?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
For several decades the incidence of cutaneous malignant melanoma (CMM) steadily increased in fair-skinned, indoor-working people around the world. Scientists think poor tanning ability resulting in sunburns initiate CMM, but they do not understand why the incidence continues to increase despite the increased use of sunscreens and formulations offering more protection. This paradox, along with lower incidences of CMM in outdoor workers, although they have significantly higher annual UV doses than indoor workers have, perplexes scientists. We found a temporal exponential increase in the CMM incidence indicating second-order reaction kinetics revealing the existence of 2 major risk factors. From epidemiology studies, we know one major risk factor for getting CMM is poor tanning ability and we now propose the other major risk factor may be the Human Papilloma Virus (HPV) because clinicians find β HPVs in over half the biopsies. Moreover, we uncovered yet another paradox; the increasing CMM incidences significantly correlate with decreasing personal annual UV dose, a proxy for low vitamin D3 levels. We also discovered the incidence of CMM significantly increased with decreasing personal annual UV dose from 1960, when it was almost insignificant, to 2000. UV and other DNA-damaging agents can activate viruses, and UV-induced cytokines can hide HPV from immune surveillance, which may explain why CMM also occurs in anatomical locations where the sun does not shine. Thus, we propose the 2 major risk factors for getting CMM are intermittent UV exposures that result in low cutaneous levels of vitamin D3 and possibly viral infection.
Abbreviations
| CMM | = | Cutaneous Malignant HERV |
| Melanoma | = | Human endogenous retrovirus |
| HPV | = | Human Papilloma Virus |
| OR | = | odds ratio |
| IARC | = | International Agency for Research on Cancer |
| UVA | = | 316–400 nm |
| UVB | = | 290–315 nm |
| UV | = | Ultraviolet 290–400 nm |
Introduction
For several decades the incidence of cutaneous malignant melanoma (CMM) has steadily increased in fair-skinned, indoor-working people around the world.Citation1-10 Scientists are not sure why CMM has steadily increased over time but from epidemiology studies, we know that the numbers of benign naevi, light skin and hair, and poor tanning ability are involved in the etiology.Citation11 We can find clues as to what may contribute toward CMM in the paradox between indoor and outdoor worker's CMM incidences and their personal annual UV (290–400 nm) doses. Outdoor workers get 3–10 times the annual UV dose that indoor workers get,Citation12 yet they have lower incidences of CMM and have half the odds ratio (OR of about 0.8) that indoor workers have (about 1.6) for getting CMM.Citation13-16 From this data, one can conclude that something other than cumulative UV dose is primarily involved in the etiology of CMM.
Most scientists believe intermittent UV exposures resulting in sunburns initiate CMM,Citation14 but the creation and use of sunscreens did not reduce the incidence. Sunscreens with primarily UVB (290–315 nm) protection were available from the early 1950s until 1988, and sunscreens with both UVB and UVA (316–400 nm) protection were available since 1988 with increasing SPF numbers over the years (http://en.wikipedia.org/wiki/Sunscreen). Some scientists think strong UVA exposures allowed by older sunscreen formulations can also initiate CMMCitation17 with unique signature mutations,Citation18 while others believe the UVA passing through the glass of officeCitation10 and car windowsCitation19 promotes it. We now know that UVA can possibly also cause both initiation and promotion of CMM because like UVB it causes similar DNA damage, i.e., cyclobutane pyrimidine dimers.Citation20 Additionally, UVA causes oxidation of DNA bases in the cytosol prior to incorporation into the genomic DNA by polymerase etaCitation21,22 and causes DNA adduct formation in the presence of photosensitizersCitation23 that people can ingest from common foods.Citation24 Sunburns probably are not involved in the initiation or propagation of melanoma because a melanoma study using the opossum animal model, Monodelphis domestica, ironically showed intense sunburn doses of UVB gave significantly fewer melanomas than sub-erythemal doses.Citation25 UVB exposures of skin creates cutaneous vitamin D3 productionCitation26 that sunscreens dramatically reduce.Citation27 Furthermore, contrary to popular belief, outdoor workers get numerous sunburnsCitation28–31 reviewed by Glanz.Citation32
The major risk factors involved in CMM had to exist prior to the first documented increase in the incidence in 1936 (Connecticut, United States).Citation33 To understand how many major risk factors are responsible for the increasing incidence of CMM, we must know the temporal incidences as far back as possible to discern if the increase is linear (one major risk factor) or exponential (interaction between 2 major risk factors). For this reason, we analyzed the fair-skinned European countries' CMM incidences over time and personal annual UV dose for males and females from 1955 to 2000 using data from the International Agency for Research on Cancer (IARC). We found an exponential increase in the incidence of CMM, implicating 2 major risk factors. In addition, we found CMM increases with decreasing personal UV dose, implicating low cutaneous vitamin D3 levels as one of the major risk factors. We suggest the other major risk factor, besides the germline incorporated Human Endogenous Retrovirus (HERV) that is already implicated in the etiology of CMM and other cancers,Citation34 may be HPV infection because clinicians found it in over half the CMM's biopsied.Citation35-38
Materials and Methods
Temporal analysis
We analyzed the CMM incidences at 5-year interval midpoints from 1955 to 2000 for European countries using IARC's age-adjusted, world population normalized data.Citation1–9 We aggregated the regional registries for England, Germany, Poland, France, and Switzerland to estimate national incidence trends.
The temporal CMM data plotted in for all European countries was from the averaged data in and the western (<17 °E) and eastern (>17°E) countries were plotted from the averaged data in and, respectively. Because we wanted to compare the European CMM incidences of white people (skin types I and II),Citation39 we excluded countries that had populations with primarily skin types III or darker (e.g., Italy, Spain, and Portugal). To show a lack of bias, we also analyzed the data of all the countries listed in and included Italy, Spain, and Portugal and found a similar exponential increase in the CMM incidences with a slightly reduced slope (results not shown). The temporal data in was plotted from the averaged data in for countries located every 5°N from 46–50°N, 51–55°N, 56–60°N to over 60°N. In , we compare female and males in the northern (averaged 46–55°N for ∼50°N) and southern (averaged all countries above 55°N for ∼60°N) regions of Europe from the averaged data in . In some of our analyses ( and ), we included a country only if it had more than 50% of the 5-year averaged CMM incidence data; countries excluded were Austria, Czech, Belarus, Belgium, Estonia, Hungary, Ireland, Latvia, Lithuania, and Romania.
Table 1A. European countries with CMM incidence data (24 countries averaged in ).
Table 1B. Western European countries <17°E with CMM incidence data (15 countries averaged in ).
Table 1C. Eastern European countries >17°E with CMM incidence data (9 countries averaged in ).
Table 2. Only European countries with more than 50% CMM incidence data averaged every 5°N ().
Table 3. Males compared with females in northern (∼60°N) and southern (∼50°N) Europe.
Figure 1. Temporal CMM incidences among fair-skinned people in Europe (plotted from the averaged data in ), western Europe <17°E (), and eastern Europe >17°E (). Note the exponential increase in CMM over the decades (semi-log plot). See statistical data in .
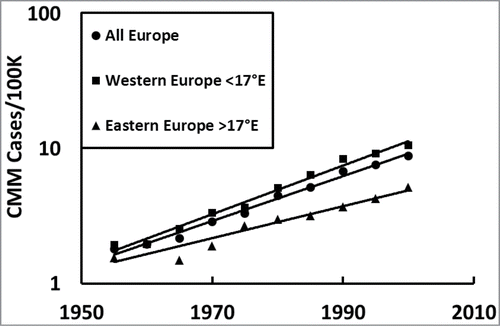
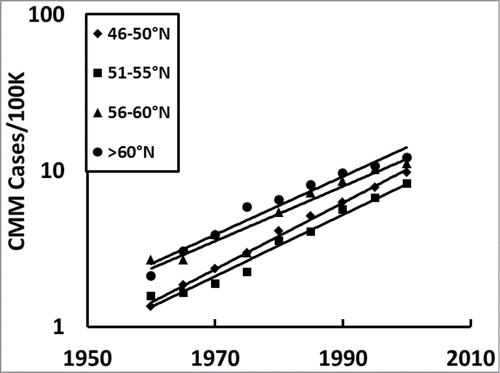
Figure 2. Temporal CMM incidences among fair-skinned people in Europe averaged every 5°N for only the European countries with more than 50% complete data sets; northern most (>60°N; mean ∼64°N), northern (∼51–55°N; mean ∼52°N), middle (56–60°N; ∼57°N), and southern Europe (∼46–50°N; mean ∼48°N) plotted from the averaged data in .
Personal annual UV dose analysis
Latitude is a proxy for personal annual UV doses. For populations living in the regions analyzed and plotted in , the average annual personal UV doses were calculated from the equation derived from the slope of the line (R2 = 0.99) from several countries known population's UV doses after geometric conversion from planar to cylinder measurements, which represent the human body.Citation40 The countries that generated this equation with average annual personal UV doses were Sweden (60°N; 5,200 J/m2), Denmark (55°N; 6,800 J/m2), the Netherlands (52.5°N; 7,000 J/m2), and the US (34°N, 10,000 J/m2 and 44°N, 12,000 J/m2):
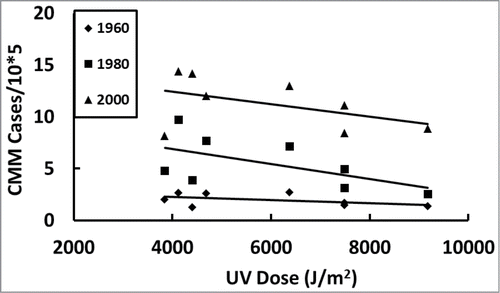
Figure 4. Average personal annual UV dose for populations of each country and CMM incidence trends in Europe over time. Eight European countries that had CMM incidence data in 1960 were followed every 20 y (; ).
Population weighting and statistical analysis
For all the temporal data, we conducted linear regression analysis using Minitab 16.2.4 (Minitab Inc.., State College, Pennsylvania) to evaluate the association between personal annual UV doses (independent variable) and log(CMM) incidence rate (dependent variable). We then performed multiple linear regressions to simultaneously assess the role of time and latitude in predicting log(CMM) incidence rate ().
Table 4. CMM incidences in 8 countries that had data in 1960 were followed every 20 y
Table 5A. Statistics for multiple linear regression of log(CMM incidence) data versus time and UV dose in all of Europe (N = 151).
Using Minitab® 16.2.4 (Penn State, State College, Pennsylvania, US), we performed weighted regression of the cancer incidences on the different personal annual UV doses in the European countries by population ().Citation45
Table 5B. Statistics for females and males in northern and southern Europe
Results
We wanted to know how fast CMM is increasing in the white populations over time and how CMM correlates with personal annual UV dose in Europe.
First, we asked how the CMM incidences changed over time in all of Europe. shows that CMM has been exponentially increasing over the decades in all of Europe (). It also shows the western European countries () have a consistently higher incidence than the eastern European countries ().
Next we asked how the southern (46–50°N), middle (51–55°N), northern (56–60°N), and northernmost (>60°N) European countries' CMM incidences changed over time to learn if the increase is linear or exponential in every latitudinal region. We only included countries with more than 50% of the available data () in so as not to skew the slopes of the trendlines by data that was only collected in the later years.
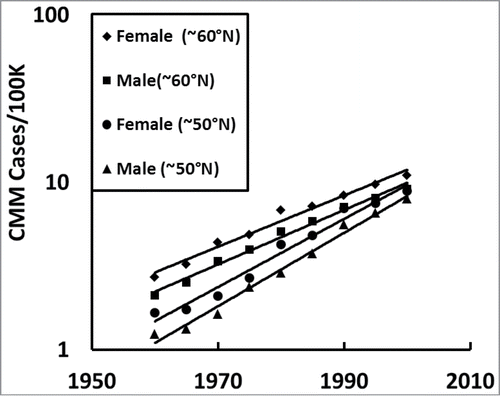
We then asked how females and males CMM rates compare in northern (∼60°N) and southern (∼50°N) Europe (). shows females always have higher incidences of CMM than males in both northern (∼60°N) and southern (∼50°N) Europe and that both sexes have temporal exponential increases in CMM.
Then we asked how the incidence of CMM varied with personal annual UV dose, starting at 1960 and assessing every 20 years, to see if CMM correlated with increasing or decreasing latitude. shows all the countries with available data in 1960 that we followed every 20 y (). In 1980 and 2000, the CMM incidence significantly increased with decreasing personal annual UV dose.
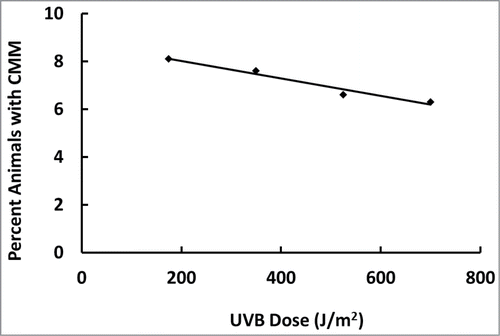
Because we found CMM incidence increases with decreasing UV dose in Europe, we plotted out Robinson et al.'s (1998) UVB-dose response dataCitation25 for the marsupial animal model because the data was obtained in a controlled experimental environment (e.g., food intake and light/dark cycles, etc.). shows the percentage of animals having CMM actually decreased with increasing UVB doses, including those producing sunburns, in a dose-dependent linear fashion (R2 = 0.96).
Figure 5. Percentage of animals with CMM decreases with increasing UVB dose in a linear dose-dependent manner (R2 = 0.96). Plotted from the data of Robinson et al.Citation25
Finally, we asked if our European CMM observations were significant or not. shows that the significance of the exponential increase in CMM over time (p < 10–30) and decreasing with UV dose (p < 2.0 × 10–7) have truly remarkably small P values. shows that both females and males in northern (∼60°N) and southern (∼50°N) Europe have significantly lower CMM incidences with higher UV dose (p < 10−6).
Discussion
We analyzed the CMM incidences in Europe to know how the rate increased over time and how it correlated with personal annual UV dose, which is a proxy for vitamin D status. We found the incidence of CMM increased at an exponential rate in Europe over time (; and ) with the western European countries having higher rates () than the eastern European countries (), possibly due to cultural differences (e.g., more time indoors) or over diagnosis. However, because this exponential increase over time is seen in both western and eastern Europe it is unlikely to be from over diagnosis, as suggested by some scientists, because it is not possible to increasingly over diagnose at an increasing rate to that extent for more than 5 decades (P < 10–7). Exponential increases occur every 5°N in Europe (;), which shows the increase is not specific to any particular latitudinal region. In addition, CMM is significantly higher for females than for males in both northern (∼60°N) and southern Europe (∼50°N;, and ). Counterintuitively, the increasing incidences of CMM significantly correlate with decreasing personal annual UV dose, a proxy for decreasing personal vitamin D3, which is most notable after 1960 when it was not significant (; ). The significant increasing slope in the incidence of CMM with decreasing UV dose over time, especially from 1980 to 2000, reveals that the gradation of lighter skin color with increasing latitude in Europe is not a reasonable explanation because it remained fairly constant. Furthermore, although the ratio of UVA to UVB increases with increasing latitude, which affects mortality of CMM,Citation46 this is not a reasonable explanation for temporal incidence increases from 1960 to 2000 because the ratio of UVA to UVB does not change very much over time. Finally, some investigators have suggested that increasing people's skin surface area from wearing smaller bathing suits over time is responsible, but this is also not a reasonable explanation because people did not expose more skin in an exponential fashion over time. Moreover, exposing more skin only increases the UV dose to those areas of the body, which is irrelevant because CMM also occurs where the sun does not shine. Instead, the temporal exponential increase shows second order reaction kinetics in the CMM incidence rates ( and ), which reveals 2 major risk factors and suggests an infectious agent or agents are involved in the etiology.
From epidemiology studies, we know one major risk factor for indoor workers‘ getting CMM is intermittent UV exposures that were believed to involve sunburn episodes based on long-term memory recall surveys.Citation47 Scientists believed outdoor workers did not get sunburns, especially blistering sunburns, like indoor workers get because outdoor workers were found to have lower incidences of CMM and half the odds ratio (about 0.8) that indoor workers have for getting it (about 1.6).Citation13-16 This belief arose because outdoor workers are exposed to UV every day, unlike indoor workers, so that their skin acclimates by thickening the stratum corneum and by producing the pigment melanin (tan). However, contrary to this popular belief, outdoor workers can get numerous sunburns and can also experience blistering eventsCitation28–31 reviewed by Glanz.Citation32 For example, the study by Buller et al.Citation30 found 45% of the ski area employees got sunburned and 8% received blistering sunburns. Another study measured alpine skiers who got 0.5–7.6 times the minimum erythemal dose (MED), or the minimum amount of UV dose needed to produce a mild sunburn, for white individuals with skin type II and 10% got more than 1 MED/h during peak exposure times.Citation48 Furthermore, Robinson et al.Citation25 found that sunburn doses of UVB yielded fewer animals with melanoma than suberythemal UVB doses (175 J/m2), which yielded the greatest percentage of opossums with melanomas. In fact, the percentage of animals with melanomas decreased with increasing UVB dose in a linear dose-dependent manner (see , R2 = 0.96). However, the decisive factor was published by Vainio et al.Citation49 concerning an official IARC report that declared sunscreens significantly reduce the incidence of sunburns but that they do not reduce the OR for getting melanoma below 1.0 like outdoor workers’ continual UV exposures do (OR∼0.8), as shown by multiple independent studies.Citation13-16 So how can sunburns be responsible for initiating melanoma?
Rather than sunburns, we propose that intermittent UV exposures result in low levels of cutaneous vitamin D3 because only UVB radiation can make vitamin D3Citation26 and UVB decreases with increasing latitudeCitation12 while the incidence of CMM increases with increasing latitude (; ). Because outdoor workers are chronically exposed to noontime UVB radiation (∼11 a.m. to 3 pm), they make plenty of vitamin D3 in their skin and have healthy blood levels. In contrast, indoor workers get intermittently exposed to UVB radiation (weekends and vacations) so that they do not have high levels of vitamin D3 in their skin or blood. Outdoor workers (gardeners) who get about 5 times the solar UV dose that indoor workers get have about twice the vitamin D blood levels that indoor workers have.Citation50 Additionally, indoor workers get exposed to only UVA radiation that passes through glass windows while they work in their officesCitation10 and drive in their cars.Citation19 UVA cannot make vitamin D3Citation26 but rather breaks it down in the skin, capillaries, and when bound to the vitamin D-binding protein.Citation51 Recently, the UVA passing through airplane windows has been implicated as the cause for pilots and flight attendants having twice the incidence of CMM as the general population.Citation52
Vitamin D3 is important in the etiology of CMM because melanoma cells can convert it to the hormone, 1,25 dihydroxyvitamin D3, or calcitriol,Citation53 and initiate an apoptotic cell death mechanismCitation54 via the nuclear vitamin D receptor (VDR).Citation55 We can find evidence of vitamin D-induced suicidal death of melanoma cells from VDR polymorphisms that result in an increased risk for CMM, reviewed by Denzer et al.Citation56 and decreased survival of patients.Citation57 Moreover, melanoma patients, who get regular, moderate sun exposures live longer than those who do notCitation58 and CMM patients were found to have deficient vitamin D levels when compared with patients who did not have CMM.Citation59 Furthermore, calcitriol controls the growth of melanoma cells,Citation60-62 inhibits tumor promotionCitation63 and angiogenesis,Citation64 and boosts the immune system.Citation65–67 Recently, the importance of vitamin D in T cell activation has been revealed; they cannot kill virally infected cells or cancer cells unless they have enough vitamin D.Citation65
Other than our data suggesting low vitamin D3 levels as one of 2 major risk factors for getting CMM, we believe the other major risk factor is HPV infection. Although we do not present any data to support our belief, we think HPV is involved because HPV-38 is found in over half the CMM biopsiesCitation35–38 and HPV infection is increasing at an exponential rate in Europe.Citation68 Clinicians found different strains of type b HPV, primarily HPV-38 and 16/18, in CMM and they found other strains of HPV, primarily HPV-77, in non-melanoma skin cancers.Citation69 The presence of HPV shortens the latency period of squamous cell carcinoma from ∼15–20 y to ∼2–5 y69 and it might also shorten the latency period of CMM, which could explain why clinicians observe a seasonal diagnostic pattern.Citation70–72 HPV incorporates into the host's genomic DNA during the DNA damage repair processesCitation73–75 and once incorporated deploys its carcinogenic regime of E6 and E7 proteins that inactivate p53Citation76 and Bak,Citation77 respectively, so that the cell cannot die via either the DNA-damage or receptor-initiated apoptotic pathways.Citation78 However, vitamin D3/calcitriol can initiate a p53-independent apoptotic cell death pathwayCitation79 circumventing HPV's plan for cellular immortalizationCitation80 by destroying the infected cell. Note that one of HPV's survival strategies is the production of E2 protein that causes the infected cell to release IL-10Citation81 helping to conceal HPV from immune surveillance.Citation82 Moreover, the opossums that got melanomas in the Robinson et al. 1998 studyCitation25 were infected with PV, as demonstrated by UVB-induced papillomas.Citation83 In addition, it now appears that HPV can establish a latent infectionCitation84 that may also have consequence both in the UV interaction and in the disruption of normal skin, which has been implicated in melanoma.Citation85 HPV may represent the first step in the transformation process because it's E6 and E7 proteins immortalize the melanocyteCitation80 setting it up for accumulating DNA mutations over time and possibly activating HERV, as other viruses are known to do.
Herein we present plausible explanations for the paradoxes observed over the decades from the second order reaction kinetics that reveals the existence of 2 major risk factors in the etiology of CMM. From epidemiology studies, we know intermittent UV exposures are a risk factor for getting CMM; we propose low cutaneous levels of vitamin D3, rather than sunburns are a risk factor for getting CMM and we suggest the other major risk factor, along with HERV, may be HPV infection.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Drs. Michael F Holick and Dale R Tavris for helpful scientific discussions.
Funding
Under an agreement with the United States Department of Energy, the Oak Ridge Institute for Science and Education supported SJ Merrill's work at the Food and Drug Administration.
References
- Doll R, Payne P, Waterhouse JAH, eds. Cancer Incidence in Five Continents, Vol. I. Geneva: union internationale contre le cancer, 1966.
- Doll R, Muir CS, Waterhouse JAH, eds. Cancer Incidence in Five Continents, Vol. II. Geneva: union internationale contre le cancer, 1970.
- Waterhouse J, Muir CS, Correa P, Powell J, eds. Cancer Incidence in Five Continents, Vol. III; IARC Scientific Publications, No. 15. Lyon: IARC, 1976.
- Waterhouse J, Muir CS, Shanmugaratnam K, Powell J, eds. Cancer Incidence in Five Continents, Vol. IV; IARC Scientific Publications, No. 42. Lyon: IARC, 1982.
- Muir CS, Waterhouse J, Mack T, Powell J, Whelan SL, eds. Cancer Incidences in Five Continents, Vol. V; IARC Scientific Publications, No. 88. Lyon: IARC, 1987.
- Parkin DM, Muir CS, Whelan SL, Gao Y-T, Ferlay J, Powell J, eds. Cancer Incidence in Five Continents, Vol. VI; IARC Scientific Publications, No. 120. Lyon: IARC, 1992.
- Parkin DM, Whelan SL, Ferlay J, Raymond L, and Young J, eds. Cancer Incidence in Five Continents, Vol. VII; IARC Scientific Publications, No. 143. Lyon: IARC, 1997.
- Parkin DM, Whelan SL, Ferlay J, Teppo L, and Thomas DB, eds. Cancer Incidence in Five Continents, Vol. VIII; IARC Scientific Publications, No. 155. Lyon: IARC, 2002.
- Curado M P, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, and Boyle P, eds. Cancer Incidence in Five Continents, Vol. IX; IARC Scientific Publications, No. 160. Lyon: IARC, 2007.
- Godar DE, Landry RJ, Lucas AD. Increased UVA exposures and decreased cutaneous vitamin D(3) levels may be responsible for the increasing incidence of melanoma. Med Hypotheses 2009; 72:434-43; PMID:19155143; http://dx.doi.org/10.1016/j.mehy.2008.09.056
- Holman CD, Armstrong BK, Heenan PJ, Blackwell JB, Cumming FJ, English DR, Holland S, Kelsall GR, Matz LR, Rouse IL, et al. The causes of malignant melanoma:results for the west australian lions melanoma research project. Recent Results Cancer Res 1986; 102:18-37; PMID:3738185; http://dx.doi.org/10.1007/978-3-642-82641-2_3
- Godar DE. UV doses worldwide. Photochem Photobiol 2005; 81:736-49; PMID:15819599; http://dx.doi.org/10.1562/2004-09-07-IR-308R.1
- Kennedy C, Bajdik CD, Willemze R, de Gruijl FR, Bouwes Bavinck J. Leidin cancer study:the influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J Invest Dermatol 2003; 120:1087-93; PMID:12787139; http://dx.doi.org/10.1046/j.1523-1747.2003.12246.x
- Gandini S, Sera F, Cattaruza MS, Pasquini P, Picconi O, Boyle P. Meta-analysis of risk factors for cutaneous melanoma: II. sun exposure. Eur J Cancer 2005; 41:45-60; PMID:15617990; http://dx.doi.org/10.1016/j.ejca.2004.10.016
- Chang YM, Barrett JH, Bishop DT, Armstrong BK, Bataille V, Bergman W, Berwick M, Bracci PM, Elwood JM, Ernstoff MS, et al. Sun exposure and melanoma risk at different latitudes: a pooled analysis of 5700 cases and 7216 controls. Int J Epidemiol 2009; 38:814-30; PMID:19359257; http://dx.doi.org/10.1093/ije/dyp166
- Radespiel-Tröger M, Meyer M, Pfahlberg A, Lausen B, Uter W, Gefeller O. Outdoor work and skin cancer incidence: a registry-based study in Bavaria. Int Arch Occup Env Health 2009; 82:357-63; PMID:18649084; http://dx.doi.org/10.1007/s00420-008-0342-0
- Setlow RB, Grist E, Thompson K, Woodhead AD. Wavelengths effective in induction of malignant melanoma. Proc Natl Acad Sci U S A 1993; 90:6666-70; PMID:8341684; http://dx.doi.org/10.1073/pnas.90.14.6666
- Halliday GM, Agar NS, Barnetson RS, Ananthaswamy HN, Jones AM. UV-A fingerprint mutations in human skin cancer. Photochem Photobiol 2005; 81:3-8; PMID:15335275; http://dx.doi.org/10.1562/2004-07-27-IR-247.1
- Moehrle M, Soballa M, Korn M. UV exposure in cars. Photodermatol Photoimmunol Photomed 2003; 19:175-81; PMID:12925188; http://dx.doi.org/10.1034/j.1600-0781.2003.00031.x
- Tewari A, Sarkany RP, Young AR. UVA1 induces cyclobutane pyrimidine dimers but not 6-4 photoproducts in human skin in vivo. J Invest Dermatol 2011; 132:394-400; PMID:21975824
- Shimizu M, Gruz P, Kamiya H, Masutani C, Xu Y, Usui Y, Sugiyama H, Harashima H, Hanaoka F, Nohmi T. Efficient and erroneous incorporation of oxidized DNA precursors by human DNA polymerase eta. Biochemistry 2007; 46:5515-22; PMID:17439242; http://dx.doi.org/10.1021/bi062238r
- Hidaka K, Yamada M, Kamiya H, Masutani C, Harashima H, Hanaoka F, Nohmi T. Specificity of mutations induced by incorporation of oxidized dNTPs into DNA by human DNA polymerase eta. DNA Repair (Amst) 2008; 7:497-506; PMID:18242151; http://dx.doi.org/10.1016/j.dnarep.2007.12.005
- Gange RW, Levins P, Murray J, Anderson RR, Parrish JA. Prolonged skin photosensitization induced by methoxsalen and subphototoxic UVA irradiation. J Invest Dermatol 1984; 82:219-22; PMID:6699425; http://dx.doi.org/10.1111/1523-1747.ep12260043
- Sayre RM, Dowdy JC. The increase in melanoma: are dietary furocoumarins responsible? Med Hypotheses 2008; 70:855-9; PMID:17881138; http://dx.doi.org/10.1016/j.mehy.2007.07.029
- Robinson ES, Hubbard GB, Colon G, Vandeberg JL. Low-dose UV exposure early in development can lead to widespread melanoma in the opossum model. Int J Exp Pathol 1998; 79:235-44; PMID:9797719
- MacLaughlin JA, Anderson RR, Holick MF. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science 1982; 216:1001-3; PMID:6281884; http://dx.doi.org/10.1126/science.6281884
- Matsuoka LY, Wortsman J, Hanifan N, Holick MF. Chronic sunscreen use decreases circulating concentrations of 25-hydroxyvitamin D. A preliminary study. Arch Dermatol 1988; 124:1802-4; PMID:3190255; http://dx.doi.org/10.1001/archderm.1988.01670120018003
- Geller AC, Glanz K, Shigaki D, Isnec MR, Sun T, Maddock J. Impact of skin cancer prevention on outdoor aquatics staff: the Pool Cool Program in Hawaii and Massachusetts. Preventive Med 2001; 33:155-61; PMID:11522155; http://dx.doi.org/10.1006/pmed.2001.0870
- Gies P, Wright J. Measured solar ultraviolet radiation exposures of outdoor workers in Queensland in the building and construction industry. Photochem Photobiol 2003; 78:342-8; PMID:14626661; http://dx.doi.org/10.1562/0031-8655(2003)078%3c0342:MSUREO%3e2.0.CO;2
- Buller DB, Andersen PA, Walkosz BJ, Scott MD, Cutter GR, Dignan MB, Zarlengo EM, Voeks JH, Giese AJ. Randomized trial testing a worksite sun protection program in an outdoor recreation industry. Health Edu Behavior 2005; 32:514-35; PMID:16009748; http://dx.doi.org/10.1177/1090198105276211
- Wright CY, Brogniez CKPN, Sivakumar V, Coetzee G, Metzger JM, Auriol F, Deroo C, Sauvage B. Sunburn risk among children and outdoor workers in South Africa and Reunion Island coastal sites. Photochem Photobiol 2013; 89:1226-33; PMID:23809057; http://dx.doi.org/10.1111/php.12123
- Glanz K, Buller DB, Saraiya M. Reducing ultraviolet radiation exposure among outdoor workers: state of the evidence and recommendations. Environ Health 2007; 6:22; PMID:17686155; http://dx.doi.org/10.1186/1476-069X-6-22
- Roush GC, Schymura MJ, Holford TR. Patterns of invasive melanoma in the connecticut tumor registry. is the long-term increase real? Cancer 1988; 61:2586-95; PMID:3365679; http://dx.doi.org/10.1002/1097-0142(19880615)61:12%3c2586::AID-CNCR2820611233%3e3.0.CO;2-2
- Downey RFS, FJ. Wang-Johanning F. Ambs S. Giles FJ. Glynn SA. Human endogenous retrovirus K and cancer: Innocent bystander or tumorigenic accomplice? Intl J Cancer 2014; PMID:24890612; http://dx.doi.org/10.1002/ijc.29003
- Dréau D, Culberson C, Wyatt S, Holder WD Jr.. Human papilloma virus in melanoma biopsy specimens and its relation to melanoma progression. Ann Surg 2000; 231:664-71; PMID:10767787; http://dx.doi.org/10.1097/00000658-200005000-00006
- Ambretti S, Venturoli S, Mirasoli M, La Placa M, Bonvicini F, Cricca M, Zerbini M, Roda A, Musiani M. Assessment of the presence of mucosal human papillomaviruses in malignant melanomas using combined fluorescent in situ hybridization and chemiluminescent immunohistochemistry. J Dermatol 2007; 156:38-44; PMID:17199564; http://dx.doi.org/10.1111/j.1365-2133.2006.07541.x
- La Placa M, Ambretti S, Bonvicini F, Venturoli S, Bianchi T, Varotti C, Zerbini M, Musiani M. Presence of high-risk mucosal human papillomavirus genotypes in primary melanoma and in acquired dysplastic melanocytic naevi. Br J Dermatol 2005; 152:909-14; PMID:15888145; http://dx.doi.org/10.1111/j.1365-2133.2005.06344.x
- Ruer JB, Pépin L, Gheit T, Vidal C, Kantelip B, Tommasino M, Prétet JL, Mougin C, Aubin F. Detection of α- and β-human papillomavirus (HPV) in cutaneous melanoma: a matched and controlled study using specific multiplex PCR combined with DNA microarray primer extension. Exp Dermatol 2009; 18:857-62; PMID:19469900; http://dx.doi.org/10.1111/j.1600-0625.2009.00866.x
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 1988; 124:869-71; PMID:3377516; http://dx.doi.org/10.1001/archderm.1988.01670060015008
- Pope SJ, Godar DE. Solar UV geometric conversion factors: horizontal plane to cylinder model. Photochem Photobiol 2010; 86:457-66; PMID:20059727; http://dx.doi.org/10.1111/j.1751-1097.2009.00679.x
- Godar DE, Tang R, Merrill SJ. Pharyngeal and cervical cancer incidences significantly correlate with personal UV doses among whites in the united states. Anticancer Res 2014; 34:4993-5000; PMID:25202082
- Godar DE, Wengraitis SP, Shreffler J, Sliney DH. UV doses of Americans. Photochem Photobiol 2001; 73:621-9; PMID:11421067; http://dx.doi.org/10.1562/0031-8655(2001)073%3c0621:UDOA%3e2.0.CO;2
- Godar DE. UV doses of American children and adolescents. Photochem Photobiol 2001; 74:787-93; PMID:11783934; http://dx.doi.org/10.1562/0031-8655(2001)074%3c0787:UDOACA%3e2.0.CO;2
- Jones CA, Huberman E, Cunningham ML, Peak MJ. Mutagenesis and cytotoxicity in human epithelial cells by far- and near-ultraviolet radiations: action spectrum. Radiation Res 1987; 110:224-54; PMID:3575654; http://dx.doi.org/10.2307/3576902
- European Commission. Population statistics 2004. Luxembourg 2004. http://ec.europa.eu/eurostat/web/population-demography-migration-projections/population-data/main-tables, accessed February 25, 2015.
- Garland CFG FC. Gorham ED. Epidemiological evidence for different roles of ultraviolet A and B radiation in melanoma mortality rates. Annal Epidemiol 2003; 13:395-404; PMID:12875796; http://dx.doi.org/10.1016/S1047-2797(02)00461-1
- Elwood JM, Gallagher RP, Hill GB, Pearson JCG. Cutaneous melanoma in relation to intermittent and constant sun exposure – the western canada melanoma study. British J Cancer 1985; 35:427-33; PMID:3988369
- Rigel EG, Lebwohl MG, Rigel AC, Rigel DS. Ultraviolet radiation in alpine skiing: magnitude of exposure and importance of regular protection. Arch Dermatol 2003; 139:60-2; PMID:12533166; http://dx.doi.org/10.1001/archderm.139.1.60
- Vainio H, Miller AB, Bianchini F. An international evaluation of the cancer-preventive potential of sunscreens. Int J Cancer 2000; 88:838-42; PMID:11072258; http://dx.doi.org/10.1002/1097-0215(20001201)88:5%3c838::AID-IJC25%3e3.0.CO;2-X
- Devgun MS, Paterson CR, Johnson BE, Cohen C. Vitamin D nutrition in relation to season and occupation. Am J Clin Nutr 1981; 34:1501-4; PMID:7270473
- Webb AR, deCosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab 1989; 68:882-7; PMID:2541158; http://dx.doi.org/10.1210/jcem-68-5-882
- Sanlorenzo M, Wehner MR, Linos E, Kornak J, Kainz W, Posch C, Vujic I, Johnston K, Gho D, Monico G, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol 2014; PMID:25517516; http://dx.doi.org/10.1001/jamadermatol.2014.4643
- Reichrath J, Rech M, Moeini M, Meese E, Tilgen W, Seifert M. In vitro comparison of the vitamin D endocrine system in 1,25(OH)2D3-responsive and -resistant melanoma cells. Cancer Biol Ther 2007; 6:48-55; PMID:17172823; http://dx.doi.org/10.4161/cbt.6.1.3493
- Danielson C, Fehsel K, Polly P, Carlberg C. Differential apoptotic response of human melanoma cells to 1α,25-dihydroxyvitamin D3 and its analogues. Cell Death Different 1998; 5:946-51; PMID:9846181; http://dx.doi.org/10.1038/sj.cdd.4400437
- Deeb KK, Trump DL, Johnson CS. Vitamin D signaling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 2007; 7:684-700; PMID:17721433; http://dx.doi.org/10.1038/nrc2196
- Denzer N, Vogt T, Reichrath J. Vitamin D receptor (VDR) polymorphisms and skin cancer: a systematic review. Dermatoendocrinol 2011; 3:205-10; PMID:22110781; http://dx.doi.org/10.4161/derm.16519
- Köstner K, Denzer N, Müller CS. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res 2009; 29:3511-36; PMID:19667145
- Berwick M, Armstrong BK, Ben-Porat L, Fine J, Kricker A, Eberle C, Barnhill R. Sun exposure and mortality from melanoma. J Nat Cancer Inst 2005; 97:195-9; PMID:15687362; http://dx.doi.org/10.1093/jnci/dji019
- Cornwell ML, Comstock GW, Holick MF, Bush TL. Prediagnostic serum levels of 1,25-dihydroxyvitamin D and malignant melanoma. Photodermatol Photoimmunol Photomed 1992; 9:109-12; PMID:1300138
- Eisman JA, Barkla DH, Tutton PJ. Suppression of in vivo growth of human cancer solid tumor xenografts by 1,25-dihydroxyvitamin D3. Cancer Res 1987; 47:21-5; PMID:3024816
- Colston K, Colston MJ, Feldman D. One,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinol 1981; 108:1083-6; PMID:6257495; http://dx.doi.org/10.1210/endo-108-3-1083
- Frampton RJ, Omond SA, Eisman JA. Inhibition of human cancer growth by 1,25-dihydroxyvitamin D3 metabolites. Cancer Res 1983; 43:4443-7; PMID:6307514
- Chida K, Hashiba H, Fukushima M, Suda T, Kuroki T. Inhibition of tumor promotion in mouse skin by 1 α, 25-dihydroxyvitamin D3. J Cancer Res 1985; 45:5426-30; PMID:3840412
- Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE. 1α,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circulation Res 2000; 87:214-20; PMID:10926872; http://dx.doi.org/10.1161/01.RES.87.3.214
- von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol 2010; 11:344-9; PMID:20208539; http://dx.doi.org/10.1038/ni.1851
- Yang S, Smith C, DeLuca HF. One α, 25-dihydroxyvitmain D3 and 19-nor-1 α, 25-dihydroxyvitmain D3 suppress immunoglobulin production and thymic lymphocyte proliferation in vivo. Biochem Biophys Acta 1993; 1158:279-86; PMID:8251528; http://dx.doi.org/10.1016/0304-4165(93)90026-5
- Yang S, Smith C, Prahl JM, Luo X, DeLuca HF. Vitamin D deficiency suppresses cell-mediated immunity in vivo. Arch Biochem Biophys Acta 1993; 303:98-106; PMID:8489269; http://dx.doi.org/10.1006/abbi.1993.1260
- Näsman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, Ahrlund-Richter S, Marklund L, Romanitan M, Lindquist D, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer 2009; 125:362-6; PMID:19330833; http://dx.doi.org/10.1002/ijc.24339
- Hengge UR. Role of viruses in the development of squamous cell cancer and melanoma. Adv Exp Med Biol 2008; 624:179-86; PMID:18348456; http://dx.doi.org/10.1007/978-0-387-77574-6_14
- Akslen LA, Hartveit F. Cutaneous melanoma–season and invasion? A preliminary report. Acta Dermato-Venereologica 1988; 68:390-4; PMID:2461021
- Braun MM, Tucker MA, Devesa SS, Hoover RN. Seasonal variation in frequency of diagnosis of cutaneous malignant melanoma. Melanoma Res 1994; 4:235-41; PMID:7950359; http://dx.doi.org/10.1097/00008390-199408000-00005
- Blum A, Ellwanger U, Garbe C. Seasonal patterns in the diagnosis of cutaneous malignant melanoma: analysis of the data of the German Central Malignant Melanoma Registry. British J Dermatol 1997; 136:968-9; PMID:9217839; http://dx.doi.org/10.1111/j.1365-2133.1997.tb03947.x
- Bouwes Bavinck JN, Neale RE, Abeni D, Euvrard S, Green AC, Harwood CA, de Koning MN, Naldi L, Nindl I, Pawlita M, et al. Multicenter study of the association between betapapillomavirus infection and cutaneous squamous cell carcinoma. Cancer Res 2010; 70:9777-86; PMID:21098702; http://dx.doi.org/10.1158/0008-5472.CAN-10-0352
- Viarisio D, Mueller-Decker K, Kloz U, Aengeneyndt B, Kopp-Schneider A, Gröne HJ, Gheit T, Flechtenmacher C, Gissmann L, Tommasino M. E6 and E7 from β HPV38 cooperate with ultraviolet light in the development of actinic keratosis-like lesions and squamous cell carcinoma in mice. PLoS Pathog 2011; 7; PMID:21779166; http://dx.doi.org/10.1371/journal.ppat.1002125
- Williams VM, Filippova M, Soto U, Duerksen-Hughes PJ. HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Future Virol 2011; 6:45-57; PMID:21318095; http://dx.doi.org/10.2217/fvl.10.73
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993; 75:495-505; PMID:8221889; http://dx.doi.org/10.1016/0092-8674(93)90384-3
- Jackson S, Harwood C, Thomas M, Banks L, Storey A. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev 2000; 14:3065-73; PMID:11114894; http://dx.doi.org/10.1101/gad.182100
- Godar DE. Light and death: photons and apoptosis. J Investig Dermatol Symp Proc 1999; 4:17-23; PMID:10537002; http://dx.doi.org/10.1038/sj.jidsp.5640175
- Beer TM, Myrthue A. Calcitriol in cancer treatment: from the lab to the clinic. Mol Cancer Ther 2004; 3:373-81; PMID:15026558
- Le Poole IC, van den Berg FM, van den Wijngaard RM, Galloway DA, van Amstel PJ, Buffing AA, Smits HL, Westerhof W, Das PK. Generation of a human melanocyte cell line by introduction of HPV16 E6 and E7 genes. Vitro Cell Dev Biol Anim 1997; 33:42-9; PMID:9028834; http://dx.doi.org/10.1007/s11626-997-0021-6
- Bermúdez-Morales VH, Peralta-Zaragoza O, Alcocer-González JM, Moreno J, Madrid-Marina V. IL-10 expression is regulated by HPV E2 protein in cervical cancer cells. Mol Med Rep 2011; 4:369-75; PMID:21468579; http://dx.doi.org/10.3892/mmr.2011.429
- McLaughlin-Drubin ME, Meyers J, Munger K. Cancer associated with human papillomaviruses. Curr Opin Virol 2012; 2:459-66; PMID:22658985; http://dx.doi.org/10.1016/j.coviro.2012.05.004
- Kusewitt DF, Applegate LA, Ley RD. Ultraviolet radiation-induces skin tumors in a South American opossum (Monodelphis domestica). Vet Pathol 1991; 28:55-65; PMID:2017828; http://dx.doi.org/10.1177/030098589102800108
- Doorbar J. Latent papillomavirus infections and their regulation. Curr Opin Virol 2013; 3:416-21; PMID:23816390; http://dx.doi.org/10.1016/j.coviro.2013.06.003
- Haass NK, Meenhard H. Normal human melanocyte homeostasis as a paradigm for understanding melanoma. J Invest Dermatol Symp Proc 2005; 10:153-63; PMID:16358819; http://dx.doi.org/10.1111/j.1087-0024.2005.200407.x