 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Sexual dimorphism has been reported in multiple pre-clinical and clinical studies on pain. Previous investigations have suggested that in at least some states, rodent dorsal root ganglion (DRG) neurons display differential sex-dependent regulation and expression patterns of various proteins involved in the pain pathway. Our goal in this study was to determine whether sexual dimorphism in the biophysical properties of voltage-gated sodium (Nav) currents contributes to these observations in rodents. We recently developed a novel method that enables high-throughput, unbiased, and automated functional analysis of native rodent sensory neurons from naïve WT mice profiled simultaneously under uniform experimental conditions. In our previous study, we performed all experiments in neurons that were obtained from mixed populations of adult males or females, which were combined into single (combined male/female) data sets. Here, we have re-analyzed the same previously published data and segregated the cells based on sex. Although the number of cells in our previously published data sets were uneven for some comparisons, our results do not show sex-dependent differences in the biophysical properties of Nav currents in these native DRG neurons.
Introduction
Chronic pain is the most prevalent health problem in the world [Citation1–3]. Pain has two components, which include nociception, in which the neural encoding of actual or impending tissue damage is signaled, and the emotional and psychological component, in which the nociceptive signal is perceived in the central nervous system (CNS) as an unpleasant experience that is called pain [Citation4]. An added layer of complexity in pain physiology is related to sexual dimorphism [Citation3,Citation5–7].
Dorsal root ganglion (DRG) neurons are among the most complex biological cell types, whose diversity and role as critical effector organs of the sensory pathway, including pain, is well-established [Citation8–11]. DRG neurons express many different types of ion channel and receptor ensembles in various stoichiometries [Citation8,Citation9,Citation12–16]. Among these proteins, the voltage-gated sodium (Nav) channel family are particularly interesting, as their role within the excitability pathway in DRG neurons is well-substantiated [Citation10,Citation14,Citation17]. Previous studies have shown that the complement of Nav channels (Nav1.1/6–9) expressed in DRG neurons varies with cell size. Nav1.7 and Nav1.8 are expressed in neurons of different sizes, but the remaining subtypes that are sensitive to tetrodotoxin (TTX) are predominantly found in larger neurons, and those that are resistant to TTX are found in smaller cells [Citation14,Citation18,Citation19].
The presence of sex-dependent differences in chronic pain has been long suggested by numerous large-scale studies that have investigated various types of pain, including musculoskeletal and arthritic pain, among many others [Citation20,Citation21]. Epidemiological studies suggest that women generally report more chronic pain than men [Citation3]. Furthermore, preclinical studies suggest that sex-dependent differences in the hormonal regulation of nociceptors could contribute to variation in pain phenotypes among sexes [Citation22–25]. Previous studies have shown that rodent DRG neurons display differential sex-dependent regulation and expression patterns of various proteins involved in the pain pathway. However, sex-dependent effects on biophysical properties of Nav channels within DRG neurons have not been previously investigated.
One approach to determining whether the biophysical properties of the Nav channels is different in DRG neurons from males versus females would be to assess sodium currents via a head-to-head and unbiased comparison of DRG neurons under identical conditions. This type of analysis has previously been challenging to achieve because of the low throughput nature of manual patch-clamp electrophysiology, and because of the presence of multiple subpopulations of sensory neurons [Citation12,Citation13].
We recently developed a new method using an automated high-throughput patch-clamp platform, where we functionally characterized the Nav channel properties of freshly isolated rodent DRG neurons [Citation9]. The published results in our previous study combined data from naïve wild-type (WT) adult C57BL/6 (7–9 weeks old) male and female mice, analyzed in an unbiased manner but not segregated on the basis of sex. In the present study we sought to determine whether Nav channel biophysical properties in DRG neurons varied as a function of sex. In this study, we have re-analyzed our previously published data, segregated by sex, and report that this analysis did not reveal sex-dependent differences in the biophysical properties of the Nav currents in populations of freshly isolated DRG neurons from male versus female adult C57BL/6 mice. To our knowledge, this is the first head-to-head comparison of Nav channel biophysical properties in sensory neurons from male versus female animals.
Materials and methods
Re-analysis of previously published data
All data presented in the manuscript were previously published [Citation9]. Because experiments were done in separate cohorts of naive male and female mice, in this present manuscript, we divided up the previously published data into sexes, and re-plotted and re-analyzed, the data, which was re-binned (based on distribution mean). Methodological information entailing how data were acquired and analyzed is below.
Preparation of DRG neurons from adult mouse
DRGs (at least 24 DRGs from each mouse) were harvested and immediately put in ice-cold complete saline solution (CSS) (in mM: 137 NaCl, 5.3 KCl, 1 MgCl2, 25 sorbitol, 3 CaCl2, and 10 HEPES, adjusted to pH 7.2 with NaOH). After all the DRGs were harvested, DRGs were transferred to 37°C enzyme solution −0.5 U/mL Liberase TM (Roche) and 0.6 mM EDTA in CSS for a 20-min incubation at 37°C, followed by a 15-min incubation at 37°C in another enzyme solution − 0.5 U/mL Liberase TL (Roche), 0.6 mM EDTA, and 30 U/mL papain (Worthington Biochemical) in CSS. DRGs were then centrifuged and triturated in 0.5 mL of 1.5 mg/mL BSA (low endotoxin) and 1.5 mg/mL trypsin inhibitor (Sigma) in DRG media [DMEM/F12 (Invitrogen) with 100 U/ml penicillin, 0.1 mg/ml streptomycin (Invitrogen), 2 mM L-glutamine (Invitrogen), and 10% fetal bovine serum (Hyclone)]. After trituration, un-dissociated pieces were removed by filtering through a 70-μm mesh (Becton Dickinson). To remove small supporting cells and small pieces of dissociated axons and myelin, density gradients with 15% BSA were applied twice. Cells were pelleted and re-suspended with 1 ml DRG media, layered on top of 15% BSA solution and centrifuged at 250 g for 10 min at 4°C; pelleted cells were then re-suspended with DRG media and went through a second round of 15% BSA purification. The cell pellet was re-suspended with 1 ml of DMEM/F12 (4°C) to get single-cell suspension. Five µl of cell suspension was counter stained with five µl of trypan blue to check neuronal number, viability, and purity. Single-cell suspension [225 ± 75 K (mean ± SD) live neurons total] was diluted to 3 ml in DMEM/F12 (4°C) before delivering to the 384 well chip on the Qube-384 instrument (Sophion A/S, Copenhagen, Denmark). An aliquot of the cell suspension was plated on a 35-mm Tissue Culture-treated culture dish and imaged using a Nikon microscope (Eclipse TE2000-U Inverted Microscope).
Automated patch-clamp
Automated patch-clamp recording was used for all experiments. Sodium currents were measured in the whole-cell configuration using a Qube-384 automated voltage-clamp system. Intracellular solution contained (in mM): 120 CsF (or KF for CC experiments), 10 NaCl, 2 MgCl2, 10 HEPES, adjusted to pH7.2 with CsOH. The extracellular recording solution contained (in mM): 145 NaCl, 3 KCl, 1 MgCl2, 1.5 CaCl2, 10 HEPES, adjusted to pH7.4 with NaOH. Liquid junction potentials calculated to be ~7 mV were not adjusted for. Currents were low pass filtered at 5 kHz and recorded at 25 kHz sampling frequency. Series resistance compensation was applied at 100% and leak subtraction enabled. The Qube-384 temperature controller was used to maintain recording for all experiments at 22 ± 2°C at the recording chamber. Appropriate filters for series resistance (<10 MOhm) and Nav current magnitude (more than baseline in the inward direction, 0 nA) were routinely applied to exclude poor quality recordings. Data analysis was performed using Analyzer (Sophion) and Prism (GraphPad Software Inc., La Jolla, CA, USA) software. More relevant information could be found in Supplemental Information.
Activation protocols
To determine the voltage-dependence of activation, we measured the peak current amplitude at test pulse potentials ranging from −120 mV to +30 mV in increments of +5 mV for 500 ms. Channel conductance (G) was calculated from peak INa:
where GNa is conductance, INa is peak sodium current in response to the command potential V, and ENa (measured on IV relationships) is the Nernst equilibrium potential. Calculated values for conductance were fit with the Boltzmann equation:
where G/Gmax is normalized conductance amplitude, Vm is the command potential, V1/2 is the midpoint voltage and k is the slope.
Steady-state inactivation protocols
The voltage-dependence of fast-inactivation was measured by preconditioning the channels from −120 to +30 mV in increments of 5 mV for 500 ms, followed by a 10 ms test pulse during which the voltage was stepped to −20 mV. Normalized current amplitudes from the test pulse were fit as a function of voltage using the Boltzmann equation:
where Imax is the maximum test pulse current amplitude.
Double Boltzmann for both activation and inactivation
Double Boltzmann fit was using the following equations:
Where y0 is offset, A is span, x01 and x02 are midpoints, k1 and k2 are slope factors, and p is fraction.
Recovery from inactivation protocols
Recovery from inactivation was measured by holding the channels at −120 mV, followed by a depolarizing pulse to 0 mV, then the potential was returned to −120 mV for different time periods. This was followed by a depolarizing 10 ms test pulse to 0 mV to measure availability. Recovery from inactivation was measured after pre-pulse durations of 20 ms and 500 ms and fit with a bi-exponential function of the form:
where t is time in seconds, Y0 is the Y intercept at t = 0, KFast and KSlow are rate constants in units the reciprocal of t, PercentFast the fraction of the Y signal attributed to the fast-decaying component of the fit.
Quantification and statistical analysis
Normalization was performed in order to control the variations in sodium channel expression and inward current amplitude and in order to be able to fit the recorded data with a Boltzmann function (for voltage-dependences) or a biexponential function (for time courses of inactivation). The Sophion Qube is an automated electrophysiology instrument that is blinded to cell selections and experimentation, and selection is performed in a randomized manner. All subsequent data filtering and analysis is performed in a non-biased manner, in which automated filters are applied to the entire dataset from a given Qube run. Fitting and graphing were done using Prism 9 software (Graphpad Software Inc., San Diego, CA), unless otherwise noted. We performed the following two-stage statistical procedure for all group comparisons. First, we performed testing for normality using the Shapiro–Wilks test. If the underlying data distribution was normal, we performed parametric statistical testing – One-way analysis of variance (ANOVA) with Bonferroni correction: when each condition was being compared other conditions; or t-test: when overall 2 conditions were being compared. If the distribution of data was non-normal, we compared groups using non-parametric testing using the Kruskal Willis test with Bonferroni correction (when there were more than 2 groups) or the Mann–Whitney U-test (for 2 groups) [Citation26]. A level of significance α = 0.05was used with p-values less than 0.05 being considered to be statistically significant. All values are reported as means ± standard error of means (SEM) or errors in fit, when appropriate, for n recordings/samples. Values are presented as mean ± SEM with probability levels less than 0.05 considered significant. The declared group size is the number of independent values, and that statistical analysis was done using these independent values. The numbers of cells in each of the experiments is provided in .
Table 1. Number of cells in each experiment/condition for male and females.
Results
Nav channel activation in rodent DRG neurons is not sex-dependent
Each of our experimental preparations included pooled neurons from 3 to 4 adult male or female mice. Upon the completion of cell preparation, the freshly isolated pooled neurons were placed on our automated patch-clamp platform. The experimenter was blinded to the sex of the mice that were used in each run until the completion of data analysis, at which point the data were decoded. All electrophysiological experiments, including cell selection, and subsequent analyses were performed in an unbiased and automated manner using the novel approach that we recently developed (see: Ghovanloo et al. 2023) [Citation9].
The first biophysical property we investigated was activation. To do this, we used a standard step-pulse to elicit peak conductance at membrane potentials between −120 and +30 mV (). We used capacitance of cells as a proxy for size [Citation27]. As Nav channels have different voltage-dependence and kinetic properties, and because DRG neurons of different sizes are known to express different Nav channel isoforms, we fit the normalized conductance-voltage (GV) relationship of every neuron with both a single and a double Boltzmann function. Depending on whether the datum was better fit with either equation, the midpoints (VD1 and VD2, for double Boltzmann, and Vs for single Boltzmann) were binned together [Citation9]. In , we show the individual GV relationships for cells that came from either male or female mice. We also show the averaged relationships for both sexes, which indicate an overlap of mean curves (). In , we show that both the capacitances, and hence neuronal sizes between the population of cells between sexes, and all activation voltage-dependent midpoints are not different from one another. These data suggest that in our cohort of neurons from males and females, the tested populations were of similar diameters, and that within these diameters, the activating properties of Nav channels were not significantly different (p > 0.05).
Figure 1. Activation of Nav channels in freshly isolated DRG neurons. a-b) all the GV data from each neuron. Each relationship displays normalized conductance as a function of membrane potential. c) averaged GV curves. d) distribution of individual midpoints on the left axis, and capacitances on the right axis. e-f) Current densities for each sex. g) averaged current densities. h) peak current densities measured at −30 mV.
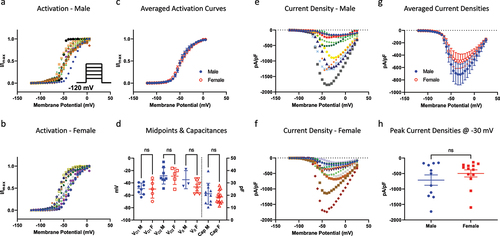
display the current densities associated with this cohort of cells. This was measured as peak sodium current divided by membrane capacitance (pA/pF), and plotted as a function of membrane potential (). Consistent with the activation data, the resulting peak current densities were not significantly different between sexes (p > 0.05) ().
No significant differences in the voltage-dependence of steady-state inactivation (SSI) between sexes
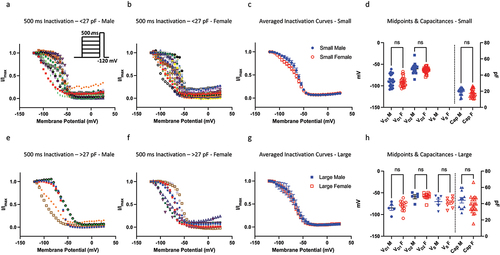
Next, we assessed the voltage-dependence of SSI of DRG neurons obtained from male and female mice from a 500-ms pre-pulse duration (). A 500-ms duration is considered to trigger an overall macroscopic intermediate inactivation, in which some channels undergo fast, and others undergo slower inactivated states [Citation9,Citation28,Citation29]. In these experiments, we recorded from neurons ranging from about 10 to 75 pF, with the mean capacitance for both sexes being around 27 pF. Based on this range of capacitances, we divided up the neurons into two size bins, in which <27 pF were considered small and >27 pF were considered large. Each of the two sizes were further divided by sex. We applied the same single vs. double Boltzmann analysis to every datum, which expectedly suggested that overall, most cells are better described with double Boltzmann equation. Within the small bin, where a greater diversity of Nav channel expression (i.e. TTX-R/TTX-S ratios) is expected, every cell was better fit with a double Boltzmann. We found that there were no significant differences in the mean neuronal capacitance distributions between males and females, and that the resulting voltage-dependent midpoints were also not significantly different (p > 0.05). These results indicate that DRG neurons have similar steady-state inactivating properties between males and females.
Figure 2. Steady-state inactivation of Nav channels. a-b) the current-voltage (IV) relationship for neurons within the <27 pF capacitive bin. The neurons within this bin have a clear bi-phasic distribution, and most of the Nav channels in these cells do not fully inactivate, i.e. there is a residual persistent current, as indicated but the low ends of the IV curves. c) averaged curves. d) distribution of individual midpoints on the left axis, and capacitances on the right axis. e-h) same info as above but for >27 pF.
Sex-dependent differences in the kinetics of recovery from inactivation
An important attribute of Nav channels is their kinetics of recovery from various types of inactivation. In a simplified model, Nav channels can occupy three distinctive states, which include resting, activated, and inactivated, which can be further subdivided into fast and slow [Citation30,Citation31]. Whereas the molecular underpinnings for both types of inactivation are fundamentally different, from an electrophysiological perspective, they both depend on the magnitude and time-course of a depolarizing pulse.
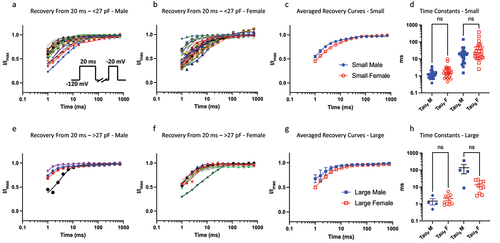
To measure recovery from fast inactivation, we held channels at −120 mV to ensure maximal Nav channel availability, then we pulsed the channels to −20 mV for 20 ms, which was followed by hyperpolarizing pulse back to −120 mV at increasing time intervals to allow recovery from inactivation. Finally, we pulsed the cells back to −20 mV as a test-pulse to measure amount of recovered Nav current (). We previously found that larger neurons have faster recovery kinetics from fast inactivation from 20 ms, as indicated by the τSlow component (but not τFast) of their recovery [Citation9]. Here, we compared these kinetic properties between males and females, and found that there are no significant differences in the τSlow between males and females (p > 0.05) in either size bins ().
Figure 3. Recovery from 20 ms inactivation. a-b) data divided up using the same bins as above for 20 ms c) displays averaged data. d) distributions of time constants. e-h) same info but for larger sized cells. The sample size in the larger bin was small for males, which may obscure statistical comparison.
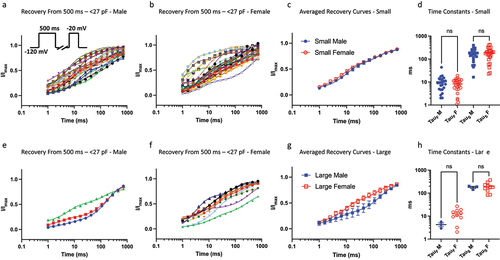
In our previous study we showed that because of the inherent variability in the gating properties of Nav channels, a 500-ms (intermediate macroscopic inactivation) pulse could cause the various Nav channel populations to become inseparable, as indicated by kinetic time constants [Citation9]. Here, we divided up the cells between sexes to determine if within the size bins, the recovery kinetics from intermediate inactivation would be different. We did not observe a significant difference between the sexes in recovery from intermediate inactivation (p > 0.05) ().
Figure 4. Recovery from 500 ms inactivation. a-b) data divided up using the same bins as above for 500 ms c) displays averaged data. d) distributions of time constants. e-h) same info but for larger sized cells. The sample size in the larger bin was small for males, which may obscure statistical comparison.
No significant differences in TTX-mediated block of Nav currents among sexes
Nav channels in DRG neurons have differential sensitivities to TTX [Citation32]. We previously showed that within our cohort of neurons, the smallest cells display variability in response to 500 nM TTX, and with the increase of cell size, the neurons show a decrease response to TTX (likely due to Nav1.8), with the largest end of the spectrum showing an increased TTX response (likely due to Nav1.6) [Citation9]. Here, we broke up the data into male vs. female, and found that the overall trends between male and female cells were similar (). Therefore, consistent with the previous results, there do not seem to be major differences in the TTX-sensitivity of Nav currents between male and female neurons.
Figure 5. Pharmacological block by 500 nM TTX; excitability using current-clamp. a-b) the fraction of Nav current inhibited by TTX vs. capacitance. In cells with capacitances from ~20–40 pF for both sexes, there is a stronger presence of TTX-R (likely Nav1.8/9). The data were fitted with a simple linear regression equation to display the trend of TTX-sensitivity of cells as a function of capacitance. The dotted lines display the 95% confidence intervals. c-d) number of AP spikes that were elicited using a standardized ramp current-clamp protocol. Sample traces for males and females.
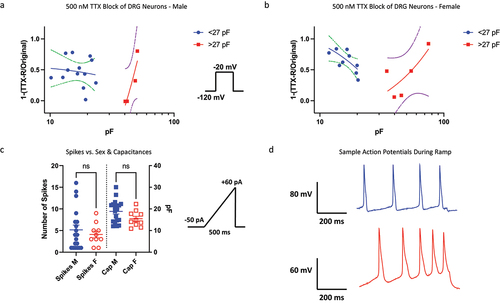
No differences in cellular excitability between sexes
Finally, we sought to determine if there are differences in the excitability of DRG neurons between sexes. We elicited action potential firing using a standard ramp current protocol in which −50 pA of current was applied the cells, which was then ramped up to +60 pA over the course of 500 ms (). We did not observe significant differences between males and females in the capacitances nor the number of spikes that were generated (p > 0.05).
Discussion
There are numerous reports of sexual dimorphism in various pain models [Citation3]. These sex-dependent differences could result in reduction of the translatability of findings from pre-clinical rodent models to larger scale clinical studies. This becomes more problematic as a similar sexual dimorphism exists in human patients. As most preclinical studies describe sex-dependent difference in injured animals [Citation3,Citation5–7], our goal in this study was to determine whether there are significant sex-dependent differences in the characteristics of Nav current in male and female WT (uninjured) mouse DRG neurons, at baseline. Given the well-established role of Nav channels within the sensory pain pathway, we focused on these channels. To achieve this objective, we reanalyzed data obtained by a new method that permits recordings from freshly isolated, diverse neurons assessed simultaneously (head-to-head) under the same conditions [Citation9].
DRG neurons are responsible for the detection of many sensory inputs [Citation33]. The membranes of these complex cells are home to a variety of different ion channel and receptor proteins, which are present at different ratios and ensembles in a given neuron. These proteins and other downstream signaling pathways work in concert to produce complex signals. A change to any of the main components of the electrogenisome within these neurons could impact their functionality [Citation10,Citation16,Citation33–35].
Our results in this study did not reveal major differences between males and females in the Nav currents within populations of DRG neurons of naive C57/BL6 mice. This is particularly important as previous studies suggest that various phenomena including injury-induced hormonal regulation of expression patterns of some vital ion channels within the sensory pathway (e.g. various TRP channels) sex-dependently [Citation22,Citation23]. It is conceivable that other ion channels and receptors could display sexual dimorphism even in naive baseline states. An important limitation in this study of previously published datasets is that the numbers of cells in some comparisons were uneven, which could have affected some of the noted conclusions. Nevertheless, given that we found no significant differences in the action potential firing of populations of neurons between sexes, we speculate that there may be no major differences in the biophysical properties of any of the vital components of the electrogenisome; this idea should be tested in future studies.
As noted in the Results section, Nav channels can exist in three fundamental states. Here, we found that, as studied on a neuronal population level, there are some but no major sex-dependent differences in these states. This has important implications for drug discovery. Given the vital role of Nav channels in pain, inhibition of these channels using different classes of compounds has been heavily pursued by academic laboratories and the pharmaceutical industry [Citation28,Citation36–38]. As such, research and development efforts often involve testing candidate compounds on primary sensory (e.g. DRG) neurons. We found that activation, steady-state inactivation, recovery from inactivation, as well as response to TTX were comparable between sexes. It is possible that the effects in large capacitance neurons could be different, but the number of neurons in our sample in this size class (>27 pF) is too small to allow us to make formal conclusions. This suggests that in vitro investigations of Nav-targeting compounds may be performed in small size WT primary cells from either sex.
In conclusion, we report that Nav currents within WT uninjured populations of DRG neurons from adult male and female mice display comparable biophysical properties. Our findings may have important implications for in vitro pharmacological studies.
Availability of materials and data
Previously published data and material are available online, and/or will be available from corresponding author.
Authors and contributors
M-R. Ghovanloo: designed study, designed protocols/experiments, assembled data, performed experiments, assay development, data analysis, figure making, funding, wrote and edited manuscript, data interpretation, and performed conceived experiments.
S. Tyagi: wrote Python code, contributed to data analysis, contributed intellectually important ideas, edited manuscript.
P. Zhao: performed neuron dissection and dissociation, and optimized cell culture.
P.R. Effraim: contributed intellectually important ideas.
S.D. Dib-Hajj: project conceptualization and funding, analysis and editing of manuscript.
S.G. Waxman: project conceptualization and funding, data curation, formal analysis, supervision, validation, investigation, methodology, writing and review and editing of manuscript, and provided project administration.
Acknowledgments
We thank the Yale StatLab for consultation on statistical testing, and Joshua Huttler for helpful discussions. This work was supported by grants from the U.S. Department of Veterans Affairs Rehabilitation Research and Development Service. The Center for Neuroscience and Regeneration Research is a Collaboration of the Paralyzed Veterans of America with Yale University. M.-R.G. is a Banting Fellow at Yale and is supported by the Canadian Institutes of Health Research (CIHR)/Grant No. 471896. S.T. was supported by the National Institutes of Health (NIH) Medical Scientist Training Program Training Grant T32GM136651.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Relieving pain in America: a blueprint for transforming prevention, care, education, and research. National Academies Press; 2011 [cited 2021 Sep 5]. Available from: https://pubmed.ncbi.nlm.nih.gov/22553896/
- James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018 [cited 2021 Sep 5];392:1789–11. Available from: http://www.thelancet.com/article/S0140673618322797/fulltext
- Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci. 2020 [[cited 2021 Aug 14]];21:353. doi: 10.1038/s41583-020-0310-6.
- Mischkowski D, Palacios-Barrios EE, Banker L, et al. Pain or nociception? Subjective experience mediates the effects of acute noxious heat on autonomic responses. Pain . 2018 [cited 2023 Apr 16];159:699. doi: 10.1097/j.pain.0000000000001132.
- Paige C, Plasencia-Fernandez I, Kume M, et al. A Female-Specific Role for Calcitonin Gene-Related Peptide (CGRP) in Rodent Pain Models. J Neurosci 2022 [cited 2023 Apr 16];42:1930–1944. Available from: https://pubmed.ncbi.nlm.nih.gov/35058371/
- Dedek A, Xu J, Lorenzo LÉ, et al. Sexual dimorphism in a neuronal mechanism of spinal hyperexcitability across rodent and human models of pathological pain. Brain. 2022 [cited 2023 Apr 16];145(3):1124–1138. Available from: https://pubmed.ncbi.nlm.nih.gov/35323848/
- Hadschieff V, Drdla-Schutting R, Springer DN, et al. Fundamental sex differences in morphine withdrawal-induced neuronal plasticity. Pain. 2020 [cited 2023 Apr 16];161(9):2022–2034. Available from: https://pubmed.ncbi.nlm.nih.gov/32345917/
- Dib-Hajj SD, Cummins TR, Black JA, et al. Sodium channels in normal and pathological pain. Annu Rev Neurosci. 2010 [cited 2018 Oct 3];33(1):325–347. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20367448
- Ghovanloo MR, Tyagi S, Zhao P, et al. High-throughput combined voltage-clamp/current-clamp analysis of freshly isolated neurons. Cell Rep Met. 2023 [cited 2023 Jan 12];3(1):100385. Available from: http://www.cell.com/article/S2667237522002909/fulltext
- Dib-Hajj SD, Yang Y, Black JA, et al. The Na v 1.7 sodium channel: From molecule to man [Internet]. Nat Rev Neurosci. 2013 [[cited 2021 Oct 21]];14:49–62. Available from: https://pubmed.ncbi.nlm.nih.gov/23232607/
- Dib-Hajj SD, Waxman SG. Sodium channels in human pain disorders: genetics and pharmacogenomics [Internet]. Annu Rev Neurosci. 2019 [[cited 2021 Oct 19]];42:87–106. Available from: https://pubmed.ncbi.nlm.nih.gov/30702961/
- Middleton SJ, Barry AM, Comini M, et al. Studying human nociceptors: from fundamentals to clinic. Brain. 2021 [cited 2023 Oct 22];144(5):1312–1335. doi: 10.1093/brain/awab048
- Zheng Y, Liu P, Bai L, et al. Deep sequencing of somatosensory neurons reveals molecular determinants of intrinsic physiological properties. Neuron [Internet]. 2019 [cited 2023 Oct 22];103(4):598–616.e7. Available from: https://pubmed.ncbi.nlm.nih.gov/31248728/
- Bennett DL, Clark XAJ, Huang J, et al. The role of voltage-gated sodium channels in pain signaling. Physiol Rev . 2019 [cited 2022 Jul 6];99:1079–1151. doi: 10.1152/physrev.00052.2017.
- Zamponi GW. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat Rev Drug Discov. 2015 [[cited 2023 Oct 22]];15:19–34. doi: 10.1038/nrd.2015.5
- Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009 [[cited 2023 Oct 22]];8:55–68. Available from: https://www.nature.com/articles/nrd2757
- Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol. 2007 [[cited 2018 Oct 3]];579:1–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17158175
- Ramachandra R, McGrew SY, Baxter JC, et al. NaV1.8 channels are expressed in large, as well as small, diameter sensory afferent neurons. Channels (Austin). 2013 [cited 2022 Jul 6];7(1):34–37. Available from: https://pubmed.ncbi.nlm.nih.gov/23064159/
- Shields SD, Ahn HS, Yang Y, et al. Nav1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain. 2012 [[cited 2022 Aug 8]];153:2017–2030. Available from: https://pubmed.ncbi.nlm.nih.gov/22703890/
- Fillingim RB, King CD, Ribeiro-Dasilva MC, et al. Gender, and pain: a review of recent clinical and experimental findings. J Pain [Internet]. 2009 [[cited 2023 Apr 16]];10:447–485. Available from: https://pubmed.ncbi.nlm.nih.gov/19411059/
- Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012 [cited 2023 Apr 16];13(12):859–866. Available from: https://pubmed.ncbi.nlm.nih.gov/23165262/
- Vandewauw I, Owsianik G, Voets T. Systematic and quantitative mRNA expression analysis of TRP channel genes at the single trigeminal and dorsal root ganglion level in mouse. BMC Neurosci. 2013 [[cited 2021 Aug 16]];14. doi: 10.1186/1471-2202-14-21
- Patil MJ, Ruparel SB, Henry MA, et al. Prolactin regulates TRPV1, TRPA1, and TRPM8 in sensory neurons in a sex-dependent manner: contribution of prolactin receptor to inflammatory pain. Am J Physiol Endocrinol Metab . 2013 [cited 2021 Aug 16];305:E1154–E1164. Available from: https://journals.physiology.org/doi/abs/10.1152/ajpendo.00187.2013.
- Patil M, Belugin S, Mecklenburg J, et al. Prolactin Regulates Pain Responses via a Female-Selective Nociceptor-Specific Mechanism. iScience 2019 [cited 2023 Apr 16];20:449–465. Available from: https://pubmed.ncbi.nlm.nih.gov/31627131/
- Paige C, Barba-Escobedo PA, Mecklenburg J, et al. Neuroendocrine mechanisms governing sex differences in hyperalgesic priming involve prolactin receptor sensory neuron signaling. J Neurosci. 2020 [[cited 2023 Apr 16]];40:7080–7090. Available from: https://pubmed.ncbi.nlm.nih.gov/32801151/
- Rochon J, Gondan M, Kieser M. To test or not to test: preliminary assessment of normality when comparing two independent samples. BMC Med Res Methodol . 2012 [cited 2023 Nov 20];12:1–11. doi: 10.1186/1471-2288-12-81.
- Limón A, Pérez C, Vega R, et al. Ca2±activated K±current density is correlated with soma size in rat vestibular-afferent neurons in culture. J Neurophysiol. 2005 [[cited 2022 Jul 7]];94:3751–3761. Available from: https://pubmed.ncbi.nlm.nih.gov/16107534/
- Ghovanloo M-R, Estacion M, Higerd-Rusli GP, et al. Inhibition of sodium conductance by cannabigerol contributes to a reduction of dorsal root ganglion neuron excitability. Br J Pharmacol . 2022 [cited 2022 Mar 17];179:4010–4030. doi: 10.1111/bph.15833.
- Gawali VS, Todt H. Mechanism of inactivation in voltage-gated Na+ channels. Curr Top Membr. 2016;78:409–450.
- Ghovanloo M-R, Aimar K, Ghadiry-Tavi R, et al. Physiology and pathophysiology of sodium channel inactivation. Curr Top Membr. 2016;78:479–509.
- Fouda MA, Ghovanloo M-R, Ruben PC. Late sodium current: incomplete inactivation triggers seizures, myotonias, arrhythmias, and pain syndromes [Internet]. J Physiol. [cited 2022 Apr 18];600:2835–2851. Available from: https://pubmed.ncbi.nlm.nih.gov/35436004/
- Hille B. Ion channels of excitable membranes. MA, USA: Sinauer; 2001.
- Waxman SG. Sodium channels, the electrogenisome and the electrogenistat: lessons and questions from the clinic. J Physiol. 2012 [[cited 2022 Nov 10]];590:2601–2612. Available from: https://pubmed.ncbi.nlm.nih.gov/22411010/.
- Ghovanloo MR, Effraim PR, Yuan JH, et al. Nav1.7 P610T mutation in two siblings with persistent ocular pain after corneal axon transection: impaired slow inactivation and hyperexcitable trigeminal neurons. J Neurophysiol. 2023 [[cited 2023 Feb 3]];129:609–618. Available from: https://pubmed.ncbi.nlm.nih.gov/36722722/
- Waxman SG, Zamponi GW. Regulating excitability of peripheral afferents: emerging ion channel targets. Nat Neurosci [Internet]. 2014 [cited 2023 Oct 23];17(2):153–163. Available from: https://pubmed.ncbi.nlm.nih.gov/24473263/
- Ahuja S, Mukund S, Deng L, et al. Structural basis of Nav1.7 inhibition by an isoform-selective small-molecule antagonist. Science. 2015;350:aac5464–aac5464. doi: 10.1126/science.aac5464.
- Alsaloum M, Higerd GP, Effraim PR, et al. Status of peripheral sodium channel blockers for non-addictive pain treatment. Nat Rev Neurol. 2020 [cited 2022 Dec 12];16(12):689–705. Available from: https://pubmed.ncbi.nlm.nih.gov/33110213/
- Bankar G, Goodchild SJ, Howard S, et al. Selective NaV1.7 antagonists with long residence time show improved efficacy against inflammatory and neuropathic pain. Cell Rep. 2018 [cited 2018 Oct 3];24(12):3133–3145. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30231997
