective Cys-loop receptor [Citation28]. These articles laid the foundation for studying the structure and mechanism of nAChR channels. Albuquerque EX and Karlin A systematically reviewed nAChR channels, including nAChR subunit structure, nAChR expression, nAChR function, and relationship with disease [Citation29,Citation30]. And these reviews provided a theoretical basis for the study of nAChR channels.
Research area analysis
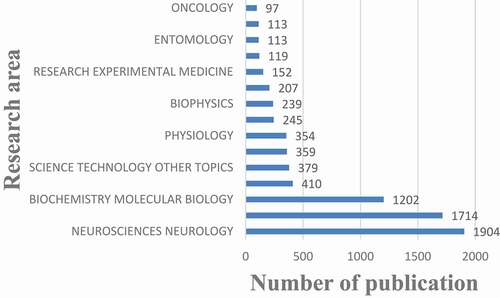
shows the top 15 research areas that appeared in publications related to nAChR channel research from 2000 to 2020. NEUROSCIENCES NEUROLOGY, PHARMACOLOGY PHARMACY, and BIOCHEMISTRY MOLECULAR BIOLOGY are the top three areas where nAChR channels are more studied.
Keyword co-occurrence and burst
Keywords represent the crucial content of research. Keyword co-occurrence analysis provides a reasonable description of research hotspots, and burst keywords can represent research frontiers over a period of time [Citation31].
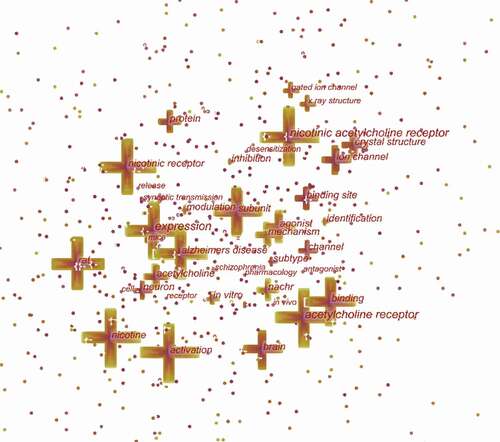
CiteSpace 5.7.R3 was used to construct an acknowledge map of keyword co-occurrence () and identified the top 20 keywords in nAChR channel research articles from 2000 to 2020 (), according to frequency. The top keywords were “nicotinic acetylcholine receptor,” “acetylcholine receptor,” “expression,” “nicotine,” “activation,” “nicotinic receptor,” “subunit,” “rat,” “binding,” “Alzheimers disease,” “brain,” “agonist,” “binding site,” “ion channel,” “acetylcholine,” “crystal structure,” “mechanism,” “protein,” “neuron,” “nachr.” Therefore, research hotspots can be summarized in the following aspects:
Table 5. Top 20 keywords in terms of frequency in nAChR channel research
Subunit and structure of nAChR
The AChR is an integral membrane protein that responds to the binding of acetylcholine. nAChR is a pentamer with five homomeric or heteromeric subunits, consisting of 10 α subunits (α1–10), four β subunits (β1–4), and γ, δ, and ε subunits. Although these subunits combine to form various nAChR subtypes, only two subtypes are highly expressed in the central nervous system. One is α4β2 heteromeric subunits, which have a high affinity for nicotine. Another is five α7 homomeric subunits, referred to as α7nAChR, mainly contribute to α-bungarotoxin [Citation32,Citation33].
The structure of nAChR subunits is composed of a large extracellular N-terminal domain (NTD; the location of the Cys-loop), three hydrophobic transmembrane regions (M1-M3), a variable intracellular loop, a fourth transmembrane region (M4), and a short extracellular C-terminus. The M1, M3, andM4 segments separate the pore-lining region from the hydrophobic membrane [Citation2,Citation33,Citation34].
Activation/agonist of nAChR channel
The cyclic C tyrosines and the cyclic B tryptophan behave similarly at all of the sites; the cyclic A tyrosine and γW55 are larger energy sources at αγ; αY190 provides most of the free energy from the affinity change in adult AChRs, but in Fetal αγ site and γW55 rank first together [Citation35]. And in terms of agonist energy, the effects of the two agonist sites are almost independent in adult and fetal AChR because the sum of the energy measured from one site is approximately equal to the total energy of the two sites AChRs [Citation36,Citation37]. However, Mukhtasimova N. once proposed that there were two states before the opening of nAChR: the first state elicits brief openings, whereas the second elicits long-lived openings in 2009. And he found whether with or without agonists, long-lived openings and related activation states could be detected and showed the same dynamic characteristics [Citation38].
Changes in nAChRs with alzheimer’s disease
George AA demonstrated that oligomeric forms of Aβ1-42 (oAβ42) interact with α7β2-containing nAChR, and altered the intrinsic excitability of specific populations of basal forebrain cholinergic neurons (BFCNs). And he found α7β2-nAChR signaling could weaken spatial reference memory deficits in the APP/PS1 mouse model of AD [Citation39].
Cholinesterase inhibitors, such as galantamine, are first approved to treat mild to moderately severe Alzheimer’s disease. However, the clinical potency of these drugs does not correlate well with their activity as cholinesterase inhibitors nor is their action as short-lived as purely symptomatic treatment. A subgroup of cholinesterase inhibitors can directly interact with nAChR, sensitize nicotinic receptors by increasing channel opening probability and by slowing down receptor desensitization [Citation40].In 2008, Kowal NM evaluated galantamine actions at α4β2 and α7 nAChR in Xenopus laevis oocytes and subjected them to two-electrode voltage-clamp electrophysiological experiments. Finally, he concluded that galantamine is not a positive allosteric modulator of α7 or α4β2 receptors [Citation41].
A new study found SK family, calcium-sensitive potassium channels, mediated inhibition exert powerful negative feedback on nicotinic excitation, dampening attention-relevant signaling in the TgCRND8 brain. These findings may contribute to a novel therapeutic target for early attention deficits in AD [Citation42].
Keywords were identified and analyzed using strong citation bursts () to explore the frontiers of research. As shown in , the red line indicates the period time during which the burst keyword appears [Citation34]. The keywords that had strong bursts after 2015 were “discovery” (2015–2020), “neuropathic pain” (2015–2020), “allosteric modulation” (2016–2020), “alpha 7 nicotinic acetylcholine receptor” (2016–2020), “alpha 7 nAChR” (2017–2020), and “neuroinflammation” (2018–2020). The three research frontiers of nAChR channel research were as follows:
Table 6. Top 25 keywords with the strongest citation bursts
Neuropathic pain
Neuropathic pain is usually chronic, caused by a lesion or disorders of the peripheral or the central nervous system. And it influences 7–10% of the general population [Citation43]. The most common conditions of neuropathic pain include trigeminal neuralgia, peripheral nerve injury, painful polyneuropathy, postherpetic neuralgia, and painful radiculopathy [Citation44]. Studies show that neuropathic pain seriously impairs quality of life [Citation45,Citation46] and cause enormous economic losses to the country and society [Citation47].
Neuroinflammation
Neuroinflammation is common in the majority of neurological conditions. It can be both an inducement and a secondary reaction to nervous system insult [Citation48]. The Neuroinflammation process is marked by the production of pro-inflammatory cytokines, such as IL-1β, IL-6, IL-18 and tumor necrosis factor (TNF), chemokines, small-molecule messengers, and reactive oxygen species by innate immune cells in the CNS, and microglia and astrocytes mainly involved in this process [Citation49–51]
α7 nACHR
The α7 nAChR is a ligand-gated ion channel and is highly expressed in the brain regions (e.g., the cerebral cortex and hippocampus) responsible for cognitive functions. The α7 nAChR is distributed both presynaptically and postsynaptically, to activate intracellular signaling cascades [Citation32].
Burn injury (BI) pain consists of inflammatory and neuropathic components. BI significantly increased spinal cord microgliosis, microglia activation, and pain-transducer (protein and/or messenger RNA) expression. Zhou Yinhui found that GTS-21, a selective α7AChR agonist, mitigated pain-transducer changes, while the α7AChR antagonist could nullify the beneficial effects of GTS-21 [Citation51].
Chronic cerebral hypoperfusion (CCH) induces an inflammatory response and contributes to cognitive impairment. It is demonstrated that activating of α7nAChR and its downstream JAK2-STAT3 pathway could promote cognitive function and improve neuroprotective effects against inflammation in CCH rats [Citation52].
Conclusions
Based on the WOSCC database, bibliometric and Visual analysis was used to study the characteristics of nAChR channel research results from 2000 to 2020. Since 2004, the number of publications on nAChR channels has maintained approximately 300 per year. The three hot spots of nAChR channel research were “subunit and structure of nAChR,” “activation/agonist of nAChR channel,” and “Changes in nAChRs With Alzheimer’s Disease.” The top three research frontiers were “neuropathic pain,” “neuroinflammation,” and “α7 nAChR.” Bibliometric analysis of the literature on the nAChR channels contributes researchers to identify cooperations, find research hotspots, and predict the frontiers of nAChR channel research.
Acknowledgments
The authors would like to express their appreciation to Professor CM Chen, who invented CiteSpace, which is free to use. Xueping Zhu and Yan Zhou are the co-first authors. Yuanhui Hu is co-corresponding author.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wu T, Wang Y, Shi W, et al. A variant in the nicotinic acetylcholine receptor alpha 3 subunit gene is associated with hypertension risks in hypogonadic patients. Front Genet. 2020 Nov 27;11:539862.
- Phillips MB, Nigam A, Johnson JW. Interplay between gating and block of ligand-gated ion channels. Brain Sci. 2020 Dec 1;10(12):928.
- Vernino S, Amador M, Luetje CW, et al. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992 Jan;8(1):127–134.
- Dani JA, Eisenman G. Monovalent and divalent cation permeation in acetylcholine receptor channels. Ion transport related to structure. J Gen Physiol. 1987 Jun;89(6):959–983.
- Kini RM. Toxins for decoding interface selectivity in nicotinic acetylcholine receptors. Biochem J. 2019 May 28;476(10):1515–1520.
- Koukouli F, Rooy M, Tziotis D, et al. Nicotine reverses hypofrontality in animal models of addiction and schizophrenia. Nat Med. 2017 Mar;23(3):347–354.
- Fukuyama K, Fukuzawa M, Shiroyama T, et al. Pathogenesis and pathophysiology of autosomal dominant sleep-related hypermotor epilepsy with S284L-mutant α4 subunit of nicotinic ACh receptor. Br J Pharmacol. 2020 May;177(9):2143–2162.
- Gu S, Matta JA, Lord B, et al. Brain α7 nicotinic acetylcholine receptor assembly requires NACHO. Neuron. 2016 Mar 2;89(5):948–955.
- Xu S, Yang B, Tao T, et al. Activation of α7-nAChRs protects SH-SY5Y cells from 1-methyl-4-phenylpyridinium-induced apoptotic cell death via ERK/p53 signaling pathway. J Cell Physiol. 2019 Aug;234(10):18480–18491.
- Gilhus NE, Tzartos S, Evoli A, et al. Myasthenia gravis. Nat Rev Dis Primers. 2019 May 2;5(1):30.
- Arias HR, Tae HS, Micheli L, et al. Coronaridine congeners decrease neuropathic pain in mice and inhibit α9α10 nicotinic acetylcholine receptors and CaV2.2 channels.. Neuropharmacology. 2020 Sep 15;175:108194.
- Chen C, Dubin R, Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update (2000-2014). Expert Opin Biol Ther. 2014 Sep;14(9):1295–1317.
- Wang Q, Yang Z, Yang Y, et al. A bibliometric analysis of research on the risk of engineering nanomaterials during 1999-2012. Sci Total Environ. 2014 Mar 1;473-474:483–489.
- Chen C, Song M, Glanzel W. Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS One. 2019 Oct 31;14(10):e0223994.
- Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004 Apr 6;101 Suppl 1(Suppl1):5303–5310.
- van Eck NJ, Waltman L. Software survey: vOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010 Aug;84(2):523–538.
- Briganti M, Delnevo CD, Brown L, et al. Bibliometric analysis of electronic cigarette publications: 2003–2018. Int J Environ Res Public Health. 2019 Jan 24;16(3):320.
- Ghorbani F, Feizabadi M, Farzanegan R, et al. An investigation of topics and trends of tracheal replacement studies using co-occurrence analysis. Tissue Eng Part B Rev. 2017 Apr;23(2):118–127.
- McIntosh JM, Absalom N, Chebib M, et al. Alpha9 nicotinic acetylcholine receptors and the treatment of pain. Biochem Pharmacol. 2009 Oct 1;78(7):693–702.
- McIntosh JM, Plazas PV, Watkins M, et al. A novel alpha-conotoxin, PeIA, cloned from Conus pergrandis, discriminates between rat alpha9alpha10 and alpha7 nicotinic cholinergic receptors. J Biol Chem. 2005 Aug 26;280(34):30107–30112.
- Papke RL, Garai S, Stokes C, et al. Differing activity profiles of the stereoisomers of 2,3,5,6TMP-TQS, a putative silent allosteric modulator of α7 nAChR. Mol Pharmacol. 2020 Oct;98(4):292–302.
- Papke RL, Lindstrom JM. Nicotinic acetylcholine receptors: conventional and unconventional ligands and signaling. Neuropharmacology. 2020 May;15(168):108021.
- Papke RL, Peng C, Kumar A, et al. NS6740, an α7 nicotinic acetylcholine receptor silent agonist, disrupts hippocampal synaptic plasticity. Neurosci Lett. 2018 Jun 11;677:6–13.
- Brejc K, van Dijk WJ, Klaassen RV, et al. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001 May 17;411(6835):269–276.
- Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003 Jun 26;423(6943):949–955.
- Hilf RJ, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008 Mar 20;452(7185):375–379.
- Bocquet N, Nury H, Baaden M, et al. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009 Jan 1;457(7225):111–114.
- Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011 Jun 2;474(7349):54–60.
- Albuquerque EX, Pereira EF, Alkondon M, et al. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009 Jan;89(1):73–120.
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002 Feb;3(2):102–114.
- Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. 2006;57(3):359–377.
- Ma KG, Qian YH. Alpha 7 nicotinic acetylcholine receptor and its effects on Alzheimer’s disease. Neuropeptides. 2019 Feb;73:96–106.
- Dani JA. Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int Rev Neurobiol. 2015;124:3–19.
- Papke RL. Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem Pharmacol. 2014 May 1;89(1):1–11.
- Auerbach A. Agonist activation of a nicotinic acetylcholine receptor. Neuropharmacology. 2015 Sep;96(Pt B):150–156.
- Jha A, Auerbach A. Acetylcholine receptor channels activated by a single agonist molecule. Biophys J. 2010 May 19;98(9):1840–1846. PMID: 20441747; PMCID: PMC2862204.
- Gupta S, Purohit P, Auerbach A. Function of interfacial prolines at the transmitter-binding sites of the neuromuscular acetylcholine receptor. J Biol Chem. 2013 May 3;288(18):12667–12679.
- Mukhtasimova N, Lee WY, Wang HL, et al. Detection and trapping of intermediate states priming nicotinic receptor channel opening. Nature. 2009 May 21;459(7245):451–454.
- George AA, Vieira JM, Xavier-Jackson C, et al. Implications of oligomeric amyloid-beta (oAβ42) signaling through α7β2-nicotinic acetylcholine receptors (nAChR) on basal forebrain cholinergic neuronal intrinsic excitability and cognitive decline. J Neurosci. 2021 Jan 20;41(3):555–575.
- Maelicke A, Samochocki M, Jostock R, et al. Allosteric sensitization of nicotinic receptors by galantamine, a new treatment strategy for Alzheimer’s disease. Biol Psychiatry. 2001 Feb 1;49(3):279–288.
- Kowal NM, Ahring PK, Liao VWY, et al. Galantamine is not a positive allosteric modulator of human α4β2 or α7 nicotinic acetylcholine receptors. Br J Pharmacol. 2018 Jul;175(14):2911–2925.
- Proulx É, Power SK, Oliver DK, et al. Apamin improves prefrontal nicotinic impairment in mouse model of Alzheimer’s Disease. Cereb Cortex. 2020 Mar 21;30(2):563–574.
- Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002.
- Scholz J, Finnerup NB, Attal N, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019 Jan;160(1):53–59.
- Sun X, Lin H, Jit M, et al. Incidence and disease burden of herpes zoster in the population aged ≥50 years in China: data from an integrated health care network. J Infect. 2020 Dec 21;S0163-4453(20):30774–X.
- Verriotis M, Peters J, Sorger C, et al. Phenotyping peripheral neuropathic pain in male and female adolescents: pain descriptors, somatosensory profiles, conditioned pain modulation and child-parent reported disability. Pain. 2020 Dec 15; Publish Ahead of Print. doi:https://doi.org/10.1097/j.pain.0000000000002172
- Harvey M, Prosser LA, Rose AM, et al. Aggregate health and economic burden of herpes zoster in the United States: illustrative example of a pain condition. Pain. 2020 Feb;161(2):361–368.
- Dendrou CA, McVean G, Fugger L. Neuroinflammation - using big data to inform clinical practice. Nat Rev Neurol. 2016 Dec;12(12):685–698.
- DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016 Oct;139 Suppl 2(Suppl2):136–153.
- Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol. 2020 Dec 14.
- Zhou Y, Leung-Pitt Y, Deng H, et al. Nonopioid GTS-21 mitigates burn injury pain in rats by decreasing spinal cord inflammatory responses. Anesth Analg. 2021 Jan;132(1):240–252.
- Cao Y, Wang L, Lin LT, et al. Acupuncture attenuates cognitive deficits through α7nAChR mediated anti-inflammatory pathway in chronic cerebral hypoperfusion rats. Life Sci. 2020 Nov 6;266:118732.