Abstract
Pyrolysis of nitrogen containing biofuels generates isocyanic acid (ICA) and we here studied if ICA also is present in cauterization smoke. Air sampling was performed when animal technicians that had developed airway symptoms worked with dehorning. Tissue heated in a laboratory model was used to mimic cauterization. ICA in air at the workplace exceeded 10 times the national exposure limit. In the laboratory, the ICA generated per mg tissue from heated hair, horn and nail was 13.9 ± 7.8, 24.0 ± 4.1 and 32.0 ± 2.9 µg, respectively. Three workers were medically examined and two were diagnosed with asthma and a third had severe airway problem that resembled asthma. The study shows that high levels of ICA are generated during cauterization of nitrogen-containing tissue. If this could trigger airway symptoms deserves to be investigated further.
Introduction
The monoisocyanates isocyanic acid (ICA) and methyl isocyanate (MIC) are highly reactive electrophilic compounds that in the body will cause damage to and dysfunction of proteins.Citation1–3 ICA is a strong organic acid that, together with other pollutants, has been found in fire smoke, diesel emissions and in emissions from heated products that contain urea-formaldehyde resins as binders.Citation4–9 ICA and hydrogen cyanide (HCN) have been shown during high-temperature pyrolysis of biofuels with a nitrogen content that varied between 2.9 and 15.5%. The formation of ICA did then increased proportionally with the nitrogen content of the fuel and no ICA was found from bark with only 0.4% nitrogen.Citation10 The chemistry behind the formation of ICA under these conditions is complex. It is however likely that the heating or combustion of nitrogen-containing products first gives rise to HCN, (together with ammonia or possibly other nitrogen-containing gases) and that this HCN then via the intermediate hydrogen isocyanide, is oxidized to ICA.Citation11–13
Electrocautery is a commonly used technique in hospitals and veterinary stations. The procedure will cause vaporization of tissue protein and create a smoke that contains high levels of ultrafine particles and several toxic volatile compounds.Citation14 This surgical smoke is an acknowledged risk and does therefore have to be evacuated with an appropriate exhaust ventilation system.Citation15,Citation16 As the burnet tissue contains proteins with on average 15% nitrogen, surgical smoke has also been found to contain HCN.Citation17 Other situations where nitrogen containing tissue is burnt are during hot iron dehorning of calves and hot shoeing of horses. Dehorning of cattle conveys many benefits and is therefore performed worldwide. In the United States, 94% of dairy cattle producers do this and the majority (68%) does then use hot iron dehorning.Citation18 The procedure is performed by veterinarians or specially trained animal technicians but is also often carried out by the farm owner.Citation19
We have recently examined workers (animal technicians) that have been exposed to dehorning smoke and that all develop severe respiratory symptoms. As isocyanates are asthmagenic and ICA previously have been found after high-temperature pyrolysis of nitrogen containing biofuels we have here studied if ICA, MIC and HCN also is generated during hot iron cauterization of calves (dehorning) and if it is present in the smoke formed when hair, horn and nails tissue are heated in a laboratory model at a temperature of 500 °C.
Methods
Dehorning
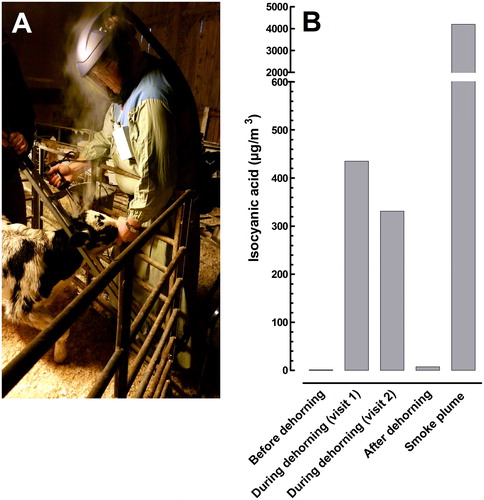
Visits and air sampling took place on three occasions in December 2017 and January 2018 when two animal technicians worked with dehorning at a local farm. The location was a room (part of a barn) with a length, width and ceiling height of approximately 5, 8 and 4 m but one side of the room was open to the rest of the barn. During a normal workday the animal technicians visited several farms and therefore consequently also conducted many dehorning sessions. The calves (normally 8-20 at each session) were anesthetized 10 minutes before the dehorning that was done with an electric soldering iron (Kerbl, Germany, no. 17460) that was pressed against the horn bud on each side during 15–20 seconds (). The temperature at the horn bud during dehorning was measured with an infrared-thermometer (Fluke 62 MAX, IR Thermometer) and was close to 500 °C.
Figure 1. An animal technician working with dehorning of a calf (A) and the levels of ICA monitored with the personal and stationary samplers (B). During the personal samplings the samplers were mounted on the patient’s chest just under the respiratory headpiece. The Swedish short-term exposure limit for ICA is 36 μg/m3. The calves were anesthetized 10 minutes before dehorning.
Air sampling
Dehorning smoke
To monitor ICA and MIC, personal and stationary air sampling was performed by drawing air through dibuthylamine-impregnated glass fiber filters with a flow rate of 0.2 L/min. The samplers were prepared in the laboratory according to a method that was previously described for end-filters in a study by Marand et al.Citation20 The personal samplers were mounted in the breathing zone on the workers chest and samplings lasted for 15 minute periods. Stationary samplings were performed in the smoke plume (over the workers head) and in the workspace before and after the working period. Midget Impinger flasks (30 mL from Werner-Glas & Instrument, Stockholm, Sweden) were used to sample HCN and air was then drawn through 10 mL of 12.5 mM borax buffer (pH 9.5) with a flow rate of 1.0 L/min. The air pumps used for samplings were calibrated with a TSI 4100 Series flow meter (TSI, MN, USA) and a BIOS DryCal DC-Lite Primary Flow Meter (BIOS, NJ, USA). Analysis of mass concentration and number of particles with diameters between 0.3 and 10 µm were performed with a Lighthouse 3116-IAQ particle monitor (Lighthouse Worldwide Solutions Fremont CA, USA) that was mounted on a tripod placed three to four meters from the worker. Data were recorded 4 times/min and logged.
Smoke from hair, horn and nails heated in a tube oven
Tissue samples (8.7–11.9 mg) of hair from one of the disbudded calves, pieces of bovine horn and fine cut human fingernails were weighed into aluminum weighing boats that were placed in a 20 × 600 mm2 glass tube that fitted an oven that was set at 500 °C (ENTECH ETF 30-6, Energiteknik AB, Ängelholm, Sweden). The two impinger flasks for sampling of ICA + MIC and HCN, respectively, were then connected to the tube via a T-tubing connector and the tube was finally inserted into the oven so that the sampling could begin. The flow through each sampler was 1.0 L/min and the sampling time was 10 minutes. Samplers for ICA and MIC were filled with 10 mL 10 mmol/L dibutylamine in toluene and the samplers for HCN with 10 mL of 12.5 mmol/L borax buffer (pH 9.5). Each impinger flask was connected with a SureSeal black polypropylene filter cassette (SKC, 225-3-23) with a 25 mm glass fiber filter (Munktell MG 160). After the sampling this filter was transferred to and mixed with the solution in the impinger flask. The air pumps that were used for sampling were from Casella CEL (Apex Pro Personal Air Sampler). The flow was calibrated with the flow meters mentioned above. A photo of the setup during sampling of smoke from the tube oven is found in supplementary material Figure S1.
Analysis
Isocyanates
ICA and MIC were analyzed with liquid chromatography-tandem mass spectrometry (LC-MS/MS). The used standards were DBA Isocyanate Monomers Mix (CRM40569) and D9-DBA Isocyanate Monomers Internal Standard Mix (CRM40570) that both were purchased from Sigma-Aldrich. Preparation of samples collected at the workplace (dry samples) was performed according to Marand et al.Citation20 Of the samples collected from the tube oven (in 10 mL 10 mM dibutylamine in toluene) 500 µL was transferred from the impinger flasks to micro tubes where they were evaporated with nitrogen and finally dissolved in 80 µL mobile phase. Analysis was performed on a TSQ Quantum Access Max (Thermo Scientific). Ten microliter of each sample was injected and the chromatographic separation was done on an Ascentis Express column (75 × 2.1 mm, 2.7 µm, Supelco) and the flow rate was 200 µL/min. The mobile phase consisted of acetonitrile:water (80:20) and 0.1% formic acid. Detection was performed with the mass spectrometer in ESI-positive mode with a capillary voltage of 3.0 kV. Vaporization and capillary temperatures were 325 and 300 °C, respectively and the collision energy was set at 15 V and the Q2 pressure was 1.5. Parent and product masses for ICA-DBA and ICA-DBA-d9 were 173.3 → 130.6 and 182.3 → 139.6 and those for MIC-DBA and MIC-DBA-d9 were 187.3 → 130.6 and 196.3 → 139.6, respectively.
Hydrogen cyanide
HCN was analyzed with a modification of a previously described method.Citation21 Here, 40 µL of samples from impinger flasks were mixed with 20 µL of 2 mM naphthalene-2,3-dicarboxaldehyde in the wells of a white 384-well plate (Nunc Maxisorp). After 4 minutes, 20 µL of 50 mM taurine was added and after four more minutes the fluorescence was read in a CLARIOstar plate reader (BMG Labtech) with excitation and emission wavelengths set at 409 nm and 460 nm. Four standards with CN-concentrations between 0.0064 and 4 µg/mL made from KCN diluted in a borax buffer were analyzed together with the samples. All chemicals were purchased from Sigma-Aldrich.
Statistics
Differences between the groups presented in were calculated using the Student’s t-test and a P value of <.05 was considered statistically significant. The correlation coefficient for the generated amounts of ICA and HCN were calculated with the Pearson’s test. The software used for calculations and preparation of graphs was Graph Pad Prism 4 (GraphPad Software, CA, USA).
Table 1. Formation of ICA, MIC and HCN from hair, horn and nails heated for 10 minutes in a tube oven set at 500 °C.
Results
Dehorning smoke and smoke from heated hair, horn and nails
The level of ICA that could be determined after personal sampling at the first and second visit was 435 and 331 µg/m3, respectively (). This was 12 and 9 times higher than the national 5-minute, short-term exposure limit. Levels in the smoke plume were 4,195 µg/m3 and those in the working space before and 10 minutes after the working period were <1 and 7 µg/m3, respectively. A mean level from the personal samplings of MIC was 1.3 µg/m3 and this was 36 times under the short-term exposure limit. HCN was measured and in two 10-minute samplings, the levels were found to be 154 and 213 µg/m3, respectively. This is 26 and 19 times under the national short-term exposure limit.
Regarding particles, the maximum mass concentration of PM10 in the workspace 3 to 4 m behind the worker was 1.7 mg/mL and the number of particles with a diameter between 0.3 and 0.5 μm exceeded 4 × 105/m3 (supplementary material Figure S2). However, during parts of the dehorning session this number would probably have been higher as the concentration limit of the instrument seems to have been exceeded.
The formation of ICA, MIC and HCN in experiments with the tube oven is shown in . Highest formation of ICA was generated from heated nail tissue followed by horn. The formation from hair was significantly lower and less than half of what was generated from nails. HCN followed the same trend as ICA and heated horn and nail tissue generated significantly more ICA than hair tissue (2.4 and 2.6 times more). There was also a significant correlation between the generated amounts of ICA and HCN (r2=076, P = .002, n = 9). A small amount of MIC was generated from all three types of tissue.
Examined workers
The first worker was a 62-year-old nonsmoker, a previously healthy man without any known allergy or hereditary predisposition for asthma or other lung diseases. He had been working in the same farm service company since 1993 and he had for 5–6 years felt increasing work-related airway symptoms when he dehorned calves. Since late 2016, he experienced increased upper airways irritations, dry coughs, breathlessness and fatigue. He was diagnosed with asthma in April 2017 and now regularly used inhalation steroids and a bronchodilator which helped quite well. Before the debut of his asthma, he had been using a simpler respirator for particles and had then also experienced problems with leakage of smoke into the headpiece. After the diagnosis, he changed to a new fan-assisted respirator with filters that trapped particles and organic, inorganic and acetic gases (). The change to the new respirator mitigated his symptoms and he could now continue to work but only part-time.
The second worker was a former colleague, a nonsmoker and previously healthy 69-year old man, who had worked with dehorning on a daily basis for 15 years. He was also diagnosed with asthma and had used the same type of simpler respirator that was only equipped with a particle filter. He had now taken an early retirement to elude the work-related symptoms.
The third worker was a present colleague to the first worker, a nonsmoker and previously healthy 63-year old woman, who had worked with dehorning almost daily for 25 years. She had experienced increasing work-related irritation from the upper respiratory tract from 3 to 4 years ago and also suffered from breathlessness and a discomfort associated with the dehorning.
Discussion
To our knowledge, this is the first time that ICA has been shown to be present in cauterization smoke. This fact could be of great interest for many that are involved in animal husbandry. We studied smoke generated during hot iron cauterization of calves but similar smoke could also be generated in other situations. One example is hot shoeing of horses. This is performed by farriers that use the technique where a heated horseshoe is pressed against and burned into the hoof. The shoe will then fit better when nailed into the hoof wall but the procedure leads to the release of a considerable amount of smoke in the direct breathing zone of the farrier.
Both measurement at the workplace and experiments with the tube oven revealed that high levels of ICA were generated. A sample taken directly in the smoke plume showed a level that exceeded the short time, occupational exposure limit with more than two orders of magnitude. Levels of MIC and HCN from the personal samplings were however low and clearly under the Swedish short-time occupational exposure limit. In our experiments using the tube oven we also found a high formation of ICA when hair and in particular horn and nail were heated. These tissues, and also hoof tissue, contain keratin that will undergo a thermal disintegration and form HCN and ICA.
Another situation where similar smoke could be generated is during operative procedures where electrocautery or surgical smoke is formed. The fact that such smoke could constitute a hazard to health service personnel has also been recognized.Citation16,Citation17 It has been estimated that there only in the US alone could be 500,000 that are exposed,Citation22 and studies have also reviled that the use of efficient exhaust ventilation systems not are widely used, this despite authoritative guidelines and recommendations.Citation23 In animal husbandry, professionals that work with dehorning of calves or hot shoeing of horses might be unaware of the presence of toxic compounds in the smoke generated when protein containing tissue is burnt. As a consequence, this could lead to their not using respirators at all or using respirators that only trap particles resulting in a substantial exposure to ICA and other toxic smoke constituents in the gas phase.
ICA, due to its instability is not used commercially and its health effects are therefore not as well studied as diisosocyanates that are widely used and for which it has been possible to study large exposed cohorts over time.Citation24,Citation25 It is however a reactive molecule that causes protein carbamylation and protein dysfunction. An increased carbamylation of proteins in vivo has been seen in patients with chronic kidney disease, characterized by elevated levels of urea and cyanate in the circulation and a possible increased risk of atherosclerosis.Citation1,Citation26,Citation27 It has also recently been shown that eosinophil peroxidase-mediated carbamylation of proteins can alter immune responses and give rise to a triggering of inflammatory signals in a similar way as could be seen in asthma.Citation28 Furthermore, cyanates could react with, and covalently bind to, glutathione which is a major lung antioxidant that plays an important role for reducing oxidative stress and airway inflammation. This will result in dysfunction and disrupt glutathione homeostasis and studies in a mice model of allergic asthma have also shown that depletion of glutation will result in an exacerbated allergic asthma.Citation29,Citation30
Unfortunately, no personal sampling of particles was performed but our assumption is that the mass concentration and number of respirable particles must have been very high in the breathing zone, in view of the fact that they were high three to four meters behind the animal technician that performed the dehorning. Cauterization smoke and surgical smoke is a complex mixture with particles and various gaseous compounds that might act as airway irritants. In this work we focused on reactive monoisocyanates and HCN but it would have been an advantage to also have analyzed other reactive compounds in the gaseous phase.
Conclusion
Tissues such as hair, horn, nail and skin are all built up by proteins such as keratin, collagen and elastin with a nitrogen content of around 15%. Together with respirable particles and toxic gaseous constituents, we here show that smoke generated when nitrogen containing tissue is heated or burnt also contain ICA. It is therefore important that personnel who in their work are exposed to such smoke also use proper respirators that both trap particles and gases. It is possible that ICA or other toxic components in the dehorning smoke played a role in the development of the severe respiratory problems that our patients developed and this deserves to be studied further.
Supplemental Material
Download MS Word (2.6 MB)Additional information
Funding
References
- Wang Z, Nicholls SJ, Rodriguez ER, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13(10):1176–1186. DOI:10.1038/nm1637.
- Gorisse LC, Pietrement V, Vuiblet V, Schmelzer CE, et al. Protein carbamylation is a hallmark of aging. Proc Natl Acad Sci USA. 2016;113(5):1191–1196. DOI:10.1073/pnas.1517096113.
- Badar A, Arif Z, Alam K. Role of carbamylated biomolecules in human diseases. IUBMB Life. 2018;70(4):267–275. DOI:10.1002/iub.1732.
- Roberts JM, Veres PR, Cochran AK, et al. Isocyanic acid in the atmosphere and its possible link to smoke-related health effects. Proc Natl Acad Sci. USA. 2011;108(22):8966–8971. DOI:10.1073/pnas.1103352108.
- Bengtström L, Salden M, Stec AA. The role of isocyanates in fire toxicity. Fire Sci Rev 2016;5:4.
- Wentzell JJ, Liggio J, Li SM, et al. Measurements of gas phase acids in diesel exhaust: a relevant source of HNCO?. Environ Sci Technol. 2013;47(14):7663–7671. DOI:10.1021/es401127j.
- Jathar SH, Heppding C, Link MF, et al. Investigating diesel engines as an atmospheric source of isocyanic acid in urban areas. Atmos Chem Phys. 2017;17(14):8959–8970. DOI:10.5194/acp-17-8959-2017.
- Karlsson D, Dalene M, Skarping G, Marand A. Determination of isocyanic acid in air. J Environ Monit. 2001;3(4):432–436.
- Westberg H, Lofstedt H, Selden A, Lilja BG, Nayström P. Exposure to low molecular weight isocyanates and formaldehyde in foundries using hot box core binders. Ann Occup Hyg. 2005;49(8):719–725. DOI:10.1093/annhyg/mei040.
- Hansson K-M, Samuelsson J, Tullin C, Åmand L-E. Formation of HNCO, HCN, and NH3 from the pyrolysis of bark and nitrogen-containing model compounds. Combust Flame. 2004;137(3):265–277. DOI:10.1016/j.combustflame.2004.01.005.
- Glarborg P, Miller JA. Mechanism and modelling of hydrogen cyanide oxidation in a flow reactor. Combust. Flame. 1994;99(3-4):475–483. https://DOI.org/10.1016/0010-2180(94)90039-6 DOI:10.1016/0010-2180(94)90039-6.
- Dagaut P, Glarborg P, Alzueta MU. The oxidation of hydrogen cyanide and related chemistry. Prog Energy Combust Sci. 2008;34(1):1–46. DOI:10.1016/j.pecs.2007.02.004.
- Glarborg P, Marshall P. Importance of hydrogen isocyanide isomer in modelling hydrogen cyanide oxidation in combustion. Energy Fuels. 2017;31:2156–2163. DOI:10.1021/acs.energyfuels.6b02085.
- Brüske-Hohlfeld I, Preissler G, Jauch KW, et al. Surgical smoke and ultrafine particles. J Occup Med Toxicol. 2008;3(1):31. DOI:10.1186/1745-6673-3-31.
- Okoshi K, Kobayashi K, Kinoshita K, Tomizawa Y, Hasegawa S, Sakai Y. Health risks associated with exposure to surgical smoke for surgeons and operation room personnel. Surg Today 2015;45(8):957–965. DOI:10.1007/s00595-014-1085-z.
- Ulmer BC. The hazards of surgical smoke. Aorn J. 2008;87(4):721–734.
- Moot AR, Ledingham KM, Wilson PF, et al. Composition of volatile organic compounds in diathermy plume as detected by selected ion flow tube mass spectrometry. ANZ J Surg. 2007;77(1-2):20–23. DOI:10.1111/j.1445-2197.2006.03827.x.
- USDA (United States Department of Agriculture). Dairy 2007, Part IV: Reference of dairy cattle health and management practices in the United States, 2007. USDA 2009. https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy07/Dairy07_dr_PartIV.pdfhttps://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy07/Dairy07_is_PartIV_Highlights.pdf
- Gottardo F, Nalon E, Contiero B, Normando S, Dalvit P, Cozzi G. The dehorning of dairy calves: practices and opinions of 639 farmers. J Dairy Sci. 2011;94(11):5724–5734. DOI:10.3168/jds.2011-4443.
- Marand A, Karlsson D, Dalene M, Skarping G. Solvent-free sampling with di-n-butylamine for monitoring of isocyanates in air. J Environ Monit. 2005;7(4):335–343. DOI:10.1039/b414761h.
- Chinaka S, Tanaka S, Takayama N, Tsuji N, Takou S, Ueda K. High-sensitivity analysis of cyanide by capillary electrophoresis with fluorescence detection. Anal Sci. 2001;17(5):649–652. DOI:10.2116/analsci.17.649.
- Steege AL, Boiano J, Sweeney MH. NIOSH Surgical Smoke and Healthcare Worker Health and Safety. Chicago: American Public Health Association; 2016.
- Steege A, Boiano JM, Sweeney MH. Secondhand smoke in the operating room? Precautionary practices lacking for surgical smoke. Am J Ind Med. 2016;59(11):1020–1031. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5069165/pdf/nihms808033.pdf DOI:10.1002/ajim.22614.
- HSE (Health and Safety Executive), Great Britain. WATCH/2008/4 Annex 2 Assessment of the potential for isocyanic acid and other monoisocyanates to cause respiratory irritation and sensitisation. Uses, occurrence and occupational exposure to monoisocyantes. Health and Safety Executive; 2008. http://www.hse.gov.uk/aboutus/meetings/iacs/acts/watch/170608/p4ann2.pdf
- Fisseler-Eckhoff A, Bartsch H, Zinsky R, Schirren J. Environmental isocyanate-induced asthma: morphologic and pathogenetic aspects of an increasing occupational disease. IJERPH. 2011;8(9):3672–3687. DOI:10.3390/ijerph8093672.
- Gajjala PR, Fliser D, Speer T, Jankowski V, Jankowski J. Emerging role of post-translational modifications in chronic kidney disease and cardiovascular disease. Nephrol Dial Transplant. 2015;30(11):1814–1824. DOI:10.1093/ndt/gfv048.
- Verbrugge FH, Tang WHW, Hazen SL. Protein carbamylation and cardiovascular disease. Kidney Int. 2015;88(3):474–478. DOI:10.1038/ki.2015.166.
- Wang Z, DiDonato JA, Buffa J, et al. Eosinophil peroxidase catalyzed protein carbamylation participates in asthma. J Biol Chem. 2016;291(42):22118–22135. DOI:10.1074/jbc.M116.750034.
- Tor-Agbidye J, Palmer VS, Spencer PS, Craig AM, Blythe LL, Sabri MI. Sodium cyanate alters glutathione homeostasis in rodent brain: relationship to neurodegenerative diseases in protein-deficient malnourished populations in Africa. Brain Res. 1999;820(1-2):12–19. DOI:10.1016/S0006-8993(98)01343-2.
- Nadeem A, Siddiqui N, Alharbi NO, Alharbi MM, Imam F, Sayed-Ahmed MM. Glutathione modulation during sensitization as well as challenge phase regulates airway reactivity and inflammation in mouse model of allergic asthma. Biochimie 2014;103:61–70. DOI:10.1016/j.biochi.2014.04.001.
